Abstract
Adaptation of behavior and physiology to changes in the ambient light level is of critical importance to life. These adaptations include light modulation of neuroendocrine function and temporal alignment of physiology and behavior to the day:night cycle by the circadian clock. These non-image forming (NIF) responses can function independent of rod and cone photoreceptors but depend on ocular light reception, suggesting the participation of novel photoreceptors in the eye. The discovery of melanopsin in intrinsically photosensitive retinal ganglion cells (ipRGCs) and genetic proof for its important role in major NIF responses have offered an exciting entry point to comprehend how mammals adapt to the light environment. Here, we review the recent advances in our understanding of the emerging roles of melanopsin and of ipRGCs. These findings now offer new avenues to understand the role of ambient light in sleep, alertness, dependent physiologies and potential pharmacological intervention as well as lifestyle modifications to improve the quality of life.
Keywords: melanopsin (OPN4), retinal ganglion cells (RGC), intrinsically photosensitive retinal ganglion cell (ipRGC), retina, non-image forming (NIF) photoresponse, the circadian clock, opsin
Three types of photoreceptors- rod, cone and ipRGC
Rods and cones in the outer retina are the predominant photoreceptor cells of the mammalian retina. Their high temporal and spatial sensitivity to light forms the basis of image forming (IF) vision. Severe disruption of rod/cone function or rod/cone cell death leads to the loss of IF vision. However, for decades it has been known that many patients and animal models with substantial rod/cone loss could support some non-image forming (NIF) functions 1-3 (Box 1), which are abolished in subjects who have lost both eyes 4. The peak spectral sensitivity of many of these NIFs in individuals and animal models lies in ∼460-500 nm range in both normal and blind subjects with substantial rod/cone loss 5-11, thus suggesting alternative photoreceptors play an important role in NIF responses. The discovery of melanopsin in a small subset of retinal ganglion cells (RGCs) in the inner retina 12, 13, the intrinsic photosensitivity of these cells 14, 15 and the genetic proof that rod, cone and melanopsin account for all ocular photoresponses in mammals 8, 16-19 have now made it possible to comprehensively understand how both inner and outer retina photoreceptors function together to adapt to the ambient light environment. Recent advances have formally linked melanopsin function to several physiological and behavioral responses to light in various mammals. These studies will help scientists understand how effective lighting strategies, pharmacological intervention and current medical practices affect the quality of life.
Box 1. Light adaptation or NIF visual photoresponse.
In addition to the image forming function, the eye also mediates several light dependent reflexes, physiologies and behaviors. These NIF responses include:
Circadian photoentrainment: In most animals, an intrinsic circadian oscillator helps organisms temporally orchestrate behavior and physiology to the appropriate time of the day. In many organisms including mice and human, the intrinsic periodicity of the circadian clock is close to, but not exactly 24 hours. To be an effective timekeeping mechanism, the circadian clock needs to be synchronized with the ambient light:dark environment on a daily basis. Light perceived through the eye acts as a strong stimulus to entrain the circadian oscillator with the ambient light:dark cycle. The threshold sensitivity for shifting the phase the circadian clock is of several seconds or minutes – orders of magnitude less sensitive than IF vision. Such requirement for integration of light information over longer timescale helps maintain a robust circadian clock in the face of occasional light noise in nature such as lightening. Attenuation of light input to the circadian clock has been documented in the Opn4-/- mice 16, 17.
Pupillary light reflex (PLR): Acute constriction of the pupil in response to an increase in light intensity received at the retina. The response is consensual or in other words, shining a beam of light in one eye constricts the pupil in the other eye. Under high light intensity melanopsin supports sustained pupil constriction 18.
Light suppression of activity: The acute reduction in activity of nocturnal animals in response to light during their activity phase. Mice lacking melanopsin show acute activity suppression at the beginning of a light pulse, but progressively increase activity under prolonged illumination 106.
Alertness: Diurnal organisms including human show improved alertness and mood under bright light 10.
Acute suppression of pineal melatonin: In mammals, the major source of circulating melatonin is the pineal gland. In both diurnal and nocturnal animals, melatonin synthesis and circulating levels peak during the night. Light for several minutes to hours can acutely suppress pineal melatonin synthesis and secretion. Peak spectral sensitivity of this response is distinct from those of rod/cone photoreceptors 107 and is intact in retinal degeneration (rd) mice 108. Mice homozygous for the rd allele are visually blind as a result of a primary degeneration of the rods and a secondary loss of cones, but they retain melanopsin-containing RGCs. Mice deficient in both rod/cone and melanopsin system show no light suppression of pineal melatonin synthesis pathway 19.
Light modulation of sleep: Light suppresses sleep in diurnal animals, whereas it enhances sleep in nocturnal animals. A light pulse during the dark period failed to induce sleep in Opn4-/- mice. The involvement of melanopsin in the direct effects of light is restricted to the dark period. Furthermore, Opn4-/- mice sleep approximately one hour less than the wild-type mice under a 12 hr/12hr light-dark schedule 109-111.
Light exacerbation of migraine: Light exacerbates migraine headache, and this effect is intact in blind individuals with light perception but not in patients who have no light perception. Direct projections of ipRGCs to the thalamic region, which is implicated in migraine pain, offers a neural mechanism for the light exacerbation response 43.
Allodynia to light or photophobia: Several individuals and some blind people show aversion to light. Intact photophobia in blind patients implies potential involvement of the melanopsin system. Additionally, young rodent pups (less than 10-days old) show photophobia before the rod/cone system is fully functional 112. Fully active melanopsin system at this age 67 most likely mediates such photophobia.
Melanopsin is an opsin class of G-protein coupled receptor (GPCR) first discovered in the photosensitive skin melanophores of Xenopus laevis (and hence the name) 20, where it mediates adaptation of skin pigmentation to ambient light level. Subsequently, melanopsin was discovered in the retina of several vertebrates (reviewed in 21). In primates and rodents, melanopsin protein (also called OPN4) is exclusively expressed in a small subset of RGCs of the inner retina that are intrinsically photosensitive (ipRGCs) (Figure 1). Research primarily in the mouse has demonstrated that the light response properties of melanopsin and the ipRGCs are distinct from those of the photopigments and photoreceptors of the outer retina. For simplicity, we will describe the molecular functions of melanopsin and cellular roles of ipRGCs as “the melanopsin system”. Specifically, the four defining properties of the melanopsin system are: i) spectral sensitivity; ii) retinoid use; iii) signal transduction; and iv) the unusual cellular architectures of the ipRGCs. Here, we describe our current understanding of the molecular function of melanopsin, the cellular architecture of ipRGCs and the integration of rod/cone and melanopsin function in determining NIF responses. We conclude by discussing the potential implications of these responses for human health and disease.
Figure 1. Melanopsin expressing RGCs and their spectral properties.
(a) Schematic diagram of the mammalian retina showing different cell types and their connectivity. The rod and cone photoreceptors densely packed in the outer retina are the primary photoreceptors supporting IF vision. Light activated signals originating from the rod/cone cells are processed in the horizontal (H), bipolar (B) and amacrine cells (A) before reaching the RGC of the inner retina. A small percentage of RGCs express melanopsin and are intrinsically photosensitive (ipRGCs). The ipRGCs, like other RGCs also receive signals originating from the outer retina rod/cone photoreceptors. RPE: retinal pigment epithelium; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer.
(b) Melanopsin expressing RGCs in rodents exhibit diversity in their cellular architecture 68, 116. Dendrites of the M1 subtype primarily arborize in the outer half of the IPL, the sublamina a (OFF sublamina). Dendrites of the M2 subtype stratify in the inner sublamina of the IPL, the sublamina b (ON sublamina), whereas the M3 subtype stratify in both sublaminae a and b. In general, ON and OFF bipolar cells have its terminals in the sublamina b (ON sublamina) and sublamina a (OFF sublamina), respectively. However, the ON bipolar cells have unusual ectopic synaptic contacts with the M1 cell type in the OFF sublamina 70.
(c) Mouse retina flat mount stained with anti-melanopsin antibody (red). The distribution of melanopsin-staining cells is almost uniform across the mouse retina, whereas in the primate retina, the fovea is largely devoid of ipRGCs 57. The somata of melanopsin-positive RGCs have sparsely branching dendrites that are relatively long (up to several hundred microns). The dendritic fields of these RGCs in primate and mouse retina have an average diameter of 0.5 mm 57 and 0.3 mm 65, respectively. Thus, despite the limited expression of melanopsin in only 1–2% of RGCs, these RGCs form a diffuse photosensitive web that covers virtually the entire retina. Melanopsin immunoreactivity is found throughout the dendrites, soma and axons, which contrasts with rods and cones whose photopigment expression is restricted to the outer segments.
(d) Spectral sensitivity of rod, cones and ipRGCs and the spectral composition of indoor fluorescent lamps and sunlight. Maximum light sensitivities of human rods (R), S cones, M cones and L cones are to ∼500 nm, ∼420 nm, ∼530 nm and ∼560 nm wavelengths, respectively. The ipRGCs exhibit peak sensitivity at ∼480 nm. The emission spectra for popular fluorescent lamps (black line) used for indoor lighting and sunlight (grey line; two hours after sunrise in San Diego, April 2010) were measured and analyzed by EPP2000 UV-VIS spectrometer and SpectraWiz software (StellarNet Inc.).
Melanopsin photopigment
Melanopsin photopigment shows peak spectral sensitivity at ∼480 nm, which lies in the blue-cyan range of the visible light and is distinct from those of classical rod/cone opsins (Figure 1d 22). The peak sensitivity correlates with the photosensitivity of several NIF responses of animals or humans under natural condition of prolonged light exposure when rod and cones have saturated or adapted, thus suggesting an important role of melanopsin in several NIF responses.
Melanopsin, like other members of the opsin class of photopigments, uses 11-cis retinaldehyde as a chromophore (light sensing ligand. Figure 2), which upon light activation, photoisomerizes to all-trans retinal and thereby causes a conformational change in the protein and activates downstream signaling proteins. The light activated or meta-state of classical vertebrate rhodopsin photopigment is unstable at physiological temperature. Drosophila rhodopsin, on the other hand, after activation by blue light, transits into a relatively stable meta-state that continues to activate downstream signaling proteins and upon excitation with long wave length orange light, the meta-state returns to the blue-sensitive basal state (reviewed in 23). Purified melanopsin from amphioxus is similarly bistable 24. Although direct experimental evidence with purified mammalian melanopsin is still lacking, some observations suggest a bistable nature of melanopsin 25, 26. In Xenopus oocytes expressing mouse melanopsin, the melanopsin photocurrent is sustained for >10 min after lights off. However, upon coexpression of arrestin the photocurrent is returned to baseline within approximately 2 min after lights off 27. Arrestin desensitizes activated GPCRs 28, and prolonged melanopsin photocurrent in the absence of arrestin is reminiscent of prolonged depolarization of Drosophila photoreceptor cells lacking arrestin 29. Therefore, the meta-state melanopsin might be stable. If the meta-state mammalian melanopsin behaves similar to the purified amphioxus melanopsin or Drosophila rhodopsin, a long wave length light pulse might return the meta-state melanopsin to the basal-blue absorbing state. In support of this hypothesis, melanopsin driven photoresponses are potentiated by prior illumination with red-shifted light 26, 30, 31. Despite these observations, direct proof of melanopsin bistability in the intact ipRGCs and its implication in natural light environment are yet to be addressed. Clear understanding of the meta-state melanopsin will impact how changes in spectral quality of ambient light modulate melanopsin signaling.
Figure 2. The visual cycle and phototranduction in the vertebrate retina.
In the rod outer segment (ROS), light converts 11-cis retinal chromophore of rhodopsin to all-trans retinal. All-trans retinal is released from rhodopsin and undergoes an elaborate multistep enzymatic process (visual cycle) to regenerate 11-cis retinal. All-trans retinal is first reduced to all-trans retinol by retinol dehydrogenase 8 (RDH8) and RDH12. In the RPE, all-trans retinol is esterified by LRAT to all-trans retinyl esters. RPE65 mediates the conversion of all-trans retinyl esters to 11-cis retinol, which is oxidized to 11-cis retinal by RDH5. 11-cis retinal returns to the ROS where it binds to opsin to regenerate rhodopsin photopigment. In the ipRGCs, melanopsin is found in complex with 11-cis retinal 22. The source of this chromophore and the mechanism for regeneration of 11-cis- from all-trans- retinal photoproduct is currently not known. Evidence points to both photoisomerization of all-trans retinal to 11-cis by melanopsin itself and use of the RPE visual cycle.
The retinal source for melanopsin
The photochemical and spectral properties of melanopsin have clear implications for human lifestyle and disease conditions. The initial source of 11-cis retinal and the steps leading to melanopsin pigment regeneration after photoactivation are not well known. Because defects in retinoid metabolism are implicated in many human diseases and conversely, components of retinoid metabolism pathway have been the focus of several therapeutic approaches 32, understanding retinoid use by melanopsin is important. In the retina, the retinal pigment epithelium (RPE) serves as the major local store of 11-cis retinal and also a site for regeneration of cis-retinoid from all-trans retinoid (Figure 2). 11-cis retinal from the RPE supports outer retina rod/cone photoreceptor functions 32. There is some evidence that the RPE also supports melanopsin function, but there is substantial debate on the mechanism by which RPE affects melanopsin function. Mice lacking critical enzymes of RPE retinoid metabolism (Rpe65-/- or Lrat-/-) produce limited quantities of cis retinoids, 33 34 which are largely used by the outer retina rod photoreceptors, leaving little retinoid for melanopsin function. Accordingly Rpe65-/- and Lrat-/- mice show reduced melanopsin photosensitivity; reduced PLR sensitivity and reduced sensitivity of the circadian clock to light. These NIF responses can be improved by the exogenous supplementation of cis-retinal or by the genetic ablation of the outer retina photoreceptors 35-37. These studies have clearly demonstrated that disruption of RPE function or survival might also affect melanopsin function. Therefore, it is likely that in visually blind patients carrying hypomorphic or null alleles of Rpe65 38-40, severe loss of PLR and poor sleep quality could arise from attenuated melanopsin function.
The residual photosensitivity in Rpe65-/- and Lrat-/- mice suggest melanopsin or the ipRGCs might utilize a self sustaining mechanism for recycling some all-trans retinal photoproduct to 11-cis retinal 37. There is indirect evidence that melanopsin can photoisomerize all-trans retinal to 11-cis retinal and thereby can regenerate an active photopigment 27. However, purified melanopsin from mouse retina was found to be in a complex with 11-cis retinal only 22. This suggests that either the all-trans retinal photoproduct is spontaneously isomerized to 11-cis retinal and stays bound to the opsin or the melanopsin-all-trans retinal complex is unstable and the all-trans retinal dissociates after initial light activation, leading to photobleaching of melanopsin. Support for both photoisomerization and photobleaching mechanisms in the mammalian ipRGCs exists. Several labs have shown that ipRGCs can be repeatedly photoactivated without exogenous retinal, which supports photoisomerization within the ipRGCs 14, 15. However, some labs have also observed a reduction in photoresponses or photobleaching by up to 70% upon repeated photostimulation of the ipRGC 41. It is safe to conclude that some melanopsin is bleached upon illumination and regenerated in the intact retina from cell autonomous or extracellular sources, whereas some melanopsin can photoisomerize the all-trans retinal and regenerate functional photopigment. The mechanisms and molecules that determine the steady-state levels of melanopsin photopigment are yet to be discovered.
Partial bleaching of melanopsin and its dependence on the RPE for (at least some) retinal supply raises some interesting clinical issues. One of the emerging therapies for preventing or slowing the progression of some forms of blindness is based on attenuating the visual cycle in the RPE cells or reducing the availability of retinoid to the photoreceptors 42. One of such inhibitors of visual cycle, all-trans-retinylamine, acutely reduces the available 11-cis retinal and consequently attenuates rod/cone function leaving the melanopsin system almost intact 37. However, the effect of chronic administration of these drugs on melanopsin system is currently unknown and needs careful assessment. If melanopsin is photobleached under prolonged illumination, the opportunity arises to develop melanopsin inhibitors, which can outcompete cis-retinal and lock melanopsin in an inactive state. Such inhibitors might induce a “pharmacological darkness” and could alleviate melanopsin-dependent exacerbation of migraine pain in normal and blind individuals (see 43).
Melanopsin protein shares sequence and functional similarities with invertebrate opsins
Molecular interaction with immediate downstream signaling proteins, subsequent signaling intermediates and effector channels are determined by the amino acid sequence of an opsin. Melanopsin shares more sequence similarity with invertebrate rhodopsins than with vertebrate rhodopsins 12. Several features of the ipRGC photosensitivity also exhibit characteristic features of invertebrate photoreceptors (Box 2). Specifically, ipRGCs depolarize upon light activation 14, 15; photoactivation causes a transient increase in cytosolic Ca2+ levels 44 and the photocurrent generated by ipRGCs exhibits a voltage-current relationship that resembles of the transient receptor potential (TRP) class of inward-rectifying cation channels 45. Furthermore, intact melanopsin phototransduction persists in mice carrying loss-of-function mutations in vertebrate rhodopsin signaling components, such as a downstream G-protein (Gnat-/-), the signaling intermediate phosphodiesterase (Pde6b-/- or Rd1) and an effecter channel (Cng3-/-) 8, 19. Consequently, many blind patients carrying mutations in rhodopsin signaling components likely have intact ipRGC function.
Box 2. Rhodopsin phototransduction in vertebrates and invertebrates exhibit several key differences.
In vertebrates (Figure Ia), a light-activated metarhodopsin triggers a pertussis toxin-sensitive class of G protein (Gt, transducin), which in turn, activates a PDE that hydrolyzes 3′-5′ cyclic guanosine monophosphate (cGMP) to 5′ cGMP. Light-activated hydrolysis of cGMP leads to closure of the cyclic nucleotide-gated (CNG) ion channels and hyperpolarization of the photoreceptor cells. In contrast, the invertebrate cascade (Figure Ib) is initiated by the activation of the pertussis toxin-insensitive Gαq class of G protein, which in turn activates phospholipase C-β (PLCβ). Activated PLCβ catalyzes the conversion of phosphatidylinositol-4,5-bisphosphate (PIP2) to inositol-1,3,5-triphosphate (IP3) and diacylglycerol (DAG). DAG is further catalyzed to produce poly-unsaturated fatty acid (PUFA). Events downstream of activation of PLC are complex and might involve IP3, DAG and PUFA as signaling intermediates to activate TRP cation channels, which results in the influx of Na+ and Ca2+ as well as membrane depolarization (reviewed in 113). IP3 can also trigger an increase in cytosolic Ca2+ level by release from Ca2+ stores 23.
Figure I. (Box 2) Phototransduction mechanisms of classical vertebrate and invertebrate rhodopsins.
The vertebrate rod/cone opsin phototransduction signaling cascade (a) is distinct from that of invertebrate rhodopsin (b).
The scarcity of ipRGCs makes it nearly impossible to employ the same set of biochemical approaches that proved successful in characterizing rhodopsin signaling processes from native photoreceptors. Instead, Xenopus melanopsin in native melanophores and heterologously expressed mammalian melanopsin have been important starting points to study melanopsin function. In both systems, light stimulated melanopsin triggers Gαq/Gα11 activation (Box 2)27, 46, 47, which in turn signal through PLCβ to trigger opening of a TRPC class of ion channel and increase in cytosolic Ca2+. A similar signaling cascade likely functions in the native ipRGCs. Melanopsin mediated photocurrent in the ipRGCs can be blocked by specific inhibition of Gq/G11 class of G proteins and of PLCβ 48. Furthermore, light activation triggers increase in cytosolic Ca2+ in the ipRGCs 49 and the melanopsin photocurrent shows characteristic features of TRP class of ion channel 45. In summary, these observations indicate melanopsin employs a downstream signaling scheme similar to that of Drosophila rhodopsin, which is distinct from the vertebrate rod/cone signaling pathway.
According to the current model of the melanopsin signaling cascade, each critical component – the effecter G protein, the signal amplifying cascade mediated by PLC, and the effecter ion channels – is encoded by functionally redundant family members that are expressed in almost all mammalian cells. Furthermore, several GPCRs can promiscuously signal through nonpreferred G proteins and downstream signaling cascades. Accordingly, purified melanopsin also activates the transducin class of G proteins (Gt) 50 and open a CNG class of channel 30 – both of which are downstream effectors of the vertebrate rhodopsin cascade. This implies that the loss of any single signaling component might not completely abolish melanopsin initiated photoresponses. Conversely, ectopically expressed melanopsin in any mammalian cell will likely find a signaling cascade. The ectopic expression of melanopsin in RGCs renders them light sensitive with properties similar to those of the native ipRGCs 51. This raises the potential for the application of melanopsin as a therapeutic optogenetic tool (Box 3) for treating several human diseases including blindness.
Box 3. Optogentics.
Optogentics generally refers to combining optical stimulation of a light sensitive protein to probe or to alter cellular function. Prevalent applications involve expression of a bacterial or algal rhodopsin in a neuronal cell type of interest and precise millisecond scale optical stimulation. Because these rhodopsins are a natural chimera between light sensitive opsin and an effecter channel, high-speed opening and closing of the channel by millisecond scale light pulses is achieved. The sluggish response of melanopsin makes it unsuitable for such popular optogenetic applications, but offers unique advantages for specific usage. As such, melanopsin can be used to mimic signaling by any Gq/G11 class of GPCR. Signaling events downstream of melanopsin increase intracellular Ca2+ level. High intracellular Ca2+ triggers phosphorylation of cyclic AMP/Ca response element binding protein (CREB) and thus leads to light mediated transcriptional activation of CREB targets 114. Because melanopsin uses a multistep intracellular signaling cascade, significant signal amplification is naturally achieved at each step of the cascade. Recombinant adenoassociated virus-mediated expression of melanopsin in a large number of RGCs of mice with extensive degeneration of rod/cone cells restores some visual functions at normal indoor light levels 51. Use of channel rhodopsin also restores some visual functions but only at high intensity light levels equivalent to midday sunlight 115. These early successes along with the fact that melanopsin is naturally expressed in humans raises the hope that melanopsin might be the tool of choice in some optogenetics-based gene therapy approaches.
Photoresponses of the ipRGCs
In addition to the unique chromophore use and signaling properties of melanopsin, ipRGCs also have exclusive properties among photoreceptor cells. In the mammalian retina, up to 20 different types of RGCs can be distinguished based on their signaling properties and neuroanatomy 52. The defining features of ipRGCs in different species are the expression of melanopsin protein and the resultant intrinsic photosensitivity. In each human eye, up to 3,000 RGCs out of ∼1.5 million stain positively for melanopsin 53. In each mouse retina, up to 1,500 RGCs out of approximately 50,000 express melanopsin 54. Unlike the regionally concentrated rod/cone opsins in classical photoreceptor cells, melanopsin immunostaining does not show any regional preference within the cell; almost uniform melanopsin staining is observed along the soma, dendrites and to some extent in the axons of the ipRGCs 13 (Figure 1c).
The intrinsic photosensitivity of ipRGCs distinguishes them from those of the rod/cone photoreceptor cells: ipRGCs have a high threshold for activation, a long latency to respond and take long time to return to base line 15. Such response properties allow the integration of light information over long periods of illumination such that the ipRGCs function as irradiance detectors. The mechanism underlying the sluggish response of melanopsin is currently unknown. Single-photon responses of melanopsin have clearly shown that the photopigment is at least as sensitive as the classical rod/cone photopigments 41. However, unlike classical vertebrate or invertebrate photoreceptors in which the photopigments and downstream signaling components are concentrated in subcellular compartments, the diffuse distribution of melanopsin in the ipRGC membrane and of other signaling components in the ipRGCs likely leads to the sluggish response. In support of this observation, ectopic expression of one of the fastest acting photopigments – Drosophila Rh1 rhodopsin – along with its downstream signaling components in mammalian neurons produces a sluggish response qualitatively similar to melanopsin 55.
The ipRGCs, like other RGCs, also transduce rod/cone-initiated light responses 56. Recordings from primate ipRGCs show distinct rod, cone and melanopsin-initiated responses in the ipRGCs 57. Under dim light conditions (scotopic conditions), rods primarily detect light, and the rod-initiated light response depolarizes ipRGCs and triggers action potentials that are sustained throughout the duration of the light pulse. With the gradual increase in light intensity within the working range of rods, there is a corresponding increase in ipRGC firing. As the light intensity increases to levels encountered during daytime, the rods are bleached. Under such lighting, both cones and melanopsin initiated light responses are detected in the ipRGCs. The L (long wavelength) and M (medium wavelength) cone-initiated light signals cause transient depolarization of the ipRGCs at onset and offset of light and, therefore, reliably encode lights on or off, but are unreliable for encoding light intensity over long duration. The intrinsic melanopsin-mediated photoresponses begin after a few milliseconds of cone-initiated response and are sustained for the duration of illumination. The activation of rods as well as L and M cones activate (“on”) ipRGCs, and the S (short wavelength) cones trigger an “off” response. However, under natural daylight, the intrinsic sustained melanopsin response likely overrides the S-dependent off response. Altogether, the primate ipRGC responses predicted that under dim light condition (approaching the limit of human vision), rod-initiated light signal likely supports NIF responses, whereas under natural daylight condition, melanopsin-initiated responses tonically encode light intensity information and support NIF responses 57. Accordingly, cone responses alone in mice are insufficient to sustain normal NIF responses under daytime light levels 58. A recent study in humans has carefully dissected the roles of cones and melanopsin in NIF responses 59. At the beginning of a long bright light pulse, cone signals are as effective as melanopsin signals in suppressing pineal melatonin release. However, with time the cone contribution decays exponentially and melanopsin functions as the predominant NIF photopigment under natural long-duration high intensity light. Under moderate light levels, both cones and melanopsin participate in setting the phase of the human circadian clock.
How do rod, cone and melanopsin photoresponses integrate? In rodents, specific ablation of the ipRGCs leads to almost complete loss of all NIF responses leaving the IF responses nearly intact (Figure 3.). This implies that rod- and cone-initiated light signal destined for NIF responses are predominantly transmitted through the ipRGCs and hence the ipRGCs constitute the principal cellular node integrating light responses from all three photopigment systems. However, it is still unclear whether melanopsin expression in ipRGCs affects its role in mediating rod/cone-initiated responses. For example, are melanopsin and outer retina responses simply additive or does melanopsin somehow augments rod/cone-initiated responses? In summary, the net light signal through the ipRGCs supports most NIF responses, and therefore, the ipRGCs constitute the central cellular framework for NIF responses.
Figure 3. The ipRGCs as the site of signal integration.
The rod and cone photoreceptors of the outer retina signal via multisynaptic pathways to the RGCs of the inner retina. The RGCs, in turn, transfer the visual signals from the eye to the brain via their axonal projections. For NIF visual functions, the light information originating from the rods and cones are exclusively transmitted through the ipRGCs. The ipRGCs function as the node for integrating melanopsin and rod/cone initiated photoresponses. ipRGCs likely participate in the IF vision by two potential mechanisms. In the retina they also affect function of the dopamine-responsive amacrine cells, 95 which then affect adaptation of the rod/cone-initiated signals under prolonged illumination. The ipRGCs also send projections to the dorsal LGN (lateral geniculate nucleus) in the brain, which receives extensive innervations from other RGCs 57.
Ontogeny, architecture and projections of the ipRGCs
The ipRGCs are born along with other RGCs in rodents, and melanopsin expression begins in utero long before the rod/cone photoreceptors are fully functional 60. In humans, the melanopsin system is also fully functional in utero, as premature babies born after 33 weeks show clear pupil constriction in response to light 61. The genetic circuitry specifying ipRGC identity or melanopsin expression is still not understood. The master transcription factors Math5 and Brn3, which specify RGC differentiation, also specify ipRGC cell fate 62-64, although the downstream regulators specifying ipRGC identity or regulating melanopsin expression are currently unknown.
The neuroanatomy of ipRGCs holds many clues of their functions. In the primate retina, ipRGCs are among the RGC cell types with the largest soma and the most extensive dendritic arborization 57. Unlike most other RGC cell types that are arranged in a nonoverlapping cobblestone pattern, the dendrites of the ipRGCs extensively overlap with each other 13, 65. By comparing the morphology of dendrites and light responsiveness, melanopsin expressing RGCs were classified into (at least) three subtypes, M1- M3 (Figure 1b. Reviewed in 66), although a recent paper doubts the definition of M3 subtype as a separate cell type 65. Physiological responses of the mouse ipRGCs also exhibit significant diversity with respect of threshold sensitivity, the magnitude of responses and deactivation rates 67. The M1 and M2 cell types in mouse retina exhibit distinct photosensitive properties 68. Despite these detail descriptions of morphological and physiological diversity, it is still unclear whether such diversity is conserved in primates and whether each cell type serves any specific purpose.
Dendrites of the predominant M1 and M2 subtype ipRGC stratify either in the on or off sublaminae of the inner plexiform layer (IPL) where they receive synaptic inputs from bipolar and amacrine cells 69, 70. Factors that specify dendritic stratification in the retina or factors that determine projections of RGCs to the target brain areas will also have significant effect on normal function of the melanopsin system. Accordingly, mice lacking a critical factor Dscam that specifies dendritic stratification and spreading show aberrant dendritic morphology of the ipRGCs 71.
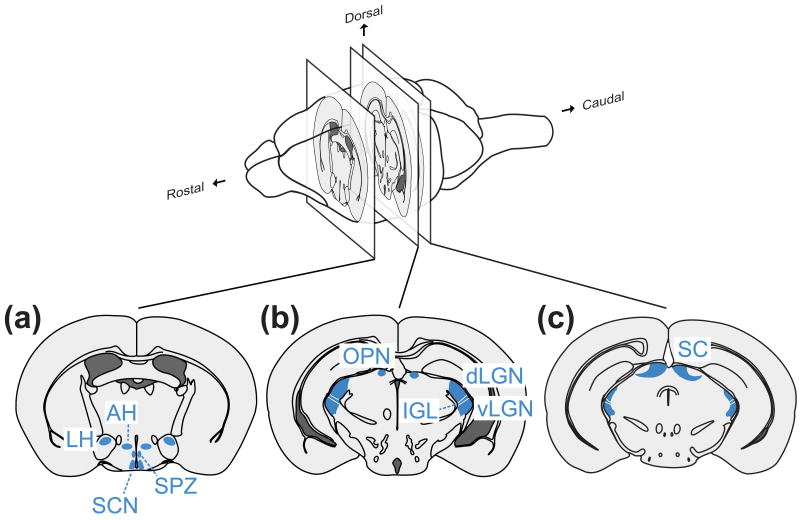
The axons of the ipRGCs exit the retina and project to distinct regions of the brain (Figure 4). Several studies in different rodents have mapped the central projections of the ipRGCs 14, 72, 73. The current and most comprehensive analysis of the central projections from the ipRGCs was done with Opn4tau:LacZ mouse, a transgenic mouse with the reporter tau:LacZ knocked into the melanopsin locus 14, in which the M1 subclass of ipRGCs are mostly labeled 74. Detailed projections of ipRGCs are described in Hattar et al 75. Unlike most other RGCs whose axons cross the optic chiasma and primarily project to the contralateral side of the brain, ipRGCs show interesting projections. Immediately after the optic chiasma, the ipRGCs from one eye almost equally innervate both left and right halves of the master circadian brain center – the suprachiasmatic nucleus (SCN). Beyond the SCN, the ipRGCs, like the other RGCs, project contralaterally to brain regions that directly or indirectly regulate other NIF processes. These regions include the intergeniculate leaflet (IGL), which indirectly entrains the circadian clock, and the olivary pretectal nucleus (OPN), the center controlling pupil constriction. Both the SCN and OPN predominantly receive ipRGC input, thus suggesting a unique axon guidance mechanism likely mediating such dominant connections. Several additional brain centers also receive sparse projections from the ipRGCs. They include: the lateral hypothalamus, the ventrolateral preoptic nucleus, the habenulla and the subparaventricular zones. These projections likely mediate effects of light on the hypothalamic regulation of sleep, behavior and physiology. Multisynaptic projections are responsible for the ipRGC regulation of pineal melatonin 76. Phenotypic assessments of mice lacking melanopsin or those with targeted ablation of ipRGCs also exhibit specific defects in light-dependent behaviors supported by these brain centers (reviewed in 66), thus validating functional significance of ipRGC projections. Recently described sparse projections of ipRGCs in the posterior thalamus found juxtaposed to dura-sensitive thalamocortical neurons are proposed to mediate light exacerbation of migraine pain 43.
Figure 4. Central projections of the ipRGCs.
A schematic diagram of summarizing the brain regions innervated with the ipRGC axons. SCN: suprachiasmatic nucleus; LH: lateral hypothalamus; AH: anterior hypothalamus, SPZ: subparaventricular zone; OPN: olivary pretectal nucleus; IGL: intergeniculate leaf; dLGN: dorsal lateral geniculate nucleus; vLGN: ventral lateral geniculate nucleus; SC: superior colliculus.
New mouse lines that comprehensively mark most ipRGCs have identified additional ipRGC brain targets. The superior colliculus (SC) and the ventral and dorsal lateral geniculate nucleus (vLGN and dLGN) receive substantial axonal projections from ipRGCs 77 (Figure 4). Both SC and LGN receive extensive innervations from other RGCs and are primary relay centers for IF vision. ipRGC projections to the LGN is also found in the primate brain 57. Accordingly, some human blind patients with substantial loss of rod/cone photoreceptors 11 have rudimentary visual perception. In normal individuals, ipRGC projections to the LGN and SC might provide brightness information for IF vision. In summary, the ipRGCs extensively innervate several brain regions mediating NIF responses and also innervate the LGN and SC where they likely encode irradiance information for IF vision.
Genetics of the melanopsin system
Our knowledge about the role of melanopsin and ipRGCs in NIF responses has largely come from rodent genetics. Comparative analyses of light-dependent phenotypes of mice lacking melanopsin (Opn4-/-), rod/cone function or ipRGCs have delineated the roles of the photopigments and of ipRGCs in NIF responses. In general, most of the NIFs that require light integration over a long period of time or acute NIF responses that activate at high-intensity light are attenuated in Opn4-/- mice. These include light modulation of the circadian clock phase, general activity, sleep and pupil constriction (reviewed in 66). These photoresponses are completely abolished in mice that lack both melanopsin and functional outer retina photoreceptors 8, 19, which implies rod/cone photoreceptors can partially compensate for the loss of melanopsin protein. Specific acute or progressive loss of ipRGCs in mice with intact and fully functional rod/cone photoreceptors also leads to loss of NIF light responses (Figure 3), thus establishing the ipRGCs as the principal carriers for transmitting the light information originating from both melanopsin and outer retina photoreceptors 78-80.
Additional mouse models with specific inactivation of rod or cone photoreceptor function are helping the elucidation of the relative roles of rods and cones in NIF responses 58, 81. As expected, both rods and cones can partially support some NIF responses including circadian photoentrainment 16. Surprisingly, even at photopic light level, cones appear to play a limited role in circadian photoentrainment. These mouse genetic studies support the overall response properties of primate ipRGCs in which a rod-initiated response supports irradiance encoding under dim light, whereas the transient responses of cones are insufficient to encode irradiance levels at high light levels. Hence, the relative roles of rod, cone and melanopsin in NIF responses are largely conserved between the nocturnal rodents and diurnal primates including humans.
Mouse genetics has also shed light on potential signaling mechanisms in the ipRGCs. Mice lacking the unconventional protein kinase C – PKCζ– phenocopy the reduced photosensitivity of the Opn4-/-mice. Coexpression of PKCζ and Opn4 in the ipRGCs raises the possibility that the kinase plays a critical role in melanopsin signaling cascade 82. Similar clues to the candidate downstream neurotransmitters in the ipRGCs have also come from mouse studies. Melanopsin expressing cells also express the neuropeptide PACAP (pituitary adeylate cyclase-activating peptide) 53, 83, 84, and PACAP receptors are expressed in the SCN 73, 85. Exogenous application of PACAP at low concentration can phase shift the SCN clock, whereas mice lacking PACAP or the PACAP receptor show reduced response of the circadian clock to a phase resetting pulse of light 86-88. The residual circadian photosensitivity in the PACAP KO mice is likely mediated via glutamate, which is also found in the ipRGCs 89. Analogous to PACAP, glutamate receptors are expressed in the SCN neurons (90 and references therein) and the exogenous application of glutamate to SCN slice culture can mimic light-induced phase shifts in the SCN clock 91, 92. Receptors for both PACAP and glutamate are excellent drug targets for several diseases 93, 94. Accordingly, specific pharmacological modulators of PACAP and glutamate signaling pathways could have significant impact on NIF responses.
Interaction between IF and NIF visual responses
Several animal models have painted a picture in which NIF responses and rod/cone-mediated IF vision are largely independent of each other; however, there are emerging data suggestive of interaction at various levels between these two systems. The melanopsin system can modulate the classical IF vision both in the retina as well as in the LGN. The ipRGCs signal to the dopaminergic amacrine cells might form a basis for the adaptation of the visual system to light intensity levels 95. Innervations of the LGN of primates 57 and rodents 77 by the ipRGCs suggest the melanopsin system might directly transmit ambient light intensity information to IF visual system.
There are several levels of potential regulation from the outer retina to the melanopsin system. As has been shown in Rpe65-/- and lrat-/- mice, general dysregulation of retinoid availability can affect the level of melanopsin protein and consequently the timing of activity rest in mice 36, 37, 96. As our knowledge on the relative contribution of rods/cones to NIF responses becomes clearer, it could have significant bearing on human health. Many diseases of the outer retina leading to blindness begins with the selective loss of RPE, rod or cone function and progress to significant cell death of the outer retina. Over a period of months and years, the surviving retina goes through profound remodeling in which the relative composition of cell types and their connectivity changes 97. Extensive remodeling of ipRGC dendritic structures in the retina has also been observed in old rats with outer retina degeneration 98. Therefore, the effects of several degenerative diseases of the outer retina on the melanopsin system might be complex and progressive.
Applications for improving health
The discovery of melanopsin now offers a mechanistic understanding of how light affects human physiology, behavior and sleep. Accordingly, effective utilization of light in improving the quality of life now offers new opportunities for interdisciplinary efforts among physicians and researchers of various branches of science that have so far remained largely nonoverlapping: circadian/endocrine biology, vision science, sleep and neuroscience, and architectural lighting. There are several areas in which the melanopsin photosystem can have direct implications in human health and diseases including (i) changing disease diagnosis procedures based on an evaluation of the melanopsin system; (ii) altering medical practices to address melanopsin-mediated photoresponses; (iii) developing pharmacological interventions for NIF responses; (iv) discovering gene alterations in patients; and (v) managing light exposure at work, home and at care-giving facilities.
Diagnostic procedures for the melanopsin system
Rodent data suggests perturbed melanopsin signaling might underlie several human disease conditions; these include sleep disorders, seasonal affective disorders, depression, aversion to light and light-exacerbated migraine pain 43. To begin to test whether the melanopsin system contributes to these ailments, it is necessary to have reliable diagnostic methods to quantify melanopsin function. However, nearly all current diagnostic procedures for the retina measure the structure and function of outer retina rod/cone photoreceptors. These procedures are unsuitable for evaluating the function of sparsely distributed ipRGCs in the ganglion cell layer of the inner retina. A promising method would be the utilization of a PLR assay. It is now well established that from rodents to primates, melanopsin specifically contributes to the persistence of pupil constriction for several seconds following a brief pulse of light 7, 18, 99. A recent study succeeded in optimizing the spectral conditions to specifically measure melanopsin function in pupil constriction. In addition, the melanopsin response is attenuated in one form of blindness (Leber's Congenital Amaurosis) while normal or enhanced melanopsin response in another form of blindness100. Such PLR response can also be used as a surrogate measure for the severity of another blinding disease - glaucoma, which involves progressive death of RGCs. In summary, evaluation of ipRGC function will be a starting point in further classifying blindness into patients with complete the loss of both NIF and IF vision and those with the loss of IF vision alone. Such classification will help determine whether normal ipRGC function in some blind patients might be beneficial in maintaining better quality of life than patients with no perception of light.
Medical practices
Several medical practices can now be evaluated in the context of melanopsin function, and these include the choice of cataract lens, pharmacological intervention aimed at the retinoid pathway for the treatment of other diseases, the decision for surgical bilateral enucleation as a prognosis for certain eye diseases including retinoblastoma, and the evaluation of gene therapy for improvement in the quality of life. For example, the human lens progressively loses transmittance in the blue range of visible light such that the lens of a 75-year old transmits 2-log units less light at 480 nm than the lens of a 5-year old 101, 102. Therefore, it is more important for elderly patients to have sufficient exposure to bright light. Furthermore, to improve visual function, to optimally activate the melanopsin system and consequently, to improve sleep quality in elderly patients, it might be ideal to implant an intraocular lens with sufficient transmittance in the blue range to restore function but minimal transmittance in the harmful near-UV range.
Pharmacological intervention
The distinct nature of melanopsin and rod/cone phototransduction pathways is encouraging ideas to manipulate signaling flux through the ipRGCs to manage human ailments dependent on lighting conditions. However, the point of intervention is still unclear. An ideal strategy would be to modulate melanopsin or a downstream signaling component in the ipRGCs without affecting the rod/cone signaling system. Compounds that either activate or inhibit the light flux through ipRGCs should mimic pharmacological light or darkness and have potential use. Activators would mimic light and offer a novel pharmacological intervention for mood uplifting effect, whereas inhibitors would mimic darkness and prevent light suppression of melatonin and hence improve sleep. Additionally, the recent observation that the ipRGCs might mediate light exacerbation of migraine pain 43 raises the possibility that pharmacological darkness alone or in combination with other drugs could offer novel strategy for pain management in normal and blind patients.
Gene and mechanism discovery from patients
Phenotypes of mice with altered signaling flux through the melanopsin system suggest a range of ailments in humans that might have an underlying defect in melanopsin function. Mouse models have also offered a range of mechanisms and genes that might affect the melanopsin system; these include melanopsin; putative downstream signaling components; and factors determining the differentiation of the ipRGCs, specifying their connectivity to the respective brain regions that mediate NIF responses and those in the outer retina affecting ipRGC function. As the cost of genome sequencing goes down and genetic association studies find loci associated with depression, seasonal affective disorders and sleep disorders, we might find genes and mechanisms implicated in the melanopsin system. Indeed, an early success of this nature has already been demonstrated. A specific amino-acid changing mutation in melanopsin associates with a small subset of patients with seasonal affective disorder (SAD) 103. SAD patients develop a form of depression that commonly begins with the short winter days and many patients find it helpful to have exposure to blue-enriched light (light therapy), thus further highlighting the relevance of light signaling for improved alertness in humans.
Light management
The general population in industrial nations is increasingly exposed to prolonged hours of artificial light that extends well into the night 104. Furthermore, most hospitals and care-giving facilities often have 24-hour lighting. Although daylight-mimicking light during the day might be beneficial, such light in the night time can adversely affect circadian clock and dependent physiologies 105. This is prompting lighting manufacturers and architects to adapt dynamic lighting for the workplace, care-giving facilities and the home.
Despite these potentials for leveraging the knowledge on melanopsin function to improving human health, significant barriers remain. Although melanopsin is expressed in the retina and is, therefore, the subject of vision science, the primary consequence of disrupted melanopsin signaling most likely lies in sleep disturbances, mood disorders, and consequent effects on metabolism, which are beyond the scope of vision science. Furthermore, these disorders are of complex etiologies and the patients are often in need of acute intervention. Nevertheless, the vision scientists have a clear role in evaluating the melanopsin system in the retina. Alternatively, sleep clinicians and psychiatrists could begin to interrogate the contribution of lighting conditions or the melanopsin signaling system to sleep and mood disorders in their patients.
Glossary Box.
Circadian clock, suprachiasmatic nucleus (SCN) and photoentrainment a vast array of physiological and behavioral changes with daily periodicity are controlled by an endogenous oscillator, the circadian clock. The master circadian clock regulating the behavioral rhythms resides in the SCN of the hypothalamus. The intrinsic period length of the circadian clock is not exactly 24 hours, and the axial tilt of the planet produces natural changes in day lengths throughout the solar year. Thus, the phase of the clock is adjusted daily primarily by light. The adjustment (entrainment) of the phase of the intrinsic clock to the phase or timing of the ambient light is generally termed as circadian photoentrainment.
Photoreceptor the cells that sense light via the expression of photopigments that induce signal transduction pathways (phototransduction) to regulate light-dependent physiologies such as vision and regulation of circadian entrainment, seasonal reproduction and body-color changes. The vertebrate retina contains three types of photoreceptors, rods, cones and ipRGCs. Each vertebrate photoreceptor contains a photopigment consisting of a protein called an opsin and a Vitamin-A-based light absorbing molecule (chromophore), 11-cis retinal (see also Figure 2).
Retinal ganglion cells (RGCs) are found in the inner most layer (ganglion cell layer; GCL) of the retina (Figure 1a), working as output neurons to send the visual information to the central nervous system. At least 10-15 types of RGCs are classified by the morphological differences, although this number varies among species 117.
Melanopsin and intrinsically photosensitive retinal ganglion cells (ipRGCs) a small subset of mammalian RGCs is intrinsically photoreceptive and these cells express an opsin protein called melanopsin (also called OPN4). Hence, they are generally referred to as ipRGCs or mRGCs.
Acknowledgments
Research in SP lab is supported by NIH grant EY16807, Pew Scholars award and Dana Foundation grant to SP. MH is a recipient of JSPS Fellowships for Research Abroad.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keeler CE. Iris movements in blind mice. American Journal of Physiology. 1927;81:107–112. [Google Scholar]
- 2.Foster RG, et al. Circadian photoreception in the retinally degenerate mouse (rd/rd) J Comp Physiol [A] 1991;169:39–50. doi: 10.1007/BF00198171. [DOI] [PubMed] [Google Scholar]
- 3.Czeisler CA, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki S, et al. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi JS, et al. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- 6.Brainard GC, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas RJ, et al. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- 8.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cajochen C, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 10.Lockley SW, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- 11.Zaidi FH, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provencio I, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provencio I, et al. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- 14.Hattar S, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berson DM, et al. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 16.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 17.Ruby NF, et al. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 18.Lucas RJ, et al. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- 19.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 20.Provencio I, et al. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayak SK, et al. Role of a novel photopigment, melanopsin, in behavioral adaptation to light. Cell Mol Life Sci. 2006;64:144–154. doi: 10.1007/s00018-006-5581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker MT, et al. Photochemistry of retinal chromophore in mouse melanopsin. Proc Natl Acad Sci U S A. 2008;105:8861–8865. doi: 10.1073/pnas.0711397105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyanagi M, et al. Cephalochordate Melanopsin: Evolutionary Linkage between Invertebrate Visual Cells and Vertebrate Photosensitive Retinal Ganglion Cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 25.Mure LS, et al. Melanopsin bistability: a fly's eye technology in the human retina. PLoS ONE. 2009;4:e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mure LS, et al. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007;22:411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panda S, et al. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 28.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 29.Dolph PJ, et al. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993;260:1910–1916. doi: 10.1126/science.8316831. [DOI] [PubMed] [Google Scholar]
- 30.Melyan Z, et al. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, et al. Melanopsin-dependent persistence and photopotentiation of murine pupillary light responses. Invest Ophthalmol Vis Sci. 2007;48:1268–1275. doi: 10.1167/iovs.06-0925. [DOI] [PubMed] [Google Scholar]
- 32.Palczewski K. Retinoids for treatment of retinal diseases. Trends Pharmacol Sci. 2010 doi: 10.1016/j.tips.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redmond TM, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 34.Batten ML, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Y, et al. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci U S A. 2005;102:10339–10344. doi: 10.1073/pnas.0501866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doyle SE, et al. From the Cover: Nonvisual light responses in the Rpe65 knockout mouse: Rod loss restores sensitivity to the melanopsin system. Proc Natl Acad Sci U S A. 2006;103:10432–10437. doi: 10.1073/pnas.0600934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu DC, et al. From the Cover: Inner retinal photoreception independent of the visual retinoid cycle. Proc Natl Acad Sci U S A. 2006;103:10426–10431. doi: 10.1073/pnas.0600917103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marlhens F, et al. Mutations in RPE65 cause Leber's congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 39.Gu SM, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17:194–197. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 40.Morimura H, et al. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc Natl Acad Sci U S A. 1998;95:3088–3093. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Do MT, et al. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radu RA, et al. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- 43.Noseda R, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekaran S, et al. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 45.Warren EJ, et al. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isoldi MC, et al. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci U S A. 2005;102:1217–1221. doi: 10.1073/pnas.0409252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 48.Graham DM, et al. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99:2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- 49.Sekaran S, et al. Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina. Curr Biol. 2005;15:1099–1107. doi: 10.1016/j.cub.2005.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newman LA, et al. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 51.Lin B, et al. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong JH, et al. Diversity of ganglion cells in the mouse retina: Unsupervised morphological classification and its limits. J Comp Neurol. 2005;489:293–310. doi: 10.1002/cne.20631. [DOI] [PubMed] [Google Scholar]
- 53.Hannibal J, et al. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004;45:4202–4209. doi: 10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez-Menendez I, et al. No loss of melanopsin-expressing ganglion cells detected during postnatal development of the mouse retina. Histol Histopathol. 2010;25:73–82. doi: 10.14670/HH-25.73. [DOI] [PubMed] [Google Scholar]
- 55.Zemelman BV, et al. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 56.Wong KY, et al. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dacey DM, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 58.Lall GS, et al. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gooley JJ, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarttelin EE, et al. Expression of opsin genes early in ocular development of humans and mice. Exp Eye Res. 2003;76:393–396. doi: 10.1016/s0014-4835(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 61.Hanita T, et al. Monitoring preterm infants' vision development with light-only melanopsin is functional. J Pediatr. 2009;155:596–596. e591. doi: 10.1016/j.jpeds.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Brzezinski JAt, et al. Loss of circadian photoentrainment and abnormal retinal electrophysiology in Math5 mutant mice. Invest Ophthalmol Vis Sci. 2005;46:2540–2551. doi: 10.1167/iovs.04-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wee R, et al. Loss of photic entrainment and altered free-running circadian rhythms in math5-/- mice. J Neurosci. 2002;22:10427–10433. doi: 10.1523/JNEUROSCI.22-23-10427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badea TC, et al. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berson DM, et al. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol. 2010;518:2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bailes HJ, Lucas RJ. Melanopsin and inner retinal photoreception. Cell Mol Life Sci. 2010;67:99–111. doi: 10.1007/s00018-009-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tu DC, et al. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belenky MA, et al. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- 70.Dumitrescu ON, et al. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuerst PG, et al. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gooley JJ, et al. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hannibal J, Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 2004;316:99–113. doi: 10.1007/s00441-004-0858-x. [DOI] [PubMed] [Google Scholar]
- 74.Baver SB, et al. Two types of melanopsin retinal ganglion cell differentially innervate the hypothalamic suprachiasmatic nucleus and the olivary pretectal nucleus. Eur J Neurosci. 2008;27:1763–1770. doi: 10.1111/j.1460-9568.2008.06149.x. [DOI] [PubMed] [Google Scholar]
- 75.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsen PJ, et al. Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. Eur J Neurosci. 1998;10:128–145. doi: 10.1046/j.1460-9568.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- 77.keding SR, Hatori M, Le H, Panda S. Comprehensive Labelling of Melanopsin Expressing Retinal Ganglion Cells and Mapping Their Central Projection in Mouse. Mol Biol Cell. 2009;20(suppl) Abstract no. 656. [Google Scholar]
- 78.Hatori M, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goz D, et al. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guler AD, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dkhissi-Benyahya O, et al. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peirson SN, et al. Microarray analysis and functional genomics identify novel components of melanopsin signaling. Curr Biol. 2007;17:1363–1372. doi: 10.1016/j.cub.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 83.Hannibal J, et al. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fahrenkrug J, et al. Expression of melanopsin during development of the rat retina. Neuroreport. 2004;15:781–784. doi: 10.1097/00001756-200404090-00008. [DOI] [PubMed] [Google Scholar]
- 85.Cagampang FR, et al. Circadian changes in PACAP type 1 (PAC1) receptor mRNA in the rat suprachiasmatic and supraoptic nuclei. Brain Res. 1998;813:218–222. doi: 10.1016/s0006-8993(98)01044-0. [DOI] [PubMed] [Google Scholar]
- 86.Colwell CS, et al. Selective Deficits in the Circadian Light Response in Mice Lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1194–1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- 87.Kawaguchi C, et al. PACAP-deficient mice exhibit light parameter-dependent abnormalities on nonvisual photoreception and early activity onset. PLoS ONE. 5:e9286. doi: 10.1371/journal.pone.0009286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hannibal J, et al. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J Neurosci. 2001;21:4883–4890. doi: 10.1523/JNEUROSCI.21-13-04883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hannibal J, et al. PACAP and glutamate are co-stored in the retinohypothalamic tract. J Comp Neurol. 2000;418:147–155. [PubMed] [Google Scholar]
- 90.Clark JP, 3rd, Kofuji P. The Stoichiometry of N-Methyl-D-Aspartate Receptors within the Suprachiasmatic Nucleus. J Neurophysiol. 2010 doi: 10.1152/jn.01069.2009. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ding JM, et al. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- 92.Harrington ME, et al. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci. 1999;19:6637–6642. doi: 10.1523/JNEUROSCI.19-15-06637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaudry D, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacological reviews. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 95.Zhang DQ, et al. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Doyle SE, et al. Retinal pathways influence temporal niche. Proc Natl Acad Sci U S A. 2008;105:13133–13138. doi: 10.1073/pnas.0801728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones BW, et al. Retinal remodelling. Clin Exp Optom. 2005;88:282–291. doi: 10.1111/j.1444-0938.2005.tb06712.x. [DOI] [PubMed] [Google Scholar]
- 98.Vugler AA, et al. Survival and remodeling of melanopsin cells during retinal dystrophy. Vis Neurosci. 2008;25:125–138. doi: 10.1017/S0952523808080309. [DOI] [PubMed] [Google Scholar]
- 99.Gamlin PD, et al. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park JC, Moura AL, Raza AS, Palmer N, Gavazi E, Tsang SH, Kardon RH, Hood DC. Toward a Clinical Protocol for Assessing Rod, Cone and Melanopsin Contributions to the Human Pupil Response. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.11-7586. 2010 E-Abstract 1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kessel L, et al. Age-related changes in the transmission properties of the human lens and their relevance to circadian entrainment. J Cataract Refract Surg. 36:308–312. doi: 10.1016/j.jcrs.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 102.Mainster MA, et al. Blue-blocking IOLs decrease photoreception without providing significant photoprotection. Surv Ophthalmol. 2010;55:272–289. doi: 10.1016/j.survophthal.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 103.Roecklein KA, et al. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114:279–285. doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hebert M, et al. Seasonal and diurnal patterns of human illumination under natural conditions. Chronobiol Int. 1998;15:59–70. doi: 10.3109/07420529808998670. [DOI] [PubMed] [Google Scholar]
- 105.Jewett ME, et al. Light-induced suppression of endogenous circadian amplitude in humans. Nature. 1991;350:59–62. doi: 10.1038/350059a0. [DOI] [PubMed] [Google Scholar]
- 106.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 107.Thapan K, et al. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lucas RJ, et al. Identifying the photoreceptive inputs to the mammalian circadian system using transgenic and retinally degenerate mice. Behav Brain Res. 2001;125:97–102. doi: 10.1016/s0166-4328(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 109.Altimus CM, et al. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci U S A. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lupi D, et al. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci. 2008 doi: 10.1038/nn.2179. [DOI] [PubMed] [Google Scholar]
- 111.Tsai JW, et al. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Routtenberg A, et al. Response of the infant rat to light prior to eyelid opening: mediation by the superior colliculus. Dev Psychobiol. 1978;11:469–478. doi: 10.1002/dev.420110510. [DOI] [PubMed] [Google Scholar]
- 113.Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–268. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- 114.Pulivarthy SR, et al. Reciprocity between phase shifts and amplitude changes in the mammalian circadian clock. Proc Natl Acad Sci U S A. 2007;104:20356–20361. doi: 10.1073/pnas.0708877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lagali PS, et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 116.Pires SS, et al. Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina. J Neurosci. 2009;29:12332–12342. doi: 10.1523/JNEUROSCI.2036-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]