Abstract
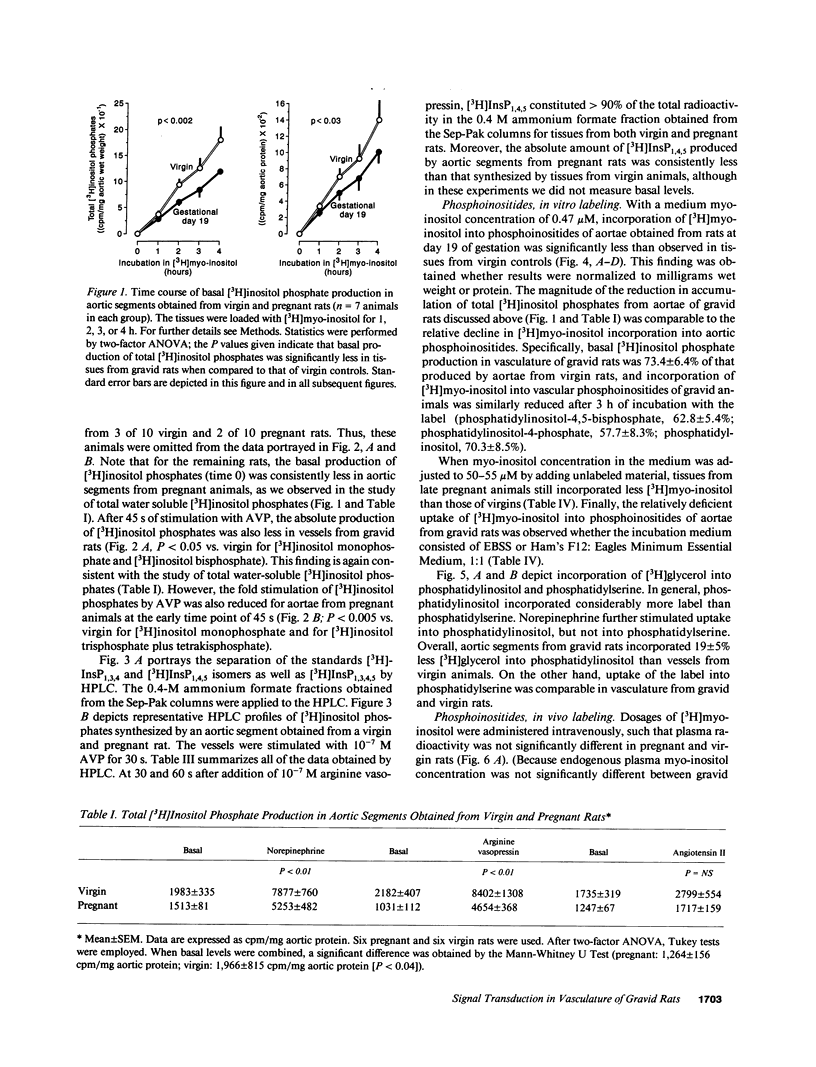
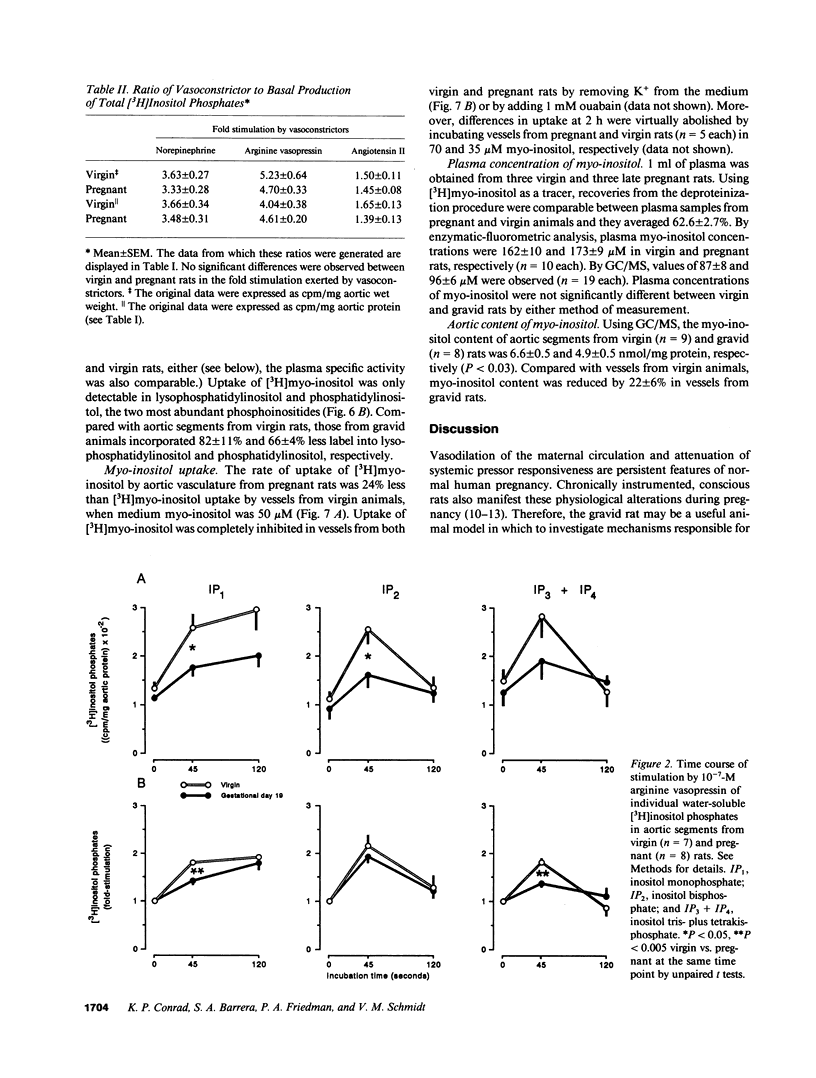
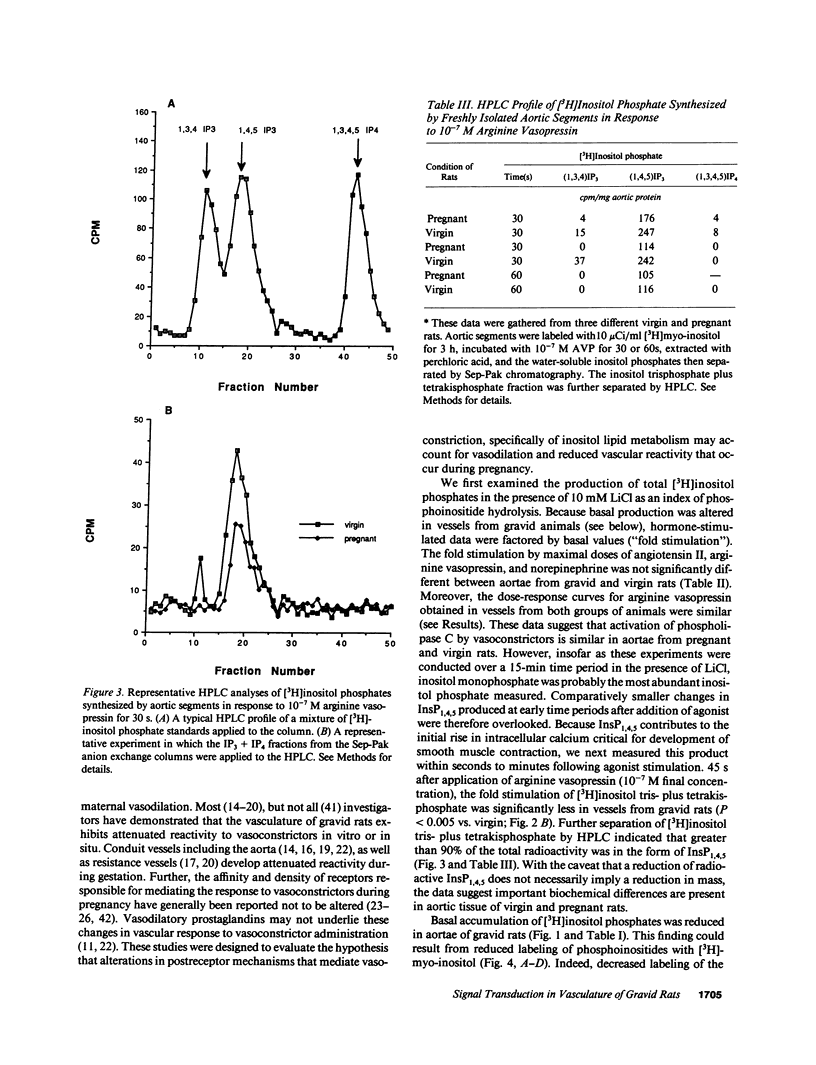
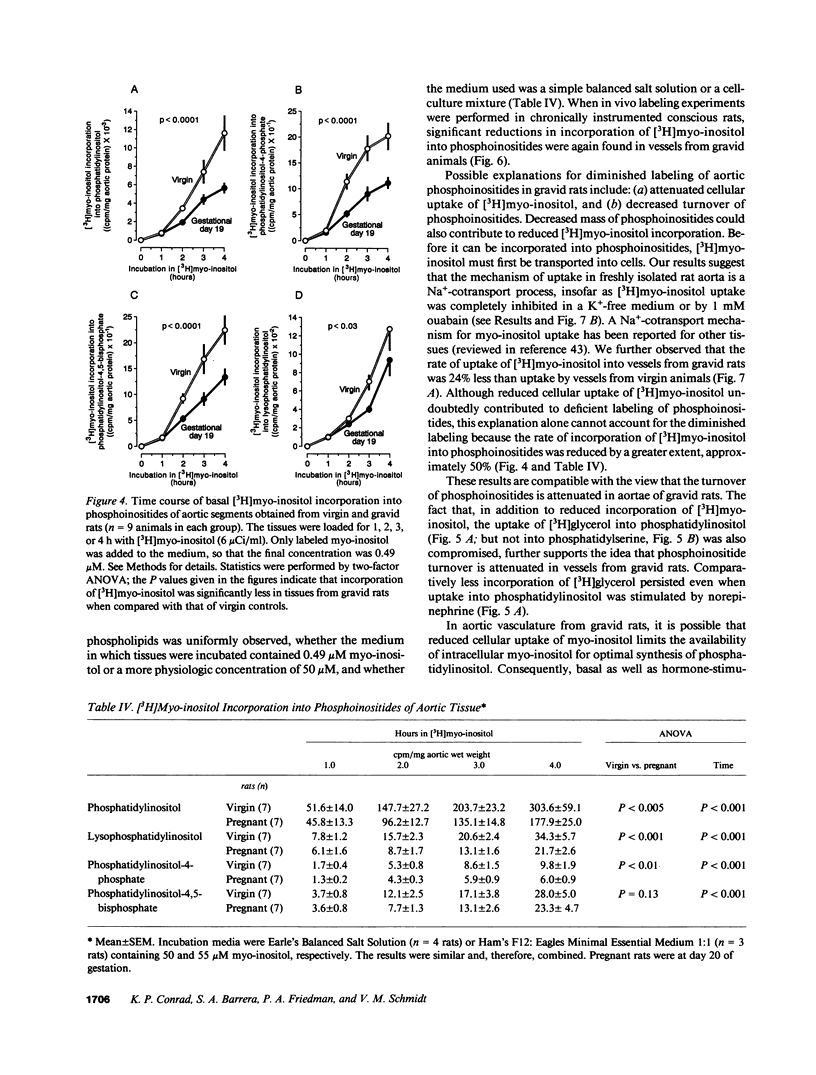
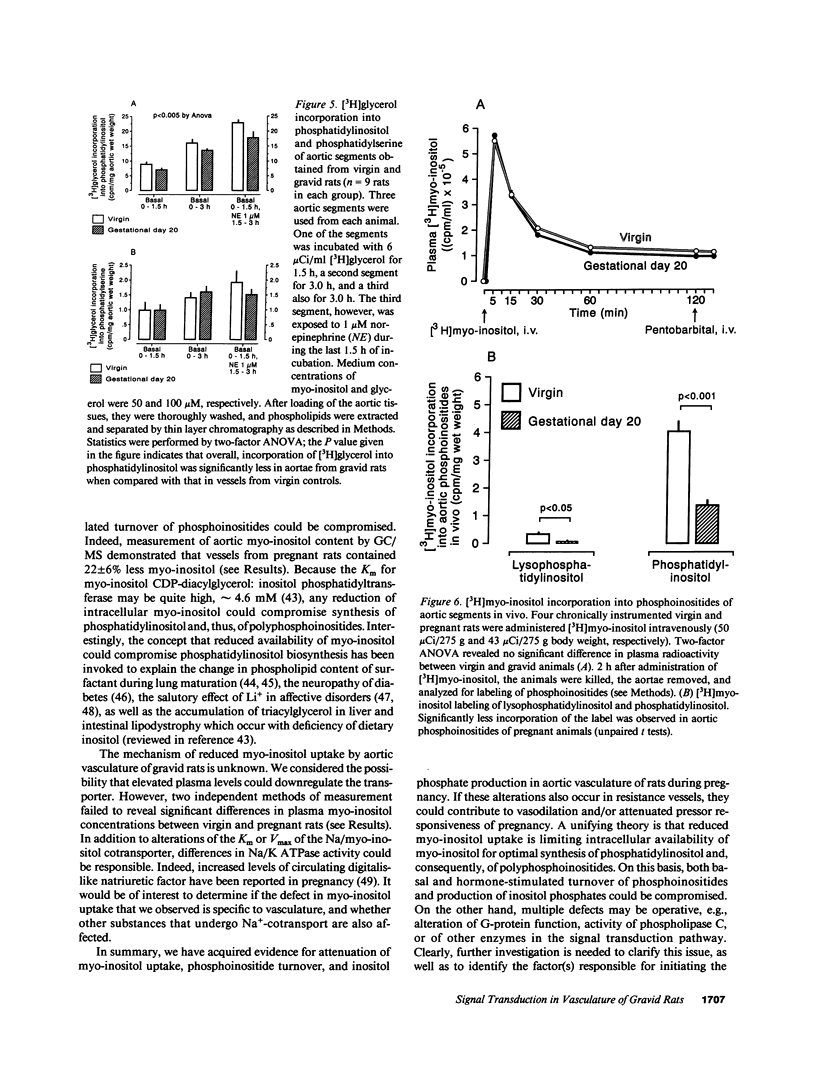
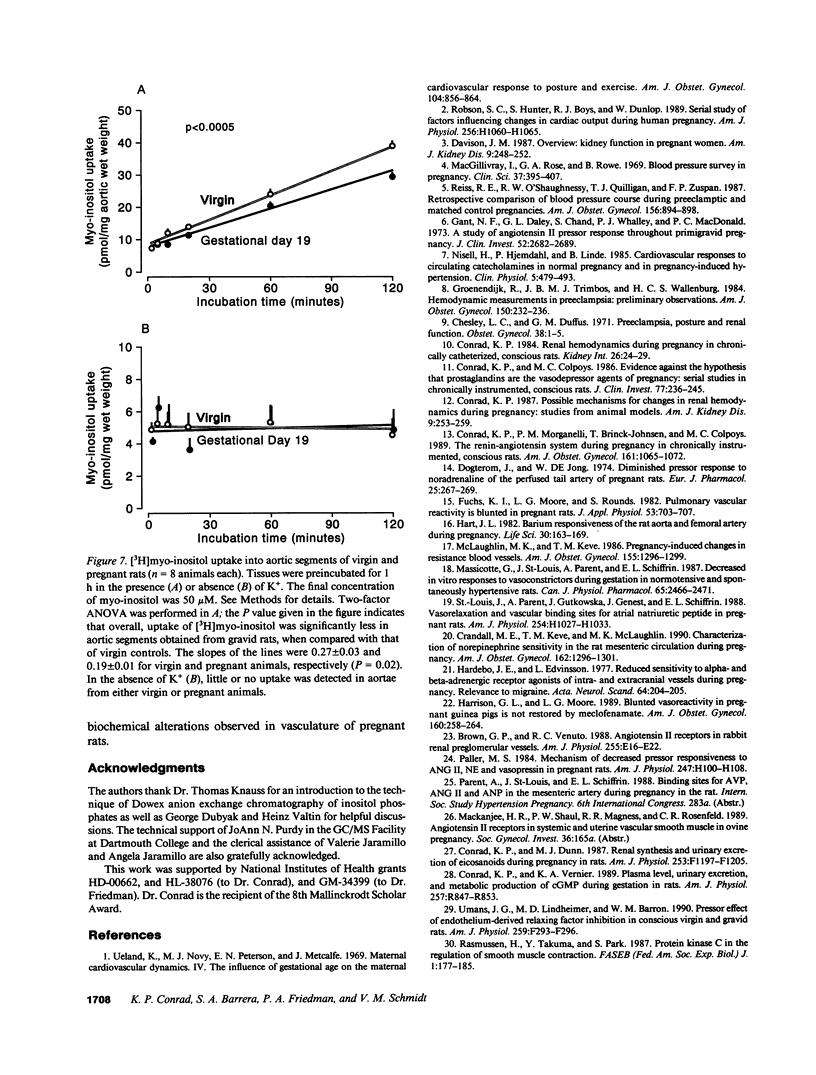
We postulated that vascular phosphoinositide metabolism is attenuated during pregnancy, and thereby could contribute to maternal vasodilation and reduced vascular reactivity. The basal rate of incorporation of [3H]myo-inositol and [3H]glycerol into phosphoinositides of aortae from pregnant rats in vitro was significantly reduced, when compared with vessels from virgin animals. After injection of [3H]myo-inositol intravenously into chronically instrumented conscious pregnant and virgin rats, the incorporation of the label by phosphatidylinositol was 66 +/- 4% less in aortae of gravid versus virgin animals (P less than 0.001), despite comparable plasma concentrations of radioactivity. Fold stimulation of total [3H]inositol phosphates by arginine vasopressin, norepinephrine, and angiotensin II over a 15-min period was not different between aortic segments from virgin and gravid rats, although both absolute basal and stimulated levels were significantly less in vessels from pregnant animals. After 45 s of incubation with 10(-7) M arginine vasopressin, however, the fold-stimulation of [3H]inositol trisplus tetrakisphosphate was reduced in aortae from gravid rats, when compared with vessels from virgin animals (P less than 0.005). By HPLC, greater than 90% of the radioactivity in the [3H]inositol trisplus tetrakisphosphate column fraction after 30 and 60 s of agonist stimulation was [3H]inositol-1,4,5-trisphosphate. We further observed that the rate of uptake of [3H]myo-inositol by aortic vasculature obtained from gravid rats was significantly (24%) less than uptake by vessels from virgin animals. Plasma myo-inositol concentrations were not significantly different, but presumably as a consequence of reduced uptake, aortic segments freshly isolated from pregnant rats contained 22 +/- 6% less myo-inositol than vessels from virgin controls as measured by gas chromatography-mass spectrometry (P less than 0.03). We conclude that myo-inositol uptake and content, phosphoinositide turnover, and inositol phosphate production are reduced in aortic vasculature of gravid rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale J. E., Maberry M. C., Quirk J. G. Myo-inositol homeostasis in foetal rabbit lung. Biochem J. 1982 Jul 15;206(1):43–52. doi: 10.1042/bj2060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleasdale J. E., Tyler N. E., Busch F. N., Quirk J. G. The influence of myo-inositol on phosphatidylglycerol synthesis by rat type II pneumonocytes. Biochem J. 1983 Jun 15;212(3):811–818. doi: 10.1042/bj2120811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. P., Venuto R. C. Angiotensin II receptor alterations during pregnancy in rabbits. Am J Physiol. 1986 Jul;251(1 Pt 1):E58–E64. doi: 10.1152/ajpendo.1986.251.1.E58. [DOI] [PubMed] [Google Scholar]
- Brown G. P., Venuto R. C. Angiotensin II receptors in rabbit renal preglomerular vessels. Am J Physiol. 1988 Jul;255(1 Pt 1):E16–E22. doi: 10.1152/ajpendo.1988.255.1.E16. [DOI] [PubMed] [Google Scholar]
- Chesley L. C., Duffus G. M. Preeclampsia, posture and renal function. Obstet Gynecol. 1971 Jul;38(1):1–5. [PubMed] [Google Scholar]
- Conrad K. P., Colpoys M. C. Evidence against the hypothesis that prostaglandins are the vasodepressor agents of pregnancy. Serial studies in chronically instrumented, conscious rats. J Clin Invest. 1986 Jan;77(1):236–245. doi: 10.1172/JCI112282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad K. P., Dunn M. J. Renal synthesis and urinary excretion of eicosanoids during pregnancy in rats. Am J Physiol. 1987 Dec;253(6 Pt 2):F1197–F1205. doi: 10.1152/ajprenal.1987.253.6.F1197. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Morganelli P. M., Brinck-Johnsen T., Colpoys M. C. The renin-angiotensin system during pregnancy in chronically instrumented, conscious rats. Am J Obstet Gynecol. 1989 Oct;161(4):1065–1072. doi: 10.1016/0002-9378(89)90785-0. [DOI] [PubMed] [Google Scholar]
- Conrad K. P. Possible mechanisms for changes in renal hemodynamics during pregnancy: studies from animal models. Am J Kidney Dis. 1987 Apr;9(4):253–259. doi: 10.1016/s0272-6386(87)80118-x. [DOI] [PubMed] [Google Scholar]
- Conrad K. P. Renal hemodynamics during pregnancy in chronically catheterized, conscious rats. Kidney Int. 1984 Jul;26(1):24–29. doi: 10.1038/ki.1984.129. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Vernier K. A. Plasma level, urinary excretion, and metabolic production of cGMP during gestation in rats. Am J Physiol. 1989 Oct;257(4 Pt 2):R847–R853. doi: 10.1152/ajpregu.1989.257.4.R847. [DOI] [PubMed] [Google Scholar]
- Crandall M. E., Keve T. M., McLaughlin M. K. Characterization of norepinephrine sensitivity in the maternal splanchnic circulation during pregnancy. Am J Obstet Gynecol. 1990 May;162(5):1296–1301. doi: 10.1016/0002-9378(90)90040-e. [DOI] [PubMed] [Google Scholar]
- Davison J. M. Kidney function in pregnant women. Am J Kidney Dis. 1987 Apr;9(4):248–252. doi: 10.1016/s0272-6386(87)80117-8. [DOI] [PubMed] [Google Scholar]
- Dogterom J., De Jong W. Diminished pressor response to noradrenaline of the perfused tail artery of pregnant rats. Eur J Pharmacol. 1974 Feb;25(2):267–269. doi: 10.1016/0014-2999(74)90062-4. [DOI] [PubMed] [Google Scholar]
- Fuchs K. I., Moore L. G., Rounds S. Pulmonary vascular reactivity is blunted in pregnant rats. J Appl Physiol Respir Environ Exerc Physiol. 1982 Sep;53(3):703–707. doi: 10.1152/jappl.1982.53.3.703. [DOI] [PubMed] [Google Scholar]
- Gant N. F., Daley G. L., Chand S., Whalley P. J., MacDonald P. C. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973 Nov;52(11):2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sastre F., Folch-Pi J. Thin-layer chromatography of the phosphoinositides. J Lipid Res. 1968 Jul;9(4):532–533. [PubMed] [Google Scholar]
- Graves S. W., Williams G. H. Endogenous digitalis-like natriuretic factors. Annu Rev Med. 1987;38:433–444. doi: 10.1146/annurev.me.38.020187.002245. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Groenendijk R., Trimbos J. B., Wallenburg H. C. Hemodynamic measurements in preeclampsia: preliminary observations. Am J Obstet Gynecol. 1984 Oct 1;150(3):232–236. doi: 10.1016/s0002-9378(84)90357-0. [DOI] [PubMed] [Google Scholar]
- Hardebo J. E., Edvinsson L. Reduced sensitivity to alpha- and beta-adrenergic receptor agonists of intra- and extracranial vessels during pregnancy. Relevance to migraine. Acta Neurol Scand Suppl. 1977;64:204–205. [PubMed] [Google Scholar]
- Harrison G. L., Moore L. G. Blunted vasoreactivity in pregnant guinea pigs is not restored by meclofenamate. Am J Obstet Gynecol. 1989 Jan;160(1):258–264. doi: 10.1016/0002-9378(89)90132-4. [DOI] [PubMed] [Google Scholar]
- Hart J. L. Barium responsiveness of the rat aorta and femoral artery during pregnancy. Life Sci. 1982 Jan 11;30(2):163–169. doi: 10.1016/0024-3205(82)90648-8. [DOI] [PubMed] [Google Scholar]
- Hart J. L., Freas W., Muldoon S. M. Neurovascular function in the rat during pregnancy. Am J Physiol. 1986 Nov;251(5 Pt 2):H1000–H1008. doi: 10.1152/ajpheart.1986.251.5.H1000. [DOI] [PubMed] [Google Scholar]
- Holub B. J. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- Kennedy E. D., Challiss R. A., Ragan C. I., Nahorski S. R. Reduced inositol polyphosphate accumulation and inositol supply induced by lithium in stimulated cerebral cortex slices. Biochem J. 1990 May 1;267(3):781–786. doi: 10.1042/bj2670781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacGillivray I., Rose G. A., Rowe B. Blood pressure survey in pregnancy. Clin Sci. 1969 Oct;37(2):395–407. [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. An enzymatic fluorimetric assay for myo-inositol. Anal Biochem. 1984 Sep;141(2):382–389. doi: 10.1016/0003-2697(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Massicotte G., St-Louis J., Parent A., Schiffrin E. L. Decreased in vitro responses to vasoconstrictors during gestation in normotensive and spontaneously hypertensive rats. Can J Physiol Pharmacol. 1987 Dec;65(12):2466–2471. doi: 10.1139/y87-391. [DOI] [PubMed] [Google Scholar]
- McLaughlin M. K., Keve T. M. Pregnancy-induced changes in resistance blood vessels. Am J Obstet Gynecol. 1986 Dec;155(6):1296–1299. doi: 10.1016/0002-9378(86)90163-8. [DOI] [PubMed] [Google Scholar]
- Nisell H., Hjemdahl P., Linde B. Cardiovascular responses to circulating catecholamines in normal pregnancy and in pregnancy-induced hypertension. Clin Physiol. 1985 Oct;5(5):479–493. doi: 10.1111/j.1475-097x.1985.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Paller M. S. Mechanism of decreased pressor responsiveness to ANG II, NE, and vasopressin in pregnant rats. Am J Physiol. 1984 Jul;247(1 Pt 2):H100–H108. doi: 10.1152/ajpheart.1984.247.1.H100. [DOI] [PubMed] [Google Scholar]
- Reiss R. E., O'Shaughnessy R. W., Quilligan T. J., Zuspan F. P. Retrospective comparison of blood pressure course during preeclamptic and matched control pregnancies. Am J Obstet Gynecol. 1987 Apr;156(4):894–898. doi: 10.1016/0002-9378(87)90347-4. [DOI] [PubMed] [Google Scholar]
- Robson S. C., Hunter S., Boys R. J., Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989 Apr;256(4 Pt 2):H1060–H1065. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- Ueland K., Novy M. J., Peterson E. N., Metcalfe J. Maternal cardiovascular dynamics. IV. The influence of gestational age on the maternal cardiovascular response to posture and exercise. Am J Obstet Gynecol. 1969 Jul 15;104(6):856–864. [PubMed] [Google Scholar]
- Umans J. G., Lindheimer M. D., Barron W. M. Pressor effect of endothelium-derived relaxing factor inhibition in conscious virgin and gravid rats. Am J Physiol. 1990 Aug;259(2 Pt 2):F293–F296. doi: 10.1152/ajprenal.1990.259.2.F293. [DOI] [PubMed] [Google Scholar]
- WEISSBACH The enzymic determination of myo-inositol. Biochim Biophys Acta. 1958 Mar;27(3):608–611. doi: 10.1016/0006-3002(58)90393-7. [DOI] [PubMed] [Google Scholar]
- WELLS W. W., CHIN T., WEBER B. QUANTITATIVE ANALYSIS OF SERUM AND URINE SUGARS BY GAS CHROMATOGRAPHY. Clin Chim Acta. 1964 Oct;10:352–359. doi: 10.1016/0009-8981(64)90066-x. [DOI] [PubMed] [Google Scholar]
- Winegrad A. I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987 Mar;36(3):396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]
- Wreggett K. A., Irvine R. F. A rapid separation method for inositol phosphates and their isomers. Biochem J. 1987 Aug 1;245(3):655–660. doi: 10.1042/bj2450655. [DOI] [PMC free article] [PubMed] [Google Scholar]


