Abstract
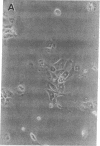
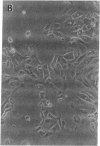
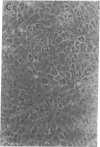
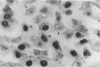
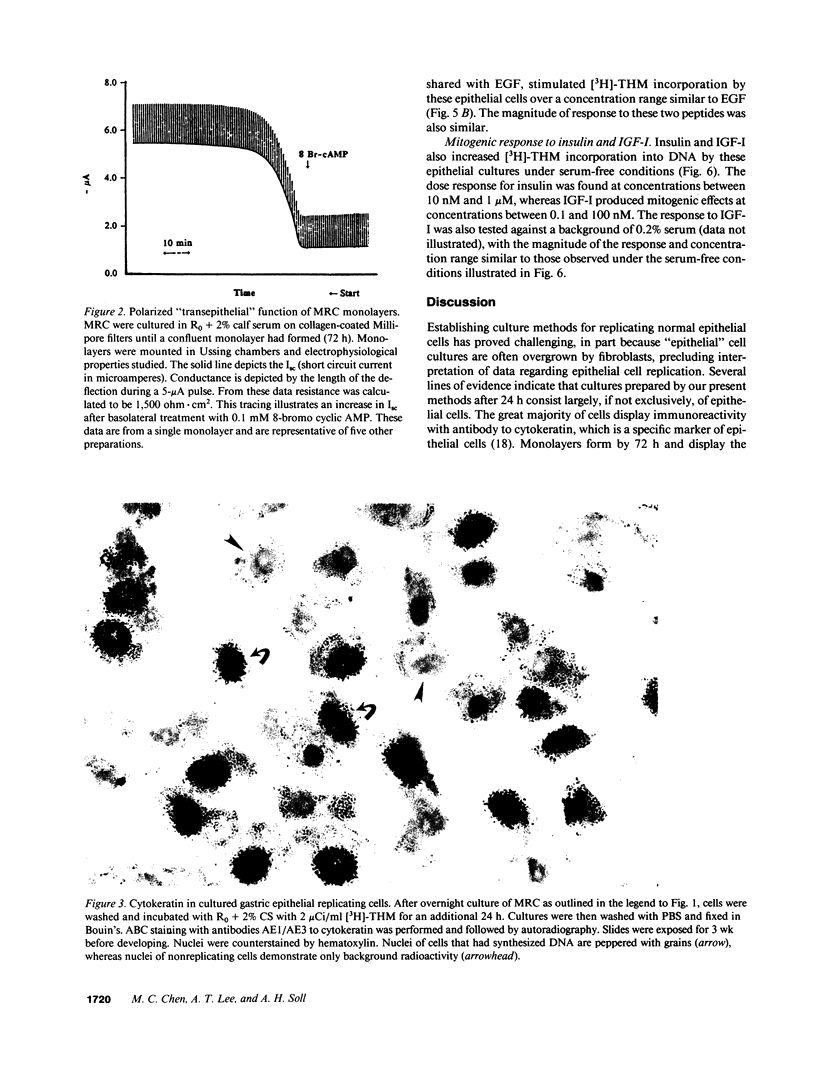
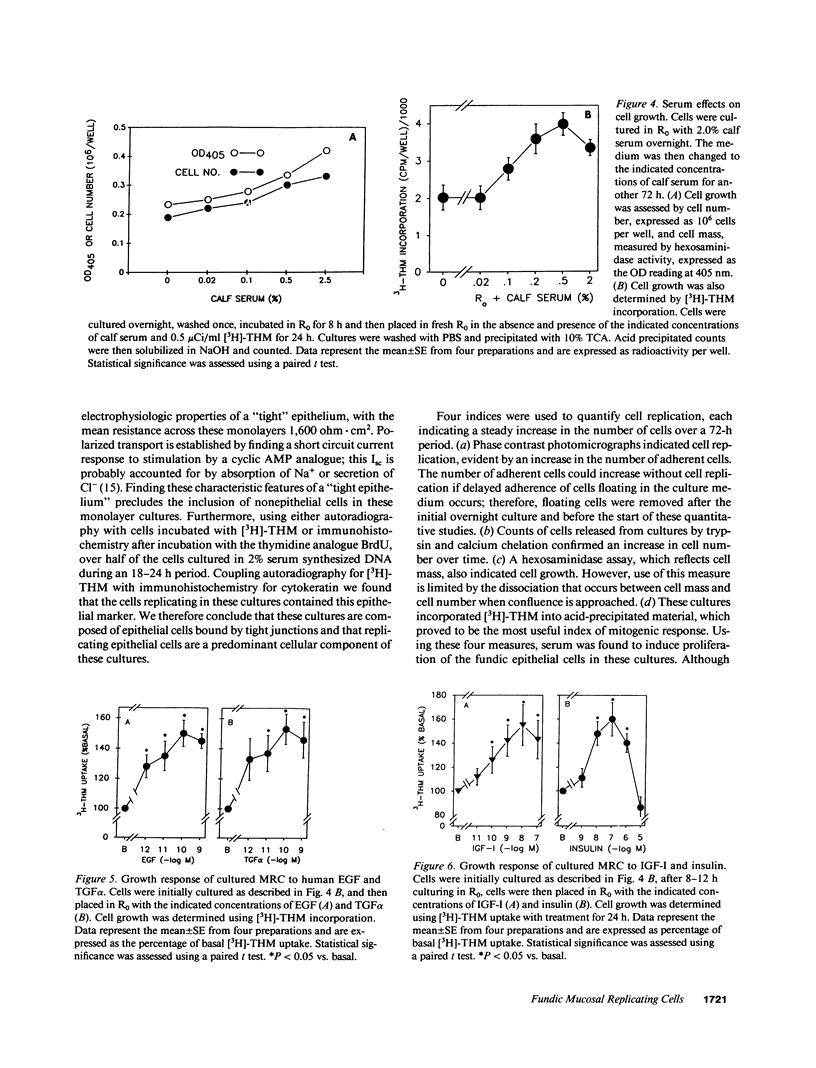
We report methods allowing the culture of rapidly dividing gastric epithelial cells to investigate the regulation of mucosal cell replication. Cells from canine fundic mucosa were dispersed by enzyme treatment, enriched by filtration and elutriation, and cultured on collagen gel in DMEM/F12 medium. After 48 h, greater than 95% of the cells displayed immunoreactivity with antibody to cytokeratin, an epithelial marker. The cells formed confluent monolayers by 72 h with a transmembrane resistance of 1,600 ohm.cm2 when mounted in a Ussing chamber indicating retention of epithelial cell characteristics. Calf serum (0.1-2%) produced a dose-dependent mitogenic effect evident by increases in [3H]-thymidine incorporation into acid-precipitated material and in cell number. After an 18-24-h incubation with [3H]-thymidine, approximately 55% of the cells cultured in 2% serum showed evidence of DNA synthesis by autoradiography and all of the replicating cells were cytokeratin positive. Using comparable culture conditions, a similar proportion of cells incubated for 18-24 h with bromodeoxyuridine displayed nuclear anti-bromodeoxyuridine immunoreactivity, thus indicating that over half of the cells in these cultures synthesized DNA during this period. As with serum, epidermal growth factor and transforming growth factor alpha (TGF alpha) (10 pM to 1 nM), insulin (10 nM to 1 microM) and insulinlike growth factor-I (IGF-I, 1-100 nM) increased [3H]-thymidine uptake. The greater potency of IGF-I, compared to insulin, suggests the presence of IGF-I receptors. We conclude that this culture preparation is composed of fundic mucosal epithelial cells and contains a predominance of dividing epithelial cells. EGF/TGF alpha and IGF-I are potential factors directly regulating proliferation of fundic mucosal cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayalon A., Sanders M. J., Thomas L. P., Amirian D. A., Soll A. H. Electrical effects of histamine on monolayers formed in culture from enriched canine gastric chief cells. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7009–7013. doi: 10.1073/pnas.79.22.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar R. S., Boes M., Booth B. A., Dake B. L., Henley S., Hart M. N. The effects of platelet-derived growth factor in cultured microvessel endothelial cells. Endocrinology. 1989 Apr;124(4):1841–1848. doi: 10.1210/endo-124-4-1841. [DOI] [PubMed] [Google Scholar]
- Beauchamp R. D., Barnard J. A., McCutchen C. M., Cherner J. A., Coffey R. J., Jr Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989 Sep;84(3):1017–1023. doi: 10.1172/JCI114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. Y., Withers H. R. Proliferative capability of parietal and zymogen cells. J Anat. 1975 Dec;120(Pt 3):421–432. [PMC free article] [PubMed] [Google Scholar]
- Chen M. C., Sanders M. J., Amirian D. A., Thomas L. P., Kauffman G., Soll A. H. Prostaglandin E2 production by dispersed canine fundic mucosal cells. Contribution of macrophages and endothelial cells as major sources. J Clin Invest. 1989 Nov;84(5):1536–1549. doi: 10.1172/JCI114330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra D. P., Siddiqui K. M., Cooney R. A. Effects of insulin, transferrin, cholera toxin, and epidermal growth factor on growth and morphology of human fetal normal colon epithelial cells. Gastroenterology. 1987 Apr;92(4):891–904. doi: 10.1016/0016-5085(87)90962-0. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Goustin A. S., Soderquist A. M., Shipley G. D., Wolfshohl J., Carpenter G., Moses H. L. Transforming growth factor alpha and beta expression in human colon cancer lines: implications for an autocrine model. Cancer Res. 1987 Sep 1;47(17):4590–4594. [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Stiles A. D., Underwood L. E. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. doi: 10.1073/pnas.81.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Feldman M., Walsh J. H., Wong H. C., Richardson C. T. Role of gastrin heptadecapeptide in the acid secretory response to amino acids in man. J Clin Invest. 1978 Feb;61(2):308–313. doi: 10.1172/JCI108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Hansen O. H., Pedersen T., Larsen J. K. Cell proliferation kinetics in normal human gastric mucosa. Studies on diurnal fluctuations and effect of food ingestion. Gastroenterology. 1976 Jun;70(6):1051–1054. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Vallgren S., Ekelund M., Rehfeld J. F., Sundler F. The vagus exerts trophic control of the stomach in the rat. Gastroenterology. 1984 Jan;86(1):28–32. [PubMed] [Google Scholar]
- Johnson L. R., Guthrie P. D. Stimulation of rat oxyntic gland mucosal growth by epidermal growth factor. Am J Physiol. 1980 Jan;238(1):G45–G49. doi: 10.1152/ajpgi.1980.238.1.G45. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S. Y., Podolsky D. K. Differential expression of transforming growth factors alpha and beta in rat intestinal epithelial cells. J Clin Invest. 1989 May;83(5):1768–1773. doi: 10.1172/JCI114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokowa M., Lynch K., Podolsky D. K. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987 Feb 13;142(3):775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984 Mar 16;67(2):379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- Larsson H., Carlsson E., Mattsson H., Lundell L., Sundler F., Sundell G., Wallmark B., Watanabe T., Håkanson R. Plasma gastrin and gastric enterochromaffinlike cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986 Feb;90(2):391–399. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- Lehy T., Puccio F. Influence of bombesin on gastrointestinal and pancreatic cell growth in adult and suckling animals. Ann N Y Acad Sci. 1988;547:255–267. doi: 10.1111/j.1749-6632.1988.tb23894.x. [DOI] [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malden L. T., Novak U., Burgess A. W. Expression of transforming growth factor alpha messenger RNA in the normal and neoplastic gastro-intestinal tract. Int J Cancer. 1989 Mar 15;43(3):380–384. doi: 10.1002/ijc.2910430305. [DOI] [PubMed] [Google Scholar]
- Matuoka K., Tanaka M., Mitsui Y., Murota S. I. Cultured rabbit gastric epithelial cells producing prostaglandin I2. Gastroenterology. 1983 Mar;84(3):498–505. [PubMed] [Google Scholar]
- Moyer M. P. Culture of human gastrointestinal epithelial cells. Proc Soc Exp Biol Med. 1983 Oct;174(1):12–15. doi: 10.3181/00379727-174-1-rc1. [DOI] [PubMed] [Google Scholar]
- Ristow H. J., Messmer T. O. Basic fibroblast growth factor and insulin-like growth factor I are strong mitogens for cultured mouse keratinocytes. J Cell Physiol. 1988 Nov;137(2):277–284. doi: 10.1002/jcp.1041370210. [DOI] [PubMed] [Google Scholar]
- Romano M., Razandi M., Sekhon S., Krause W. J., Ivey K. J. Human cell line for study of damage to gastric epithelial cells in vitro. J Lab Clin Med. 1988 Apr;111(4):430–440. [PubMed] [Google Scholar]
- Sanders M. J., Ayalon A., Roll M., Soll A. H. The apical surface of canine chief cell monolayers resists H+ back-diffusion. Nature. 1985 Jan 3;313(5997):52–54. doi: 10.1038/313052a0. [DOI] [PubMed] [Google Scholar]
- Scheving L. A., Yeh Y. C., Tsai T. H., Scheving L. E. Circadian phase-dependent stimulatory effects of epidermal growth factor on deoxyribonucleic acid synthesis in the tongue, esophagus, and stomach of the adult male mouse. Endocrinology. 1979 Dec;105(6):1475–1480. doi: 10.1210/endo-105-6-1475. [DOI] [PubMed] [Google Scholar]
- Shiraishi T., Samloff I. M., Taggart R. T., Stemmermann G. N. Slow moving proteinase in gastric cancer and its relationship to pepsinogens I and II. An immunohistochemical study. Dig Dis Sci. 1988 Nov;33(11):1466–1472. doi: 10.1007/BF01537004. [DOI] [PubMed] [Google Scholar]
- Smolka A., Helander H. F., Sachs G. Monoclonal antibodies against gastric H+ + K+ ATPase. Am J Physiol. 1983 Oct;245(4):G589–G596. doi: 10.1152/ajpgi.1983.245.4.G589. [DOI] [PubMed] [Google Scholar]
- Soll A. H., Amirian D. A., Thomas L. P., Reedy T. J., Elashoff J. D. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984 May;73(5):1434–1447. doi: 10.1172/JCI111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):370–380. doi: 10.1172/JCI108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmermann G. N., Samloff I. M., Hayashi T. Pepsinogens I and II in carcinoma of the stomach: an immunohistochemical study. Appl Pathol. 1985;3(3):159–163. [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Taub M., Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1980 Nov;105(2):369–378. doi: 10.1002/jcp.1041050220. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Domingo D. L. Anti-transferrin receptor monoclonal antibody and toxin-antibody conjugates affect growth of human tumour cells. Nature. 1981 Nov 12;294(5837):171–173. doi: 10.1038/294171a0. [DOI] [PubMed] [Google Scholar]
- Vostrejs M., Moran P. L., Seligman P. A. Transferrin synthesis by small cell lung cancer cells acts as an autocrine regulator of cellular proliferation. J Clin Invest. 1988 Jul;82(1):331–339. doi: 10.1172/JCI113591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans N. D., Trier J. S. Epithelial cell proliferation and migration in the developing rat gastric mucosa. Dev Biol. 1976 Oct 15;53(2):206–216. doi: 10.1016/0012-1606(76)90224-4. [DOI] [PubMed] [Google Scholar]