Abstract
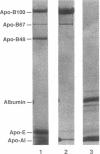
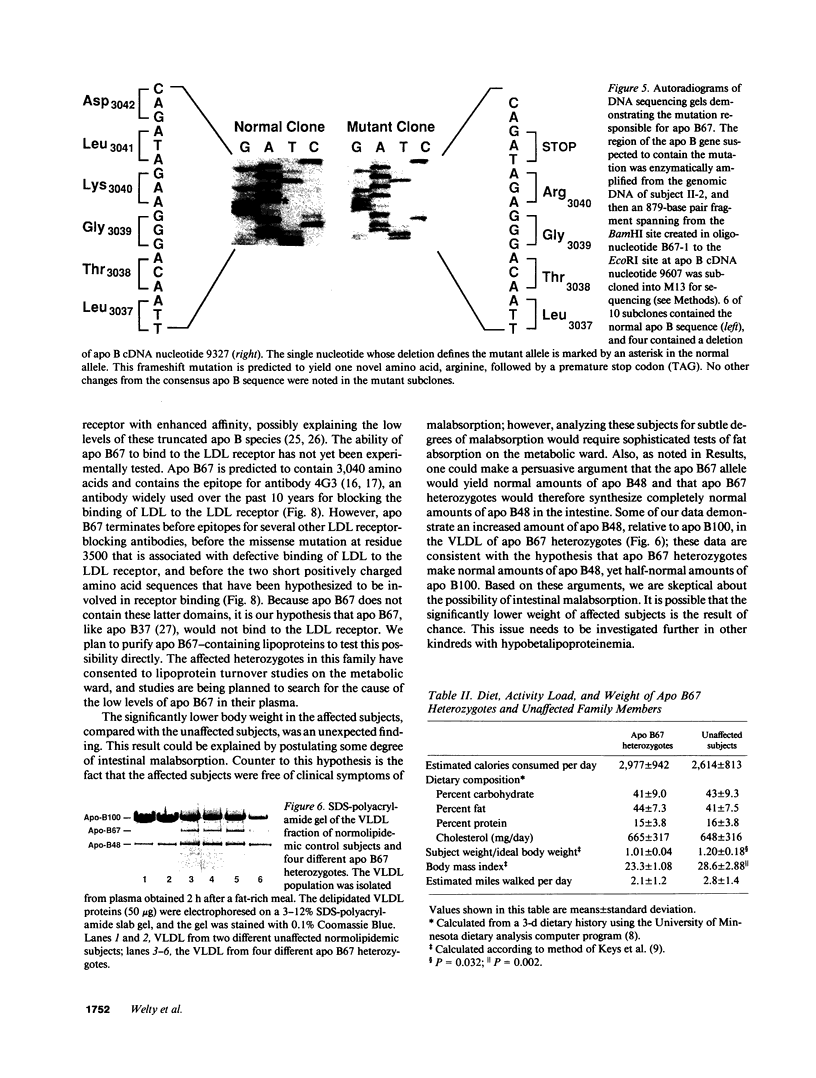
We describe a kindred in which the proband and 6 of his 12 children have hypobetalipoproteinemia. The plasma lipoproteins of the affected subjects contained a unique species of apolipoprotein (apo) B, apo B67, in addition to the normal species, apo B100 and apo B48. The size of apo B67 and immunochemical studies with a panel of apo B-specific antibodies indicated that apo B67 was a truncated species of apo B that contained approximately the amino-terminal 3,000-3,100 amino acids of apo B100. Sequencing of genomic apo B clones revealed that affected family members were heterozygous for a mutant apo B allele containing a single nucleotide deletion in exon 26 (cDNA nucleotide 9327). This frameshift mutation is predicted to result in the synthesis of a truncated apo B containing 3,040 amino acids. Apo B67 is present in low levels in the plasma but is easily detectable within the very low density lipoprotein and low density lipoprotein fractions. Examination of the proband's immediate family revealed seven normolipidemic subjects and seven subjects with hypobetalipoproteinemia. In the affected subjects, the mean total and low density lipoprotein cholesterol levels were 120 and 42 mg/dl, respectively. A significantly higher mean high density lipoprotein cholesterol level was found in the affected subjects (75 vs. 55 mg/dl). We hypothesize that the elevated high density lipoprotein cholesterol levels in subjects heterozygous for the apo B67 mutation may be metabolically linked to the low levels of apo B-containing lipoproteins in their plasma.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanche P. J., Gong E. L., Forte T. M., Nichols A. V. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim Biophys Acta. 1981 Sep 24;665(3):408–419. doi: 10.1016/0005-2760(81)90253-8. [DOI] [PubMed] [Google Scholar]
- Brown M. L., Inazu A., Hesler C. B., Agellon L. B., Mann C., Whitlock M. E., Marcel Y. L., Milne R. W., Koizumi J., Mabuchi H. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature. 1989 Nov 23;342(6248):448–451. doi: 10.1038/342448a0. [DOI] [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Immunochemical heterogeneity of human plasma apolipoprotein B. I. Apolipoprotein B binding of mouse hybridoma antibodies. J Biol Chem. 1982 Dec 25;257(24):15213–15221. [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Immunochemical heterogeneity of human plasma high density lipoproteins. Identification with apolipoprotein A-I- and A-II-specific monoclonal antibodies. J Biol Chem. 1985 Mar 10;260(5):2982–2993. [PubMed] [Google Scholar]
- Glueck C. J., Gartside P. S., Mellies M. J., Steiner P. M. Familial hypobeta-lipoproteinemia: studies in 13 kindreds. Trans Assoc Am Physicians. 1977;90:184–203. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innerarity T. L., Young S. G., Poksay K. S., Mahley R. W., Smith R. S., Milne R. W., Marcel Y. L., Weisgraber K. H. Structural relationship of human apolipoprotein B48 to apolipoprotein B100. J Clin Invest. 1987 Dec;80(6):1794–1798. doi: 10.1172/JCI113273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. A., Glueck C. J. Familial hypobetalipoproteinemia. Absence of atherosclerosis in a postmortem study. JAMA. 1978 Jul 7;240(1):47–48. doi: 10.1001/jama.240.1.47. [DOI] [PubMed] [Google Scholar]
- Keys A., Fidanza F., Karvonen M. J., Kimura N., Taylor H. L. Indices of relative weight and obesity. J Chronic Dis. 1972 Jul 1;25(6):329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- Krul E. S., Kinoshita M., Talmud P., Humphries S. E., Turner S., Goldberg A. C., Cook K., Boerwinkle E., Schonfeld G. Two distinct truncated apolipoprotein B species in a kindred with hypobetalipoproteinemia. Arteriosclerosis. 1989 Nov-Dec;9(6):856–868. doi: 10.1161/01.atv.9.6.856. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaCroix A. Z., Guralnik J. M., Curb J. D., Wallace R. B., Ostfeld A. M., Hennekens C. H. Chest pain and coronary heart disease mortality among older men and women in three communities. Circulation. 1990 Feb;81(2):437–446. doi: 10.1161/01.cir.81.2.437. [DOI] [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P., Hardman D. A., Hamilton R. L., Dalal K. B. Normotriglyceridemic abetalipoproteinemia. absence of the B-100 apolipoprotein. J Clin Invest. 1981 May;67(5):1441–1450. doi: 10.1172/JCI110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy M. J., Kane J. P. Hypolipidemia. Med Clin North Am. 1982 Mar;66(2):469–484. doi: 10.1016/s0025-7125(16)31431-6. [DOI] [PubMed] [Google Scholar]
- Milne R. W., Theolis R., Jr, Verdery R. B., Marcel Y. L. Characterization of monoclonal antibodies against human low density lipoprotein. Arteriosclerosis. 1983 Jan-Feb;3(1):23–30. doi: 10.1161/01.atv.3.1.23. [DOI] [PubMed] [Google Scholar]
- Milne R., Théolis R., Jr, Maurice R., Pease R. J., Weech P. K., Rassart E., Fruchart J. C., Scott J., Marcel Y. L. The use of monoclonal antibodies to localize the low density lipoprotein receptor-binding domain of apolipoprotein B. J Biol Chem. 1989 Nov 25;264(33):19754–19760. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schakel S. F., Sievert Y. A., Buzzard I. M. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988 Oct;88(10):1268–1271. [PubMed] [Google Scholar]
- Sigurdsson G., Nicoll A., Lewis B. Turnover of apolipoprotein-B in two subjects with familial hypobetalipoproteinemia. Metabolism. 1977 Jan;26(1):25–31. doi: 10.1016/0026-0495(77)90124-x. [DOI] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Curtiss L. K., Dubois B. W., Witztum J. L. Genetic analysis of a kindred with familial hypobetalipoproteinemia. Evidence for two separate gene defects: one associated with an abnormal apolipoprotein B species, apolipoprotein B-37; and a second associated with low plasma concentrations of apolipoprotein B-100. J Clin Invest. 1987 Jun;79(6):1842–1851. doi: 10.1172/JCI113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Curtiss L. K., Witztum J. L. Characterization of an abnormal species of apolipoprotein B, apolipoprotein B-37, associated with familial hypobetalipoproteinemia. J Clin Invest. 1987 Jun;79(6):1831–1841. doi: 10.1172/JCI113025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Scott T. M., Dubois B. W., Curtiss L. K., Witztum J. L. Parallel expression of the MB19 genetic polymorphism in apoprotein B-100 and apoprotein B-48. Evidence that both apoproteins are products of the same gene. J Biol Chem. 1986 Mar 5;261(7):2995–2998. [PubMed] [Google Scholar]
- Young S. G., Hubl S. T., Chappell D. A., Smith R. S., Claiborne F., Snyder S. M., Terdiman J. F. Familial hypobetalipoproteinemia associated with a mutant species of apolipoprotein B (B-46). N Engl J Med. 1989 Jun 15;320(24):1604–1610. doi: 10.1056/NEJM198906153202407. [DOI] [PubMed] [Google Scholar]
- Young S. G., Hubl S. T., Smith R. S., Snyder S. M., Terdiman J. F. Familial hypobetalipoproteinemia caused by a mutation in the apolipoprotein B gene that results in a truncated species of apolipoprotein B (B-31). A unique mutation that helps to define the portion of the apolipoprotein B molecule required for the formation of buoyant, triglyceride-rich lipoproteins. J Clin Invest. 1990 Mar;85(3):933–942. doi: 10.1172/JCI114522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Peralta F. P., Dubois B. W., Curtiss L. K., Boyles J. K., Witztum J. L. Lipoprotein B37, a naturally occurring lipoprotein containing the amino-terminal portion of apolipoprotein B100, does not bind to the apolipoprotein B,E (low density lipoprotein) receptor. J Biol Chem. 1987 Dec 5;262(34):16604–16611. [PubMed] [Google Scholar]
- Young S. G. Recent progress in understanding apolipoprotein B. Circulation. 1990 Nov;82(5):1574–1594. doi: 10.1161/01.cir.82.5.1574. [DOI] [PubMed] [Google Scholar]
- Young S. G., Witztum J. L., Casal D. C., Curtiss L. K., Bernstein S. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis. 1986 Mar-Apr;6(2):178–188. doi: 10.1161/01.atv.6.2.178. [DOI] [PubMed] [Google Scholar]