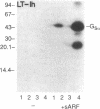
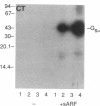
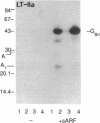
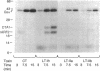
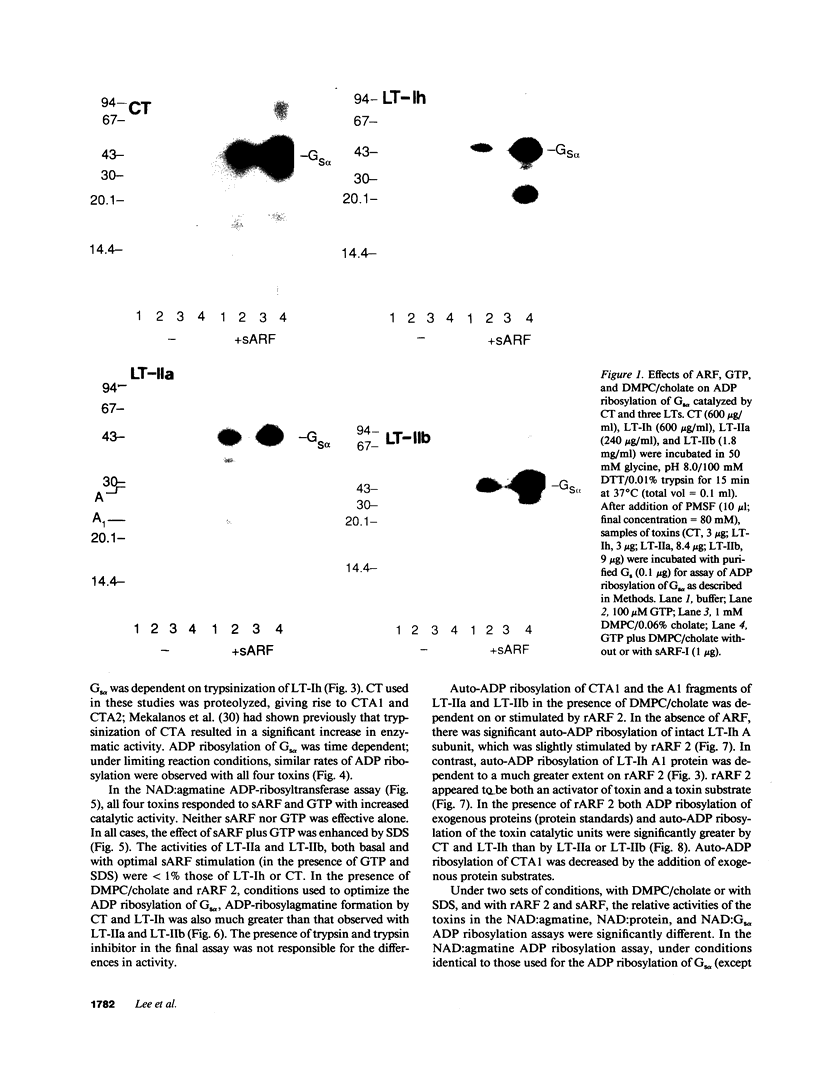
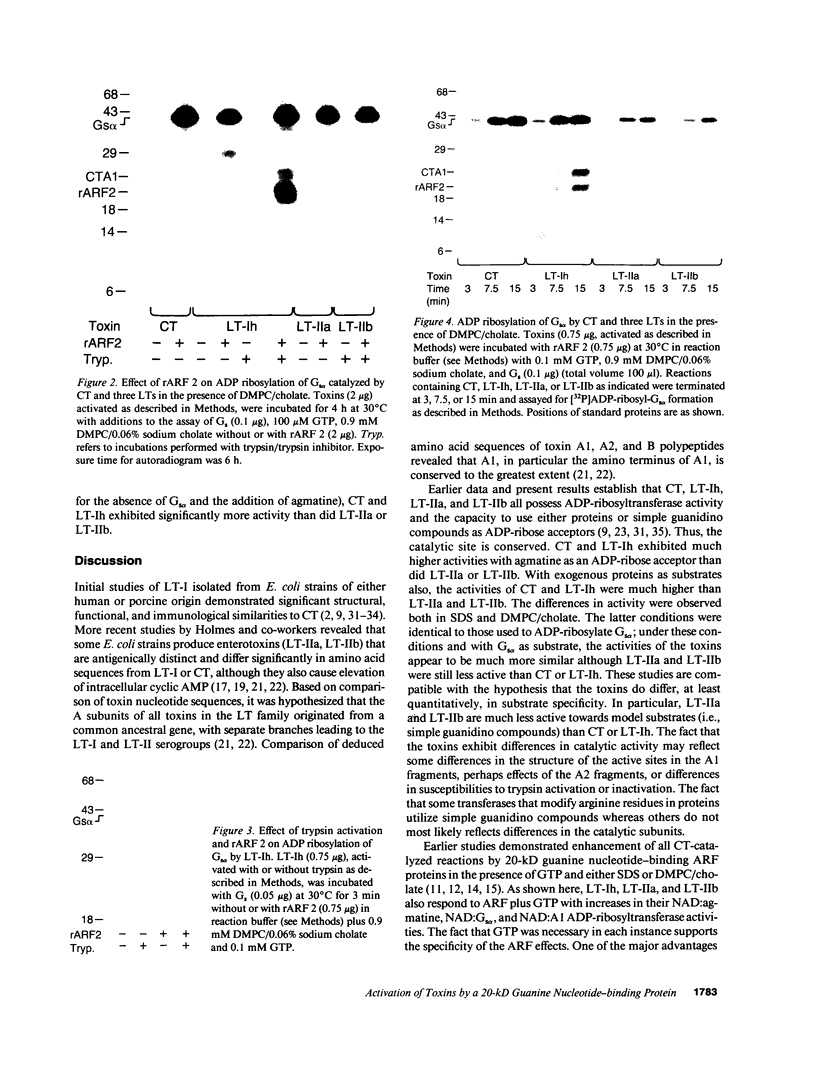
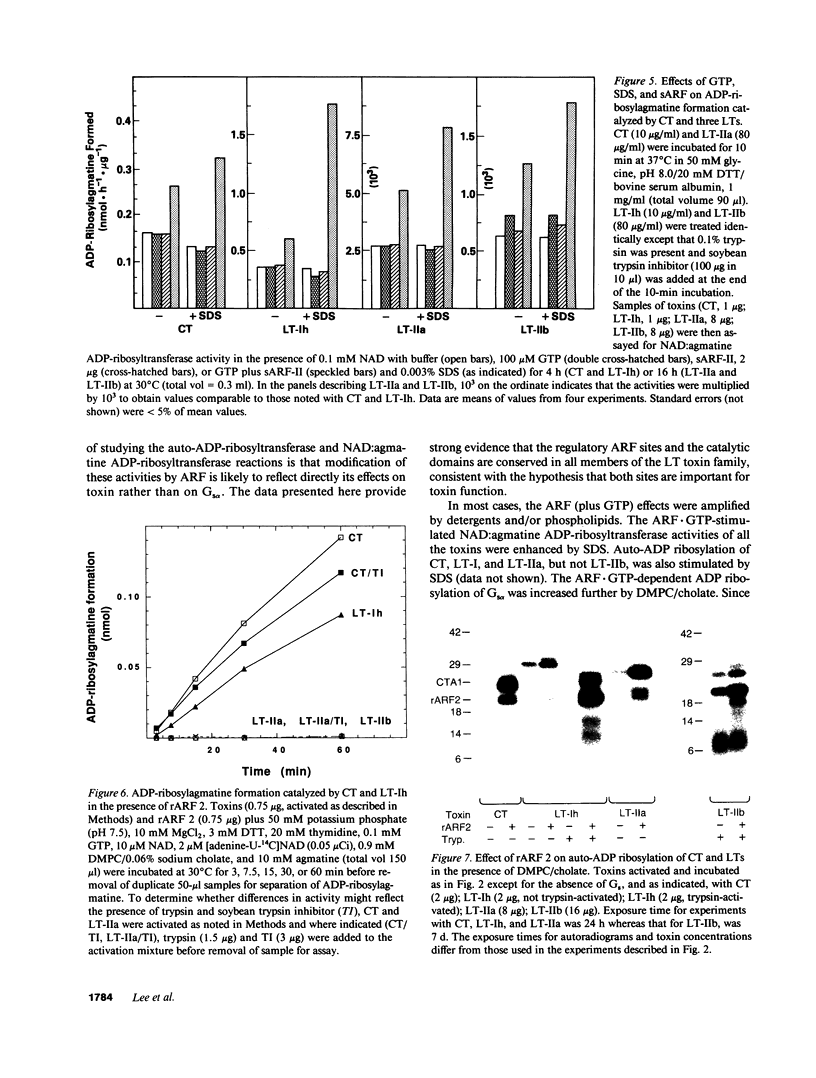
Abstract
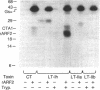
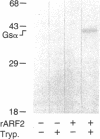
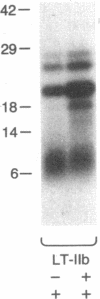
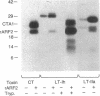
Escherichia coli heat-labile enterotoxins (LT) are responsible in part for "traveler's diarrhea" and related diarrheal illnesses. The family of LTs comprises two serogroups termed LT-I and LT-II; each serogroup includes two or more antigenic variants. The effects of LTs result from ADP ribosylation of Gs alpha, a stimulatory component of adenylyl cyclase; the mechanism of action is identical to that of cholera toxin (CT). The ADP-ribosyltransferase activity of CT is enhanced by 20-kD guanine nucleotide-binding proteins, known as ADP-ribosylation factors or ARFs. These proteins directly activate the CTA1 catalytic unit and stimulate its ADP ribosylation of Gs alpha, other proteins, and simple guanidino compounds (e.g., agmatine). Because of the similarities between CT and LTs, we investigated the effects of purified bovine brain ARF and a recombinant form of bovine ARF synthesized in Escherichia coli on LT activity. ARF enhanced the LT-I-, LT-IIa-, and LT-IIb-catalyzed ADP ribosylation of agmatine, as well as the auto-ADP ribosylation of the toxin catalytic unit. Stimulation of ADP-ribosylagmatine formation by LTs and CT in the presence of ARF was GTP dependent and enhanced by sodium dodecyl sulfate. With agmatine as substrate, LT-IIa and LT-IIb exhibited less than 1% the activity of CT and LT-Ih. CT and LTs catalyzed ADP-ribosyl-Gs alpha formation in a reaction dependent on ARF, GTP, and dimyristoyl phosphatidylcholine/cholate. With Gs alpha as substrate, the ADP-ribosyltransferase activities of the toxins were similar, although CT and LT-Ih appeared to be slightly more active than LT-IIa and LT-IIb. Thus, LT-IIa and LT-IIb appear to differ somewhat from CT and LT-Ih in substrate specificity. Responsiveness to stimulation by ARF, GTP, and phospholipid/detergent as well as the specificity of ADP-ribosyltransferase activity are functions of LTs from serogroups LT-I and LT-II that are shared with CT.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Bobak D. A., Bliziotes M. M., Noda M., Tsai S. C., Adamik R., Moss J. Mechanism of activation of cholera toxin by ADP-ribosylation factor (ARF): both low- and high-affinity interactions of ARF with guanine nucleotides promote toxin activation. Biochemistry. 1990 Jan 30;29(4):855–861. doi: 10.1021/bi00456a600. [DOI] [PubMed] [Google Scholar]
- Burns D. L., Moss J., Vaughan M. Choleragen-stimulated release of guanyl nucleotides from turkey erythrocyte membranes. J Biol Chem. 1982 Jan 10;257(1):32–34. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. P., Moss J., Twiddy E. M., Holmes R. K. Type II heat-labile enterotoxin of Escherichia coli activates adenylate cyclase in human fibroblasts by ADP ribosylation. Infect Immun. 1987 Aug;55(8):1854–1858. doi: 10.1128/iai.55.8.1854-1858.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieplak W., Burnette W. N., Mar V. L., Kaljot K. T., Morris C. F., Chen K. K., Sato H., Keith J. M. Identification of a region in the S1 subunit of pertussis toxin that is required for enzymatic activity and that contributes to the formation of a neutralizing antigenic determinant. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4667–4671. doi: 10.1073/pnas.85.13.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Demonstration of shared and unique immunological determinants in enterotoxins from Vibrio cholerae and Escherichia coli. Infect Immun. 1978 Dec;22(3):709–713. doi: 10.1128/iai.22.3.709-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowl R., Seamans C., Lomedico P., McAndrew S. Versatile expression vectors for high-level synthesis of cloned gene products in Escherichia coli. Gene. 1985;38(1-3):31–38. doi: 10.1016/0378-1119(85)90200-8. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Field M. Mechanisms of action of cholera and Escherichia coli enterotoxins. Am J Clin Nutr. 1979 Jan;32(1):189–196. doi: 10.1093/ajcn/32.1.189. [DOI] [PubMed] [Google Scholar]
- Fukuta S., Magnani J. L., Twiddy E. M., Holmes R. K., Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988 Jul;56(7):1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Griffiths S. L., Finkelstein R. A., Critchley D. R. Characterization of the receptor for cholera toxin and Escherichia coli heat-labile toxin in rabbit intestinal brush borders. Biochem J. 1986 Sep 1;238(2):313–322. doi: 10.1042/bj2380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. E., Pickett C. L., Twiddy E. M., Holmes R. K., Gomes T. A., Lima A. A., Guerrant R. L., Franco B. D., Trabulsi L. R. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun. 1986 Nov;54(2):587–589. doi: 10.1128/iai.54.2.587-589.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. E., Twiddy E. M., Trabulsi L. R., Holmes R. K. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect Immun. 1986 Nov;54(2):529–536. doi: 10.1128/iai.54.2.529-536.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L. Relationships among heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. J Infect Dis. 1974 Mar;129(3):277–283. doi: 10.1093/infdis/129.3.277. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Twiddy E. M., Pickett C. L. Purification and characterization of type II heat-labile enterotoxin of Escherichia coli. Infect Immun. 1986 Sep;53(3):464–473. doi: 10.1128/iai.53.3.464-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Fredman P., Lindblad M., Svennerholm A. M., Svennerholm L. Rabbit intestinal glycoprotein receptor for Escherichia coli heat-labile enterotoxin lacking affinity for cholera toxin. Infect Immun. 1982 Nov;38(2):424–433. doi: 10.1128/iai.38.2.424-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lindblad M., Fredman P., Svennerholm L., Myrvold H. Comparison of receptors for cholera and Escherichia coli enterotoxins in human intestine. Gastroenterology. 1985 Jul;89(1):27–35. doi: 10.1016/0016-5085(85)90741-3. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. ADP-ribosylation of Gs promotes the dissociation of its alpha and beta subunits. J Biol Chem. 1984 May 25;259(10):6235–6240. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J Biol Chem. 1984 May 25;259(10):6228–6234. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986 Jun 15;261(17):7906–7911. [PubMed] [Google Scholar]
- Kelly M. T. Cholera: a worldwide perspective. Pediatr Infect Dis. 1986 Jan-Feb;5(1 Suppl):S101–S105. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. Y. Determination of the primary structure of cholera toxin B subunit. J Biol Chem. 1977 Oct 25;252(20):7249–7256. [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Enzymic activity of cholera toxin. I. New method of assay and the mechanism of ADP-ribosyl transfer. J Biol Chem. 1979 Jul 10;254(13):5849–5854. [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Enzymic activity of cholera toxin. II. Relationships to proteolytic processing, disulfide bond reduction, and subunit composition. J Biol Chem. 1979 Jul 10;254(13):5855–5861. [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Moss J., Burns D. L., Hsia J. A., Hewlett E. L., Guerrant R. L., Vaughan M. NIH conference. Cyclic nucleotides: mediators of bacterial toxin action in disease. Ann Intern Med. 1984 Nov;101(5):653–666. doi: 10.7326/0003-4819-101-5-653. [DOI] [PubMed] [Google Scholar]
- Moss J., Garrison S., Fishman P. H., Richardson S. H. Gangliosides sensitize unresponsive fibroblasts to Escherichia coli heat-labile enterotoxin. J Clin Invest. 1979 Aug;64(2):381–384. doi: 10.1172/JCI109472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Osborne J. C., Jr, Fishman P. H., Nakaya S., Robertson D. C. Escherichia coli heat-labile enterotoxin. Ganglioside specificity and ADP-ribosyltransferase activity. J Biol Chem. 1981 Dec 25;256(24):12861–12865. [PubMed] [Google Scholar]
- Moss J., Stanley S. J. Histone-dependent and histone-independent forms of an ADP-ribosyltransferase from human and turkey erythrocytes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4809–4812. doi: 10.1073/pnas.78.8.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Tsai S. C., Adamik R., Moss J., Vaughan M. Mechanism of cholera toxin activation by a guanine nucleotide-dependent 19 kDa protein. Biochim Biophys Acta. 1990 May 16;1034(2):195–199. doi: 10.1016/0304-4165(90)90076-9. [DOI] [PubMed] [Google Scholar]
- Pickett C. L., Twiddy E. M., Belisle B. W., Holmes R. K. Cloning of genes that encode a new heat-labile enterotoxin of Escherichia coli. J Bacteriol. 1986 Feb;165(2):348–352. doi: 10.1128/jb.165.2.348-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. L., Weinstein D. L., Holmes R. K. Genetics of type IIa heat-labile enterotoxin of Escherichia coli: operon fusions, nucleotide sequence, and hybridization studies. J Bacteriol. 1987 Nov;169(11):5180–5187. doi: 10.1128/jb.169.11.5180-5187.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. Differential inhibitory effects of cholera toxoids and ganglioside on the enterotoxins of Vibrio cholerae and Escherichia coli. J Exp Med. 1973 Apr 1;137(4):1009–1023. doi: 10.1084/jem.137.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. R., Nightingale M., Tsai S. C., Williamson K. C., Adamik R., Chen H. C., Moss J., Vaughan M. Guanine nucleotide-binding proteins that enhance choleragen ADP-ribosyltransferase activity: nucleotide and deduced amino acid sequence of an ADP-ribosylation factor cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5488–5491. doi: 10.1073/pnas.85.15.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seriwatana J., Echeverria P., Taylor D. N., Rasrinaul L., Brown J. E., Peiris J. S., Clayton C. L. Type II heat-labile enterotoxin-producing Escherichia coli isolated from animals and humans. Infect Immun. 1988 May;56(5):1158–1161. doi: 10.1128/iai.56.5.1158-1161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E. K., Kavanaugh W. M., Dallas W. S., Falkow S., Konigsberg W. H., Schafer D. E. Sequence homologies between A subunits of Escherichia coli and Vibrio cholerae enterotoxins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):50–54. doi: 10.1073/pnas.78.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Tsai S. C., Noda M., Adamik R., Chang P. P., Chen H. C., Moss J., Vaughan M. Stimulation of choleragen enzymatic activities by GTP and two soluble proteins purified from bovine brain. J Biol Chem. 1988 Feb 5;263(4):1768–1772. [PubMed] [Google Scholar]
- Tsai S. C., Noda M., Adamik R., Moss J., Vaughan M. Enhancement of choleragen ADP-ribosyltransferase activities by guanyl nucleotides and a 19-kDa membrane protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5139–5142. doi: 10.1073/pnas.84.15.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnieski B. J., Bramhall J. S. Photolabelling of cholera toxin subunits during membrane penetration. Nature. 1981 Jan 22;289(5795):319–321. doi: 10.1038/289319a0. [DOI] [PubMed] [Google Scholar]