Abstract
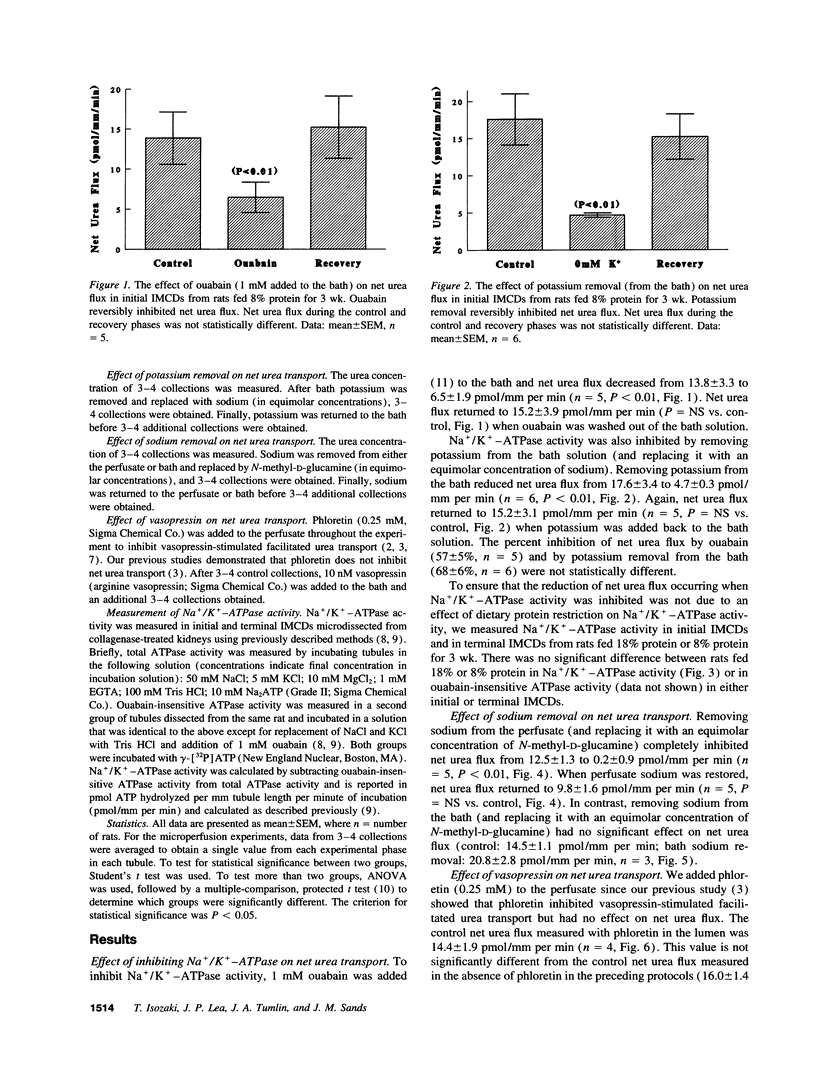
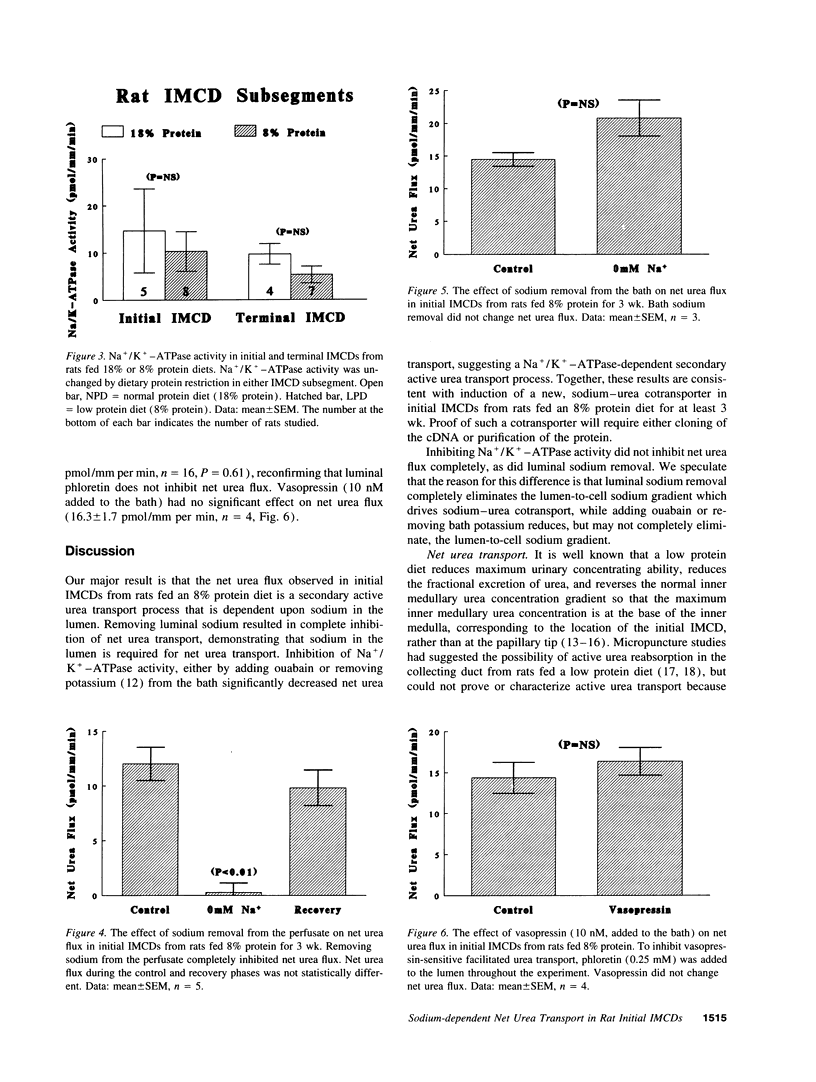
We reported that feeding rats 8% protein for 3 wk induces net urea transport and morphologic changes in initial inner medullary collecting ducts (IMCDs) which are not present in rats fed 18% protein. In this study, we measured net urea transport in microperfused initial IMCDs from rats fed 8% protein for > or = 3 wk and tested the effect of inhibiting Na+/K(+)-ATPase activity and found that adding 1 mM ouabain to the bath reversibly inhibited net urea transport from 14 +/- 3 to 6 +/- 2 pmol/mm per min (P < 0.01), and that replacing potassium (with sodium) in the bath reversibly inhibited net urea transport from 18 +/- 3 to 5 +/- 0 pmol/mm per min (P < 0.01). Replacing perfusate sodium with N-methyl-D-glucamine reversibly inhibited net urea transport from 12 +/- 2 to 0 +/- 1 pmol/mm per min (P < 0.01), whereas replacing bath sodium had no significant effect on net urea transport. Adding 10 nM vasopressin to the bath exerted no significant effect on net urea transport. Finally, we measured Na+/K(+)-ATPase activity in initial and terminal IMCDs from rats fed 18% or 8% protein and found no significant difference in either subsegment. Thus, net urea transport in initial IMCDs from rats fed 8% protein for > or = 3 wk requires sodium in the lumen, is reduced by inhibiting Na+/K(+)-ATPase, and is unchanged by vasopressin or phloretin. These results suggest that net urea transport may occur via a novel, secondary active, sodium-urea cotransporter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou C. L., Knepper M. A. Inhibition of urea transport in inner medullary collecting duct by phloretin and urea analogues. Am J Physiol. 1989 Sep;257(3 Pt 2):F359–F365. doi: 10.1152/ajprenal.1989.257.3.F359. [DOI] [PubMed] [Google Scholar]
- Danielson R. A., Schmidt-Nielsen B. Recirculation of urea analogs from renal collecting ducts of high- and low-protein-fed rats. Am J Physiol. 1972 Jul;223(1):130–137. doi: 10.1152/ajplegacy.1972.223.1.130. [DOI] [PubMed] [Google Scholar]
- Doucet A., Katz A. I., Morel F. Determination of Na-K-ATPase activity in single segments of the mammalian nephron. Am J Physiol. 1979 Aug;237(2):F105–F113. doi: 10.1152/ajprenal.1979.237.2.F105. [DOI] [PubMed] [Google Scholar]
- Dytko G., Smith P. L., Kinter L. B. Urea transport in toad skin (Bufo marinus) J Pharmacol Exp Ther. 1993 Oct;267(1):364–370. [PubMed] [Google Scholar]
- El Mernissi G., Chabardès D., Doucet A., Hus-Citharel A., Imbert-Teboul M., Le Bouffant F., Montégut M., Siaume S., Morel F. Changes in tubular basolateral membrane markers after chronic DOCA treatment. Am J Physiol. 1983 Jul;245(1):F100–F109. doi: 10.1152/ajprenal.1983.245.1.F100. [DOI] [PubMed] [Google Scholar]
- Garcia-Romeu F., Masoni A., Isaia J. Active urea transport through isolated skins of frog and toad. Am J Physiol. 1981 Sep;241(3):R114–R123. doi: 10.1152/ajpregu.1981.241.3.R114. [DOI] [PubMed] [Google Scholar]
- Gillin A. G., Sands J. M. Characteristics of osmolarity-stimulated urea transport in rat IMCD. Am J Physiol. 1992 Jun;262(6 Pt 2):F1061–F1067. doi: 10.1152/ajprenal.1992.262.6.F1061. [DOI] [PubMed] [Google Scholar]
- Isozaki T., Gillin A. G., Swanson C. E., Sands J. M. Protein restriction sequentially induces new urea transport processes in rat initial IMCD. Am J Physiol. 1994 May;266(5 Pt 2):F756–F761. doi: 10.1152/ajprenal.1994.266.5.F756. [DOI] [PubMed] [Google Scholar]
- Isozaki T., Verlander J. W., Sands J. M. Low protein diet alters urea transport and cell structure in rat initial inner medullary collecting duct. J Clin Invest. 1993 Nov;92(5):2448–2457. doi: 10.1172/JCI116852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S., Kokko J. P. Urea secretion by the straight segment of the proximal tubule. J Clin Invest. 1976 Sep;58(3):604–612. doi: 10.1172/JCI108507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y., Abe K., Igarashi Y., Kudo K., Tada K., Yoshinaga K. Direct evidence for the absence of active Na+ reabsorption in hamster ascending thin limb of Henle's loop. J Clin Invest. 1993 Jan;91(1):5–11. doi: 10.1172/JCI116199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste I., Dunel-Erb S., Harvey B. J., Laurent P., Ehrenfeld J. Active urea transport independent of H+ and Na+ transport in frog skin epithelium. Am J Physiol. 1991 Oct;261(4 Pt 2):R898–R906. doi: 10.1152/ajpregu.1991.261.4.R898. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Kaissling B., Dubach U. C. Distal tubular segments of the rabbit kidney after adaptation to altered Na- and K-intake. II. Changes in Na-K-ATPase activity. Cell Tissue Res. 1982;224(3):493–504. doi: 10.1007/BF00213747. [DOI] [PubMed] [Google Scholar]
- Peil A. E., Stolte H., Schmidt-Nielsen B. Uncoupling of glomerular and tubular regulations of urea excretion in rat. Am J Physiol. 1990 Jun;258(6 Pt 2):F1666–F1674. doi: 10.1152/ajprenal.1990.258.6.F1666. [DOI] [PubMed] [Google Scholar]
- Rapoport J., Abuful A., Chaimovitz C., Noeh Z., Hays R. M. Active urea transport by the skin of Bufo viridis: amiloride- and phloretin-sensitive transport sites. Am J Physiol. 1988 Sep;255(3 Pt 2):F429–F433. doi: 10.1152/ajprenal.1988.255.3.F429. [DOI] [PubMed] [Google Scholar]
- Sands J. M., Knepper M. A. Urea permeability of mammalian inner medullary collecting duct system and papillary surface epithelium. J Clin Invest. 1987 Jan;79(1):138–147. doi: 10.1172/JCI112774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. M., Nonoguchi H., Knepper M. A. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol. 1987 Nov;253(5 Pt 2):F823–F832. doi: 10.1152/ajprenal.1987.253.5.F823. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen B., Barrett J. M., Graves B., Crossley B. Physiological and morphological responses of the rat kidney to reduced dietary protein. Am J Physiol. 1985 Jan;248(1 Pt 2):F31–F42. doi: 10.1152/ajprenal.1985.248.1.F31. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen B., Truniger B., Rabinowitz L. Sodium-linked urea transport by the renal tubule of the spiny dogfish Squalus acanthias. Comp Biochem Physiol A Comp Physiol. 1972 May 1;42(1):13–25. doi: 10.1016/0300-9629(72)90360-x. [DOI] [PubMed] [Google Scholar]
- TRUNIGER B., SCHMIDT-NIELSEN B. INTRARENAL DISTRIBUTION OF UREA AND RELATED COMPOUNDS: EFFECTS OF NITROGEN INTAKE. Am J Physiol. 1964 Nov;207:971–978. doi: 10.1152/ajplegacy.1964.207.5.971. [DOI] [PubMed] [Google Scholar]
- Terada Y., Knepper M. A. Na+-K+-ATPase activities in renal tubule segments of rat inner medulla. Am J Physiol. 1989 Feb;256(2 Pt 2):F218–F223. doi: 10.1152/ajprenal.1989.256.2.F218. [DOI] [PubMed] [Google Scholar]
- Tumlin J. A., Sands J. M. Nephron segment-specific inhibition of Na+/K(+)-ATPase activity by cyclosporin A. Kidney Int. 1993 Jan;43(1):246–251. doi: 10.1038/ki.1993.38. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Schmidt-Nielsen B. Urea transport in the collecting duct of rats on normal and low protein diet. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(2):147–156. doi: 10.1007/BF00362746. [DOI] [PubMed] [Google Scholar]
- Ussing H. H., Johansen B. Anomalous transport of sucrose and urea in toad skin. Nephron. 1969;6(3):317–328. doi: 10.1159/000179736. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Sonnenberg H. Urea reabsorption in the medullary collecting duct of protein-depleted young rats before and after urea infusion. Pflugers Arch. 1982 Jun;393(4):302–307. doi: 10.1007/BF00581414. [DOI] [PubMed] [Google Scholar]
- You G., Smith C. P., Kanai Y., Lee W. S., Stelzner M., Hediger M. A. Cloning and characterization of the vasopressin-regulated urea transporter. Nature. 1993 Oct 28;365(6449):844–847. doi: 10.1038/365844a0. [DOI] [PubMed] [Google Scholar]


