Abstract
Summary: Helicobacter pylori is a gastric pathogen that colonizes approximately 50% of the world's population. Infection with H. pylori causes chronic inflammation and significantly increases the risk of developing duodenal and gastric ulcer disease and gastric cancer. Infection with H. pylori is the strongest known risk factor for gastric cancer, which is the second leading cause of cancer-related deaths worldwide. Once H. pylori colonizes the gastric environment, it persists for the lifetime of the host, suggesting that the host immune response is ineffective in clearing this bacterium. In this review, we discuss the host immune response and examine other host factors that increase the pathogenic potential of this bacterium, including host polymorphisms, alterations to the apical-junctional complex, and the effects of environmental factors. In addition to host effects and responses, H. pylori strains are genetically diverse. We discuss the main virulence determinants in H. pylori strains and the correlation between these and the diverse clinical outcomes following H. pylori infection. Since H. pylori inhibits the gastric epithelium of half of the world, it is crucial that we continue to gain understanding of host and microbial factors that increase the risk of developing more severe clinical outcomes.
INTRODUCTION
Helicobacter pylori
Less than 3 decades ago, Robin Warren and Barry Marshall definitively identified Helicobacter pylori by culturing an organism from gastric biopsy specimens that had been visualized for almost a century by pathologists (196). In 1994, H. pylori was recognized as a type I carcinogen, and now it is considered the most common etiologic agent of infection-related cancers, which represent 5.5% of the global cancer burden (239). In 2005, Marshall and Warren were awarded the Nobel Prize of Medicine for their seminal discovery of this bacterium and its role in peptic ulcer disease.
H. pylori is a Gram-negative bacterial pathogen that selectively colonizes the gastric epithelium. The bacterium is urease, catalase, and oxidase positive, is spiral shaped, and possesses 3 to 5 polar flagella that are used for motility. In addition, the majority of H. pylori strains express virulence factors that have evolved to affect host cell signaling pathways. Among many unique characteristics of H. pylori, one of the most remarkable is its capacity to persist for decades in the harsh gastric environment due to an inability of the host to eliminate the infection. Unlike other viruses and bacteria, H. pylori has evolved the ability to colonize the highly acidic environment found within the stomach by metabolizing urea to ammonia via urease, which generates a neutral environment enveloping the bacterium (332). Indeed, evidence now supports the tenet that H. pylori has coexisted with humans for tens of thousands of years, with genetic studies indicating that humans have been colonized with H. pylori for at least 58,000 years (176).
Approximately half of the world's population is infected with H. pylori, and the majority of colonized individuals develop coexisting chronic inflammation. In most persons, H. pylori colonization does not cause any symptoms (242). However, long-term carriage of H. pylori significantly increases the risk of developing site-specific diseases. Among infected individuals, approximately 10% develop peptic ulcer disease, 1 to 3% develop gastric adenocarcinoma, and <0.1% develop mucosa-associated lymphoid tissue (MALT) lymphoma (244). At early stages, gastric MALT lymphoma can be cured completely by eradication of H. pylori and therefore is considered the first clonal lesion which can be eliminated by treatment with antibiotics (294).
The link between H. pylori and gastric cancer was a matter of debate for a number of years. However, several studies, including a study of 1,526 Japanese patients, have now provided clear evidence that H. pylori infection significantly increases gastric cancer risk (319). Uemura et al. (319) reported that gastric cancer developed in approximately 3% of H. pylori-infected patients, compared to none of the uninfected patients. Eradication of H. pylori significantly decreases the risk of gastric cancer in infected individuals without premalignant lesions. Randomized prospective studies demonstrated that eradication significantly reduced the presence of premalignant lesions, providing additional evidence that this organism has an effect on early stages of gastric carcinogenesis (200, 342). In experimentally challenged Mongolian gerbils, eradication of H. pylori resulted in a significant attenuation of the progression toward gastric cancer (224, 264). Taken together, these studies support an unequivocal role for H. pylori in the development of gastric cancer and indicate that anti-H. pylori therapy may be an effective means of gastric cancer prevention.
Though H. pylori infection can be found in all regions of the world, rates of colonization vary considerably, with higher rates present in developing countries than in developed areas (87). Most infections are thought to be acquired in childhood via the fecal-oral or oral-oral mode of transmission (85, 87). The variable outcomes of H. pylori infection likely depend on factors such as strain-specific bacterial constituents, inflammatory responses governed by host genetic diversity, or environmental influences, which ultimately influence the interactions between pathogen and host (33).
Gastric Cancer
Almost 1 million cases of gastric cancer are diagnosed each year, establishing this disease as the fourth most common cancer worldwide. This is the second leading cause of cancer-related deaths, and approximately 700,000 people succumb each year to gastric adenocarcinoma (239). In some regions of the world, gastric carcinoma is the most common malignancy, and in Japan, the incidence of gastric cancer is almost 10-fold higher than rates observed in the United States. Typically, the diagnosis of gastric cancer is delayed by a lack of early specific symptoms, and most patients are diagnosed after cancer has invaded the muscularis propria. This may be one explanation for why the 5-year survival rate for gastric cancer in the United States is less than 15% (57).
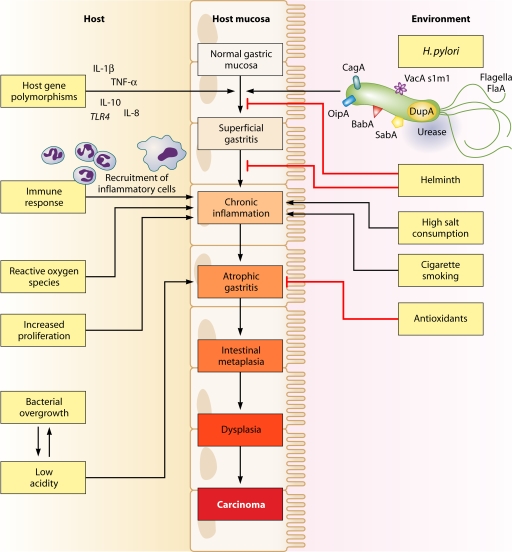
Histologically, two distinct variants of gastric carcinoma have been identified: diffuse-type gastric cancer, which consists of individually infiltrating neoplastic cells that do not form glandular structures; and intestinal-type adenocarcinoma, which progresses through a series of well-defined histological steps and was first described in 1975 (56) (Fig. 1). Intestinal-type adenocarcinoma is initiated by the transition from normal mucosa to chronic superficial gastritis; this is followed by atrophic gastritis and intestinal metaplasia, finally leading to dysplasia and adenocarcinoma (55, 289). This form of gastric cancer affects men twice as commonly as women and occurs in men with a mean age of 50.4 years and in women with a mean age of 47.7 years (59, 128). Corpus-predominant gastritis predisposes individuals toward gastric cancer, which is thought to be due in part to decreased acid secretion. In contrast, infection primarily of the gastric antrum results in increased acid production and predisposes individuals to duodenal ulcer disease, which is associated with a decreased risk of gastric cancer (23).
FIG. 1.
Multifactorial pathway leading to gastric carcinoma. Many host, bacterial, and environmental factors act in combination to contribute to the precancerous cascade leading to development of gastric cancer.
H. PYLORI VIRULENCE FACTORS
cag PAI
Due to the genetic heterogeneity present within H. pylori genomes, bacterial virulence factors likely play an important role in determining the outcome of H. pylori infection. The cag pathogenicity island (cag PAI) is a 40-kb DNA insertion element which contains 27 to 31 genes flanked by 31-bp direct repeats and encodes one of the most intensely investigated H. pylori proteins, CagA (7, 43, 60). CagA was initially identified in the early 1990s, and expression of CagA was found to be associated strongly with peptic ulceration (62, 67). Due to its association with clinical disease, the cag PAI is now a well-characterized H. pylori virulence determinant, and CagA is frequently used as an indicator of the presence of the entire cag PAI.
Approximately 60 to 70% of Western H. pylori strains and almost 100% of East Asian strains express CagA (10, 43, 310). Although all H. pylori strains induce gastritis, strains that contain the cag PAI (cag+) augment the risks for severe gastritis, atrophic gastritis, and distal gastric cancer compared to those with strains that lack the cag island (cag-deficient mutants) (34, 62, 67, 68, 167, 240, 245, 252, 265, 287, 311, 325). At least 18 cag genes encode components of a bacterial type IV secretion apparatus which functions to export bacterial proteins across the bacterial membrane and into host gastric epithelial cells.
CagA
H. pylori strains are frequently segregated into cagA-positive and cagA-negative strains, depending on the presence or absence of the terminal gene product of the cag island, CagA. The H. pylori CagA protein is a 120- to 140-kDa protein that is translocated into host cells by the type IV cag secretion system after bacterial attachment. Once inside the host cell, CagA is tyrosine phosphorylated at glutamate-proline-isoleucine- tyrosine-alanine (EPIYA) motifs and induces cell morphological changes, initially termed “the hummingbird phenotype,” which are associated with increased cellular migration (20, 226, 279, 292, 293) (see “CagA phosphorylation-dependent host cell signaling”).
To date, four distinct EPIYA motifs (EPIYA-A, -B, -C, and -D) have been identified within the carboxy-terminal polymorphic region of CagA, and they are distinguished by different amino acid sequences surrounding the EPIYA motif (128, 134, 215). EPIYA-A and -B motifs are present in strains throughout the world, whereas EPIYA-C is typically found only in strains from Western countries (Europe, North America, and Australia). The number of EPIYA-C sites can vary; however, most CagA proteins contain a single EPIYA-C site (A-B-C type), and EPIYA-A and EPIYA-B sites are phosphorylated to a lesser extent than EPIYA-C sites. In Western strains, an increased number of CagA EPIYA-C sites is an important indicator of the risk of developing gastric cancer (31). The EPIYA-D motif is found almost exclusively in East Asian strains (from Japan, South Korea, and China), and strains containing this motif induce larger amounts of interleukin-8 (IL-8) from gastric epithelial cells than do strains harboring Western A-B-C-type CagA (19, 128).
CagA phosphorylation-dependent host cell signaling.
Once it is phosphorylated by members of the Abl and Src families of kinases, phospho-CagA targets and interacts with numerous intracellular effectors. Phospho-CagA activates a eukaryotic tyrosine phosphatase (SHP-2), leading to sustained activation of extracellular signal-regulated kinases 1 and 2 (ERK1/2), Crk adaptor (133), and C-terminal Src kinase, in a tyrosine phosphorylation-dependent manner in which East Asian A-B-D-type CagA exhibits stronger binding activity for SHP-2 than Western A-B-C-type CagA (133). Interactions of phospho-CagA with C-terminal Src kinase rapidly activate a negative feedback loop to downregulate Src signaling (315).
In a human gastric adenocarcinoma cell line (AGS), translocation and subsequent phosphorylation of CagA result in the “hummingbird phenotype,” a phenotype associated with cell elongation and cell scattering (209, 279). In AGS cells, the interaction between phospho-CagA and SHP-2 increases the duration of ERK activation, in a manner independent of Ras or phosphatidylinositol 3-kinase (PI3K), and results in cell elongation (132). The interaction between CagA and SHP-2 also dephosphorylates and inactivates focal adhesion kinase (FAK), resulting in cell elongation (316). Phosphorylated CagA also induces cell elongation by inducing a defect in cell retraction; however, the signaling molecules required for this phenotype remain undefined (38). In addition, the catalytic activity of c-Src is inhibited by phosphorylated CagA, which leads to tyrosine dephosphorylation of the actin binding proteins cortactin, ezrin, and vinculin, leading to cell elongation (208, 280, 281).
CagA phosphorylation-independent host cell signaling.
Nonphosphorylated CagA also exerts effects within the cell that contribute to pathogenesis. Translocation, but not phosphorylation, of CagA leads to aberrant activation of β-catenin, disruption of apical-junctional complexes, and a loss of cellular polarity (13, 27, 101, 212, 269, 303). Nonphosphorylated CagA targets the cell adhesion protein E-cadherin, the hepatocyte growth factor receptor c-Met, phospholipase C gamma (PLC-γ), the adaptor protein Grb2, and the kinase partitioning-defective 1b/microtubule affinity-regulating kinase 2 (PAR1b/MARK2) (53, 205, 212, 269), which leads to proinflammatory and mitogenic responses, disruption of cell-cell junctions, and loss of cell polarity. Nonphosphorylated CagA associates with the epithelial tight junction scaffolding protein zonula occludens 1 (ZO-1) and the transmembrane protein junctional adhesion molecule A (JAM-A), leading to nascent but incomplete assembly of tight junctions at ectopic sites of bacterial attachment (13). Recently, CagA was shown to directly bind PAR1b/MARK2, a central regulator of cell polarity, and to inhibit its kinase activity as well as to dysregulate mitotic spindle formation, thus promoting a loss of cell polarity (see “Apical-Junctional Complexes”) (179, 269, 320). While it is evident that non-tyrosine-phosphorylated mutant forms of CagA exert effects within gastric epithelial cells, to our knowledge, there is currently no direct evidence for nonphosphorylated CagA within the host cell.
CagA is one of the most intensely investigated H. pylori proteins, and to date, it is the only bacterial effector protein known to be translocated by the type IV cag secretion system. As discussed above, studies with animal and cell culture models have provided evidence for the importance of CagA in H. pylori pathogenesis and have highlighted the vast array of host cell functions with which CagA may interfere. The generation of transgenic mice expressing CagA has now provided more direct evidence for a causal relationship between CagA and oncogenesis by demonstrating that transgenic expression of CagA leads to gastric epithelial cell proliferation and carcinoma. These changes were not observed in mice expressing phosphorylation-resistant CagA (231). Overall, there is strong evidence that CagA functions as a bacterial oncoprotein in mammals. However, experimental models that are currently available have not provided all of the answers. Cell culture and animal models often provide conflicting data, and pathological changes reported for transgenic CagA mice occurred in the absence of inflammation, which is in stark contrast to what is seen in humans (231). In addition, only a small fraction of individuals colonized by CagA-positive H. pylori develop gastric cancer. Much remains to be learned about the circumstances that coalesce to permit CagA to initiate carcinogenesis. Until very recently, it was unclear how CagA is actually delivered into the host cell. However, Murata-Kamiya and colleagues have now reported that H. pylori induces the appearance of a host phospholipid, phosphatidylserine, on the external leaflet of the plasma membrane, where CagA can specifically interact and gain entry into the cells (211). Fertile areas of future research will include studies to determine the specific mechanism by which CagA is internalized and when during chronic infection CagA is translocated into host cells.
Peptidoglycan
In addition to CagA, the cag secretion system can also deliver components of H. pylori peptidoglycan into host cells. Peptidoglycan interacts with the host intracellular pattern recognition molecule Nod1, which acts as a sensor for peptidoglycan components originating from Gram-negative bacteria. The interaction of H. pylori peptidogylcan with Nod1 leads to activation of NF-κB-dependent proinflammatory responses, such as secretion of IL-8 (323) or β-defensin-2 (37). H. pylori translocated peptidoglycan has been shown to activate other host signaling pathways that are associated with an increased risk for developing gastric cancer. For example, a recent study demonstrated that H. pylori translocated peptidoglycan can activate PI3K-AKT signaling, leading to decreased apoptosis and increased cell migration (214). Another study revealed that intracellular sensing of H. pylori peptidoglycan components triggers an intracellular signaling cascade, which culminates in the production of type I interferon (IFN) (331).
VacA Toxin
An independent H. pylori locus linked with increased disease risk is vacA, which encodes the secreted toxin VacA (61, 64, 247, 276). VacA was first identified as a proteinaceous cytotoxin that induced intracellular vacuolation of cultured cells (173). It was later purified to homogeneity from H. pylori broth culture supernatants and was identified as a protein of approximately 87 kDa in its denatured form (61). VacA suppresses T-cell responses to H. pylori, which may contribute to the longevity of infection (35, 111, 299).
The vacA gene is present in the majority of H. pylori strains; however, considerable differences in vacuolating activities are observed between strains. This variation is attributed to variations in vacA gene structures within the signal (s) region, the middle (m) region, and the more recently identified intermediate (i) region, which is located between the s and m regions (259). The s region is stratified into s1 and s2 subtypes and encodes a component of the signal peptide and the N terminus of the mature protein. The m region partially encodes the 55-kDa C-terminal subunit and is classified as either the m1 or m2 type. vacA s1/m1 chimeric strains induce greater vacuolation than do s1/m2 strains, and there is typically no vacuolating activity in s2/m2 strains (24, 61, 259, 321). In Western populations, the vacA s1/m1 allele is strongly associated with duodenal and gastric ulcer disease and with gastric cancer (24, 25, 203). East Asian strains are almost all vacA s1/m1 and, as predicted, are not associated with any specific clinical outcome. There are two i region subtypes, i1 and i2; the i region plays a functional role in vacuolating activity, since vacA s1/i1/m2 strains are vacuolating types and vacA s1/i2/m2 strains do not induce vacuolation. All s1/m1 vacA alleles are of type i1, all s2/m2 alleles are of type i2, and s1/m2 alleles can be either i1 or i2 (259). In a study of 73 H. pylori-infected Iranian patients, colonization with vacA i1 strains was strongly associated with gastric cancer. This association with gastric cancer may be stronger than associations of vacA s or m types or cag status (259). Among H. pylori strains isolated from patients in Iraq and Italy, vacA i1 strains were associated with gastric ulcer disease (31, 74, 141). However, in East Asian and Southeast Asian populations, where the incidence of gastric cancer is high, vacA i-region subtype is not associated with risk of disease (228).
Recently, a deletion of 81 bp between the m region and the i region was identified and termed the d region; d1 strains have no deletion, while strains of the d2 type contain a 69- to 81-bp deletion. For a small number of Western strains, but not East Asian strains, vacA d1 type was significantly associated with neutrophil infiltration and gastric mucosal atrophy, potentially making the d region genotype another risk locus for gastric cancer and peptic ulceration in Western strains (229).
Consequences of VacA within the host cell.
Similar to elements encoded by the cag PAI, VacA exerts multiple effects on epithelial cell structure and results in phenotypes that include disruption of gastric epithelial barrier function and modulation of the inflammatory response. Other effects of VacA include disruption of late endosomal compartments, which results in vacuole formation in vitro (173, 237), and targeting of mitochondria, leading to a decrease in mitochondrial transmembrane potential, the release of cytochrome c, activation of caspase-8 and caspase-9, and induction of apoptosis in vitro (63, 107, 190, 243, 336).
One of several receptors that VacA binds to on gastric epithelial cells is the receptor-type protein tyrosine phosphatase RPTPβ. This receptor regulates cell proliferation, differentiation, and adhesion, all of which likely play roles in ulcerogenesis (105). Oral administration of acid-activated and then neutralized VacA to wild-type RPTPβ+/+ mice has a profound effect on the gastric epithelium. Within 2 days of VacA administration, heavy bleeding develops in the stomach, which leads to development of gastric ulcers and gastric atrophy. In sharp contrast, RPTPβ−/− mice receiving VacA are resistant to the development of gastric damage (105). Interestingly, in primary cultures of cells isolated from either RPTPβ−/− or RPTPβ+/+ mice, VacA induces vacuolation. However, only cells isolated from RPTPβ+/+ mice detach from Matrigel in response to VacA, suggesting that VacA induces gastric ulcers through RPTPβ signaling, not vacuolation (99).
Recent reports suggest that CagA is able to downregulate the effects of VacA on host cell vacuolation, and conversely, VacA may downregulate CagA activity (232, 308). Mechanistically, Oldani et al. determined that tyrosine-phosphorylated CagA blocks VacA trafficking, preventing it from reaching its intracellular target and inducing vacuole formation. Via a separate mechanism, unphosphorylated CagA antagonized vacuolation by blocking VacA activity at the mitochondria (232). Conversely, VacA antagonizes the effects of CagA on cell scattering and elongation by inactivating epidermal growth factor receptor (EGFR) and HER2/Neu, which suppresses activation of ERK1/2 mitogen-activated protein (MAP) kinase and the hummingbird phenotype (308). These findings further highlight mechanisms through which H. pylori can avoid the induction of excess cellular damage and maintain long-term colonization of the gastric niche.
Adhesins and OMPs
Adherence of H. pylori to the gastric epithelium facilitates initial colonization, persistence of infection, and delivery of virulence factors to host epithelial cells. Sequence analysis of six completely sequenced H. pylori strains reveals that approximately 4% of the H. pylori genome is predicted to encode outer membrane proteins (OMPs), which is significantly more than that for other known bacterial species. OMP expression has been associated with gastroduodenal diseases (as discussed below) and therefore may heighten the risk for developing gastric cancer (73).
BabA.
Blood group antigen binding adhesin (BabA) is encoded by the babA2 gene, which binds to fucosylated Lewisb antigen (Leb) on the surfaces of gastric epithelial cells and is the most well-described H. pylori OMP (36, 113, 143). Transgenic mice that express Leb on pit and surface mucous cells have been used to demonstrate that H. pylori attaches to the surfaces of Leb-expressing cells (123). The ensuing gastritis is more severe than that seen in nontransgenic mice, despite a comparable colonization density, suggesting that Leb-mediated colonization may increase the pathogenic potential of H. pylori (123). A recent study utilizing the same system demonstrated that selective pressure is exerted by transgenic Leb expression and dictates the pattern of Lewis antigen expression on H. pylori lipopolysaccharide (LPS) from Lex and Ley toward Leb (248).
Analyses of binding specificities of H. pylori strains from across the world suggest that the BabA adhesin has evolved in response to host mucosal glycosylation patterns to permit H. pylori to adapt to its host and to maintain persistent colonization (22, 248). The presence of babA2 is associated with duodenal ulcer disease and gastric cancer, and when found in conjunction with cagA and vacA s1 alleles, it is associated with an even greater risk of developing more severe disease (113). More recent analyses of babA2 as a virulence marker have produced conflicting data on the usefulness of babA2 expression in predicting clinical outcome, which is most likely dependent on the geographic origin of the H. pylori strains. In Portuguese and Thai populations, babA2 is not a biomarker for peptic ulcer disease or gastric cancer (52, 110). However, for strains isolated from Germany, Turkey, or northern Portugal, babA2 expression is associated with the severity of gastric disease (26, 86, 113).
SabA and OipA.
Sialic acid-binding adhesin (SabA) is an H. pylori adhesin that binds to the carbohydrate structure sialyl-Lewisx antigen expressed on gastric epithelium and is associated with an increased gastric cancer risk but a reduced risk for duodenal ulceration (354). Sialyl-Lewisx expression is induced during chronic gastric inflammation, suggesting that H. pylori modulates host cell glycosylation patterns to enhance attachment and colonization (187). In vitro, H. pylori induces expression of sialyl-Lewisx antigens via induction of the gene encoding beta3 GlcNAc T5, a transferase essential for the biosynthesis of Lewis antigens (195). Furthermore, SabA is regulated by phase variation, such that SabA expression can rapidly be switched “on” or “off” to adapt to changes exerted by the gastric niche (354).
Outer inflammatory protein (OipA) is an inflammation-related outer membrane protein (353). H. pylori contains either a functional or nonfunctional oipA gene, and the presence of a functional gene is significantly associated with the presence of duodenal ulcers, gastric cancer, and increased neutrophil infiltration (102, 354). OipA expression is linked to increased IL-8 production in vitro (352). Recent work using Mongolian gerbils infected with wild-type H. pylori and an isogenic oipA mutant strain demonstrated a role for OipA in induction of the mucosal cytokines IL-1, IL-17, and tumor necrosis factor alpha (TNF-α) and in gastric mucosal inflammation (296). OipA is also involved in upregulation of matrix metalloproteinase 1 (MMP-1), an MMP associated with gastric cancer (347); in inhibition of glycogen synthase kinase 3β (GSK-3β) (304); and in β-catenin translocation to the nucleus (102). Accumulation of β-catenin in the nucleus results in the formation of heterodimers with LEF/TCF transcription factors and in transcriptional activation of genes that can influence carcinogenesis.
DupA.
Duodenal ulcer promoting gene (dupA) is located within the plasticity zone of the H. pylori genome and may be another novel virulence marker. Initial analysis of 500 H. pylori strains from Colombia, South Korea, and Japan showed an increased risk for duodenal ulcer and a decreased risk for gastric cancer in persons carrying dupA-positive strains (178). In vitro, DupA increases IL-8 production (178). However, a subsequent study focused on strains from Belgium, South Africa, China, and the United States found no significant relationships between dupA expression and duodenal ulcer but a significant association with gastric cancer (18). Comparison of strains from Iran and Iraq indicates that dupA expression is significantly associated with duodenal ulceration in strains isolated from Iraq but not in Iranian isolates (141). No association was found between dupA expression and gastric cancer or duodenal ulcer in strains from Japan (220) or Sweden (275), but correlations were observed between dupA and duodenal ulcer disease or gastric cancer in Chinese strains (275). Collectively, it seems likely that dupA may promote duodenal ulceration and/or gastric cancer in some, but not all, populations.
FlaA.
H. pylori possesses a unipolar bundle of 3 to 5 flagella, which are composed of three structural elements: the basal body, the hook, and the filament (112, 234). The filament acts as a propeller when rotated at its base and is a copolymer of the flagellin subunits FlaA and FlaB (175, 295). FlaA is the predominant subunit, and FlaB is the minor subunit. Mutation of flaA results in flagellar truncation and decreased motility in vitro (149). In vivo, FlaA and other proteins necessary for flagellar assembly are essential for persistent infection in rodent and gnotobiotic piglet models (79, 157, 160). Unlike flagellin from Salmonella or other Gram-negative pathogens that colonize mucosal surfaces, H. pylori FlaA has low intrinsic activity to activate Toll-like receptor 5 (TLR5), which may contribute to evasion of the host immune response and to persistent colonization (15, 114, 170). Isogenic mutants of motB, which encodes the MotB flagellar motor protein and is required for motility, also significantly affect colonization (235). In addition, comparing a noncarcinogenic strain of H. pylori to an in vivo-adapted carcinogenic strain, the noncarcinogenic strain was found to contain a single nucleotide mutation in FlaA rendering it less motile than the carcinogenic strain (100). Thus, motility appears to be essential for successful gastric colonization and may contribute to pathogenesis.
HOST FACTORS
Host Polymorphisms That Influence the Propensity toward Gastric Cancer Development
IL-1β.
H. pylori strain-specific constituents are not absolute determinants of virulence, as most persons colonized with disease-associated strains remain asymptomatic. This has underscored the need to define host factors that may also influence pathological outcomes. Critical host responses that influence the progression to H. pylori-induced carcinogenesis include gastric inflammation and a reduction in acid secretion (81). A host effector molecule that interacts with both of these parameters is the Th1 cytokine IL-1β, a pleiotropic proinflammatory molecule that is increased within the gastric mucosa of H. pylori-infected persons (222). The IL-1β gene cluster, consisting of IL-1β and IL-1RN (encoding the naturally occurring IL-1β receptor antagonist), contains a number of functionally relevant polymorphisms that are associated with either increased or decreased IL-1β production, which has permitted case-control studies to be performed that relate host genotypes to disease. El-Omar et al. were the first to demonstrate that H. pylori-colonized persons with high-expression IL-1β polymorphisms have a significantly increased risk for hypochlorhydria, gastric atrophy, and distal gastric adenocarcinoma compared to that for persons with genotypes that limit IL-1β expression (82). Importantly, these relationships were present only among H. pylori-colonized persons, not uninfected individuals, emphasizing the critical role of host-environment interactions and inflammation in the progression to gastric cancer. Since this initial report, similar findings have been replicated in geographically distinct regions of the world that comprise Caucasian, Asian, and Hispanic populations (106, 185).
Investigations utilizing animal models have confirmed these observational studies of humans. In H. pylori-infected Mongolian gerbils, gastric mucosal IL-1β levels increased at 6 to 12 weeks postchallenge, and this was accompanied by a reciprocal decrease in gastric acid output (305). Administration of recombinant IL-1 receptor antagonist normalized acid outputs (305), implicating IL-1β as a pivotal modulator of acid secretion within inflamed mucosa. Gastric tissue levels of IL-1β are similarly higher in colonized human patients possessing high- versus low-expression IL-1β polymorphisms, and increased IL-1β levels are significantly related to the intensity of gastric inflammation and atrophy (142). IL-1β transgenic mice overexpressing human IL-1β in parietal cells have been found to develop spontaneous gastritis and dysplasia after 1 year of age and to exhibit increased dysplasia and carcinoma when infected with Helicobacter felis (317). Importantly, these findings were linked to activation of myeloid suppressor cells (MDSCs) (317). MDSCs are Gr-1+ CD11b+ immature myeloid cells that have been associated with tumor development and growth (40, 356, 357) and with IL-1β (40, 290). Since IL-1β is a potent inhibitor of acid secretion, is profoundly proinflammatory, is upregulated by H. pylori, and is regulated by promoters with informative polymorphisms, this molecule likely plays a critical role in the development of gastric cancer.
TNF-α.
In addition to IL-1β, TNF-α is a proinflammatory acid-suppressive cytokine that is increased within H. pylori-colonized human gastric mucosa (66). Polymorphisms that increase TNF-α expression have now been associated with an increased risk of gastric cancer and its precursors (83). Oguma et al. recently identified a link between expression of this proinflammatory cytokine and aberrant β-catenin signaling by using transgenic mice that overexpress the β-catenin agonist Wnt1 and develop gastric dysplasia (229a). Within dysplastic mucosa, infiltrating macrophages were observed to be in close apposition to gastric epithelial cells harboring nuclear β-catenin. In vitro studies revealed that supernatants from activated macrophages promoted β-catenin signaling in gastric epithelial cells, which was attenuated by inhibition of binding of TNF-α to its cognate receptor on gastric epithelial cells, providing a potential mechanism through which enhanced levels of TNF-α may augment the risk for gastric cancer.
IL-10.
Polymorphisms that reduce the production of anti-inflammatory cytokines may similarly increase the risk for gastric cancer, and low-expression polymorphisms within the locus controlling expression of the anti-inflammatory cytokine IL-10 are associated with an enhanced risk of distal gastric cancer (83). The combinatorial effect of IL-1β, TNF-α, and IL-10 polymorphisms on the development of cancer has also been determined, and risk increases progressively with an increasing number of proinflammatory polymorphisms, to the point that three high-risk polymorphisms increase the risk of cancer 27-fold over baseline (83).
IL-8.
Genetic polymorphisms that affect innate immune response genes have also been linked to an increased risk of gastric cancer. High-expression alleles within the promoter region of the chemokine IL-8 gene increase the risk for severe inflammation and premalignant lesions in Caucasian and Asian populations, but this has not been confirmed in all studies. A functional polymorphism within TLR4 has also been demonstrated to increase the risk for gastric atrophy and gastric cancer in white populations, which may be related to a deficiency in production of the anti-inflammatory cytokine IL-10.
An important question raised by these studies is whether H. pylori strain characteristics augment cancer risk exerted by host genotypes. Figueiredo et al. stratified H. pylori-infected subjects on the basis of high-expression IL-1β polymorphisms and virulence genotypes of their infecting H. pylori strains (94). Odds ratios for distal gastric cancer were greatest for those persons with high-risk host and bacterial genotypes, and specifically, for persons with high-expression IL-1β alleles that were colonized by H. pylori vacA s1-type strains, the relative risk for gastric cancer was 87-fold over baseline (94), indicating that interactions between specific host and microbial characteristics are biologically significant for the development of gastric cancer.
On the basis of these case-control studies, it is apparent that H. pylori organisms are able to send and receive signals from their hosts, allowing host and bacteria to become linked in a dynamic equilibrium (161, 242). The equilibrium is likely different for each colonized individual, as determined by both host and bacterial characteristics, and may explain why certain H. pylori strains augment the risk for carcinogenesis. For example, cag+ strains induce severe gastritis, leading to increased production of proinflammatory cytokines, such as IL-1β and TNF-α, that not only can amplify the mucosal inflammatory response but also may inhibit acid production, especially in hosts with polymorphisms that permit high expression levels of these molecules. This creates an environment conducive to the growth of other bacteria that can sustain inflammation and continually induce oxidative stress, thus heightening the risk for gastric cancer.
COX-2
In addition to stimulating cytokine production, H. pylori also activates proinflammatory cyclooxygenase (COX) enzymes. Cyclooxygenases catalyze key steps in the conversion of arachidonic acid to endoperoxide (PGH2), a substrate for a variety of prostaglandin synthases that catalyze the formation of prostaglandins and other eicosanoids (122). Prostaglandins regulate a diverse array of physiologic processes, including immunity and development (122), and different isoforms of cyclooxygenase have been identified, each possessing similar activities but differing in expression characteristics and inhibition profiles for nonsteroidal anti-inflammatory drugs (NSAIDs). COX-1 is expressed constitutively in many cells and tissues (207, 337), while COX-2 expression is inducible and can be stimulated by a variety of growth factors and proinflammatory cytokines, such as TNF-α, IFN-γ, and IL-1 (337). COX-2 expression is increased in gastric epithelial cells cocultured with H. pylori (150, 263) and within infected human gastric mucosa (103, 274). COX-2 expression is further increased within gastric premalignant and malignant lesions (260, 300), and COX inhibitors such as aspirin and other NSAIDs decrease the risk of distal gastric cancer (8, 92). H. pylori also activates phospholipase A2, an enzyme that catalyzes the formation of the prostaglandin precursor arachidonic acid, both in vitro and in vivo (217, 249). The capacity of COX-2-generated products to promote neoplasia is well described, and specific mechanisms utilized by these molecules include stimulation of proliferation with inhibition of apoptosis (which leads to a heightened retention of mutagenized cells), promotion of cellular adhesion, stimulation of angiogenesis, and cellular transformation (229, 231).
Acid Secretion
Gastrin, acetylcholine, and histamine are major stimulants of gastric acid secretion. In the gastric corpus, gastrin acts directly on parietal cells and indirectly via histamine release from ECL cells, which in turn activates histamine-H2 receptors on the parietal cell to elicit the release of acid. Acetylcholine acts directly on M3 receptors on the parietal cell and indirectly through histamine release from the ECL cell and inhibition of somatostatin release from D cells (277). Parietal cell stimulation elicits an extensive conformational transformation whereby the tubulovesicles of the resting parietal cell are transformed into secretory canaliculi. The H+K+ ATPase is the primary gastric proton pump, and in unstimulated parietal cells, it is located in tubulovesicles in the cytoplasm. Upon stimulation, the H+K+ ATPase is translocated to the apical membrane to mediate secretion of acid (96).
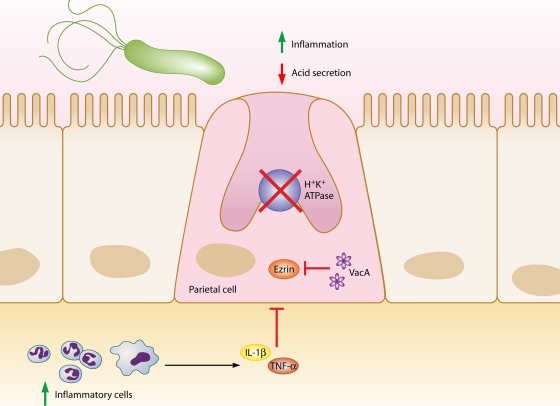
H. pylori can inhibit or stimulate acid secretion, depending on the context of infection. Acute infection is usually associated with hypochlorhydria as a result of increased production of the proinflammatory cytokine IL-1β and inhibition of H+K+ ATPase α-subunit promoter activity (277) (Fig. 2). Indeed, recent work suggests that H. felis infection leads to a decrease in acid production via increased IL-1β acting at the parietal cell IL-1β receptor, which subsequently acts to decrease sonic hedgehog gene expression and to inhibit acid secretion (326). VacA also induces hypochlorhydria by proteolysis of ezrin, which disrupts apical membrane-cytoskeleton interactions in gastric parietal cells that are required to translocate the H+K+ ATPase for acid secretion (329). H. pylori may also decrease acid secretion through repression of H+K+ ATPase α-subunit gene expression by ERK1/2-mediated activation and translocation of NF-κB to the nucleus (271).
FIG. 2.
Relationships between H. pylori, inflammation, and acid secretion. H. pylori infection can reduce acid secretion and increase inflammation via multiple intermediates. Increased production of IL-1β and TNF-α from inflammatory cells inhibits acid secretion from parietal cells. Acid secretion is also inhibited by repression of H+K+ ATPase α-subunit promoter activity, in addition to VacA-induced proteolysis of ezrin.
Chronic H. pylori infection may result in hypochlorhydria or hyperchlorhydria, depending on the severity and distribution of gastritis. Most patients infected long-term develop pangastritis associated with hypochlorydria, which may progress to gastric ulceration and/or adenocarcinoma. Conversely, antral predominant gastritis occurs in approximately 12% of chronically infected patients and is characterized by hyperchlorhydria, which may lead to duodenal ulcer disease (23).
Oxidative Damage
A potential contributing factor in the inflammation-to-carcinoma sequence is the generation of oxidative stress. Oxidative DNA damage induced by H. pylori infection has been well documented for gastritis tissues (28, 90). While this may derive from infiltrating neutrophils, DNA damage has been demonstrated in gastric epithelial cell lines directly exposed to H. pylori (225). The generation of reactive oxygen species in the gastric epithelium may also contribute to dysfunction of these cells, and the oxidative stress response in gastric epithelial cells has been linked to the presence of the cag PAI (72).
As discussed below, polyamines have been implicated in the pathogenesis of H. pylori infection. One specific aspect of this is that oxidation of the polyamine spermine by the enzyme spermine oxidase (SMO; originally termed polyamine oxidase 1) is induced by upregulation of SMO in gastric epithelial cells, and this results in generation of H2O2 (350). Various metabolites of H2O2, such as hydroxyl radicals (OH·), can be highly damaging to macromolecules within cells, including DNA. Inhibition or small interfering RNA (siRNA) knockdown of SMO blocks both apoptosis and DNA damage in gastric epithelial cells (350). In addition, H. pylori-activated macrophages also exhibit marked upregulation of SMO, which causes both apoptosis due to mitochondrial membrane depolarization and release of H2O2 into the extracellular space (48), which can contribute to oxidative stress in adjacent epithelial cells.
Role of the host immune response in H. pylori-induced carcinogenesis.
In considering the importance of host immune/inflammatory responses in the pathogenesis of H. pylori-induced gastric cancer, it is essential to evaluate the potential mechanisms for how immune dysregulation contributes to neoplastic transformation. In many diseases, including those resulting from chronic infections, dysregulation of the immune system is a central component. A signature feature of H. pylori infection is the presence of chronic active gastritis, characterized by both chronic (lymphocytic) and active (neutrophilic) forms of inflammation (119, 196). In the majority of cases, the bacterium remains in the stomach for the life of the host, indicating that the immune response is ineffective. Furthermore, the presence of inflammation for decades supports the notion that the immune response is indeed dysregulated.
General Considerations for Innate and Adaptive Immunity
Innate immunity.
Innate immunity refers to responses that do not require previous exposure to the immune stimulus and represents the first line of defense in the response to pathogens. A major advance in this field has been the elucidation of the TLRs, which are activated by recognition of pathogen-associated molecular patterns (PAMPs) (6). Nonspecific activation by stimuli from microorganisms can lead to important antimicrobial effects but can also result in inflammation and injury due to release of inflammatory mediators such as cytokines, reactive oxygen species, and nitric oxide (NO).
Adaptive immunity.
The adaptive immune response is considered a predetermined response to a previously identified immunologic stimulus. Thus, the response is specific to a particular pathogen and involves immunologic memory. However, the lines between adaptive and innate immunity are blurred by the close interactions between pathways, such that stimulation of antigen-presenting macrophages and dendritic cells (DCs) leads to activation and recruitment of lymphocytes and the development of T-helper (Th)-cell-specific responses.
Differentiation of Th cells (1) involves clonal expansion caused by engagement of the T-cell receptor (213). Th cells are believed to differentiate into two major CD4+ functional classes, namely, Th1 cells, which produce a set of cytokines that include IFN-γ and IL-2, and Th2 cells, which produce cytokines such as IL-4, IL-5, IL-10, and IL-13 (91). Th1 cells generate cell-mediated immunity, which is important in protection against intracellular parasites, while Th2 responses are associated with humoral immunity and protection against intestinal helminths (213). In addition to the Th1/Th2 paradigm, a third class of CD4+ cells has been discovered (126) and is linked to the etiopathogenesis of inflammatory bowel disease (191) and colon carcinogenesis (348). These cells are activated by IL-23 and produce Th17 cytokines, including IL-17, IL-21, and IL-22 (159).
H. pylori-induced gastritis is driven by a variety of bacterial factors that stimulate epithelial cell, macrophage, and DC activation, as well as a Th1-predominant lymphocyte response; the role of the Th17 versus Th1 response is an area of intense investigation. Colonization of H. pylori can be abrogated by immunization with bacterial components such as urease (236), indicating activation of the adaptive response, but urease is also a major inducer of innate responses in monocytes and macrophages, stimulating cytokine and NO generation (118, 188, 189). Thus, determining whether the response of a particular cell type represents purely an innate or adaptive response is difficult, and the recognition that cells such as B cells can respond to H. pylori directly or via interaction with activated T cells illustrates the complexity of the immune response.
Immune Response to H. pylori
H. pylori induces both humoral and cellular immune responses. Local and systemic antibody responses have been demonstrated that include IgA, IgM, and IgG isotypes (67, 255, 349). Early studies with mouse models demonstrated that immunization with H. pylori antigens could induce protective immunity (194).
Although H. pylori proteins have been demonstrated in the lamina propria of the stomach (188), H. pylori has generally been considered a noninvasive pathogen residing primarily in the extracellular mucus layer. However, several studies have demonstrated the ability of H. pylori to invade gastric epithelial cells both in vitro (12) and in vivo, in the stomachs of humans and monkeys (282) as well as in mice with atrophic gastritis (230). H. pylori bacteria have also been shown to be bound to erythrocytes within the microvessels of the lamina propria (21). Transmission electron microscopy and immunogold detection have been used to elegantly demonstrate that H. pylori cells are in direct contact with immune cells of the lamina propria in the majority of cases of gastritis and gastric cancer (208).
Macrophages. (i) Macrophage signaling of T cells.
Macrophages are essential as innate responders to H. pylori-derived products and signals from epithelial cells that are in direct contact with the bacterium on the surface of the mucosa. Monocytes and macrophages are important coordinators of immune responses to pathogens, and in the case of H. pylori, they are likely activators, along with DCs, of adaptive immunity by producing factors, such as IL-12 (124, 201, 202), that stimulate Th1 cells, resulting in production of cytokines such as IFN-γ (124, 201, 202). The neutrophil-activating protein (NAP) of H. pylori contributes to Th1 polarization by stimulating both IL-12 and IL-23 secretion from neutrophils and monocytes (11). IL-12 production in the gastric mucosa is linked to the development of peptic ulcers in infection with cagA-positive H. pylori strains, most likely due to stimulation of Th1 responses (131). Macrophages are also involved in amplification of the inflammatory response by production of cytokines such as IL-1, TNF-α, and IL-6 (115, 127, 189). IL-6 activation has been linked to activation of TLR4, MAP kinase, and NF-κB signaling events (241).
IL-12 and IL-23 share the common p40 subunit, with a heterodimer of p19 and p40 constituting IL-23 and a heterodimer of p40 and p70 constituting IL-12. Notably, it was recently reported that in addition to increased expression of IL-12 p40 in human H. pylori gastritis, there is also increased immunostaining for IL-23 localized to macrophages in addition to epithelial cells (306). Therefore, additional studies are needed to clarify the role of IL-12 and IL-23 in the stimulation of T-cell responses.
(ii) Macrophages as effector cells.
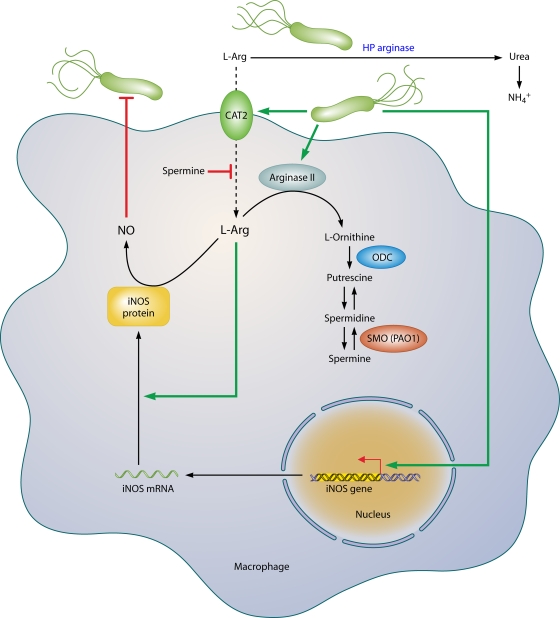
Macrophages constitute a potentially powerful line of defense against H. pylori through their own effector function, yet, intriguingly, these capabilities fail the host. One such pathway is the generation of nitric oxide (NO) derived from the enzyme inducible NO synthase (iNOS or NOS2), which has been shown to be upregulated by H. pylori in macrophages in vitro (42, 117, 118, 339) and in vivo (103, 192) (Fig. 3). Coculture studies demonstrate that H. pylori organisms can be eliminated by macrophages even when the bacteria are physically separated from these cells and that this antimicrobial defense is NO dependent (42, 117). The arginase enzyme possessed by H. pylori, encoded by the rocF gene, can compete sufficiently with macrophages for the iNOS substrate l-arginine (l-Arg) that host NO production is impaired, leading to enhanced survival of the bacterium through this mechanism (117). Moreover, this competition can deplete l-Arg sufficiently to impair the synthesis of iNOS protein, since its translation is highly dependent on l-Arg availability inside the macrophage (46). Bacterial arginase serves to generate urea from l-Arg, which is then utilized by urease to synthesize ammonia that is required to neutralize gastric acid.
FIG. 3.
Pathways involved in regulation of macrophage iNOS synthesis and NO production in response to H. pylori. The translation of iNOS protein depends on the availability of l-arginine (l-Arg). Pathogenic mechanisms that inhibit l-Arg availability for iNOS include (i) the consumption of extracellular l-Arg by H. pylori itself, through its bacterial arginase activity; (ii) the upregulation of macrophage arginase II, which depletes intracellular l-Arg; and (iii) induction of ODC that generates the polyamine spermine, which blocks uptake of l-Arg into macrophages by CAT2. The resulting effect is limitation of iNOS protein synthesis and NO production, despite high levels of iNOS mRNA. Arginase and ODC are novel targets for therapeutic intervention to enhance antimicrobial NO production and hence reduce persistent colonization that leads to chronic inflammation and cancer risk.
While the induction of iNOS in macrophages is termed classical activation of the M1 type, the alternative, M2 pathway involving the metabolism of l-Arg by arginase is also involved (Fig. 3). Exposure of macrophages to H. pylori products results in upregulation of the enzyme arginase II (Arg2) (116), which produces l-ornithine in addition to urea. Arginase induction exerts at least three potentially pathogenic roles. First, arginase depletes substrate availability for iNOS. In H. pylori-stimulated macrophages, iNOS protein translation is dependent on the level of l-Arg in culture medium and bacterial killing requires high levels of l-Arg (46). Consistent with this, increased iNOS translation and NO production occurred with inhibition of arginase or siRNA knockdown of Arg2 or in primary macrophages from Arg2−/− mice, while oral administration of an arginase inhibitor to H. pylori-infected mice increased iNOS protein expression and NO production by gastric macrophages (174). Second, Arg2 has a central role in inducing apoptosis of macrophages, which is dependent on the metabolism of its product, l-ornithine, into polyamines (116). Finally, the generation of ornithine by arginase results in increased substrate levels for the generation of polyamines by ornithine decarboxylase (ODC), which is also induced by H. pylori (48, 50, 116), and this results in inhibition of iNOS (42). Specifically, the polyamine spermine does not alter iNOS transcription, but instead, it blocks iNOS protein translation and NO production. Knockdown of ODC by RNA interference results in sufficient increases in iNOS protein expression and NO production that killing of H. pylori by macrophages can be enhanced significantly (42).
Inhibition of iNOS by spermine can also be attributed to blocking the uptake of l-Arg into macrophages by cationic amino acid transporter 2 (CAT2), required for iNOS translation, and depletion of spermine by the induction of the metabolic enzyme SMO facilitates iNOS protein synthesis and NO production (47). The biochemical pathways that limit NO production (summarized in Fig. 3) may exist to protect macrophages from the potential toxic effects of overproduction of NO in response to other pathogens, but in the case of H. pylori, the bacterium may usurp these host effectors to establish long-term colonization. These findings indicate that the dysfunction of the mucosal macrophage iNOS-derived NO synthesis plays a fundamental role in the persistence of the bacterium and, by definition, the risk for neoplastic transformation.
(iii) Macrophage apoptosis.
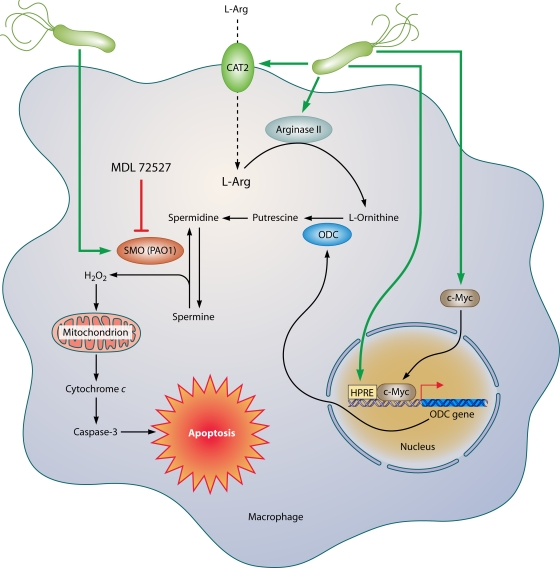
Host inflammatory responses are enhanced by macrophage activation, but apoptosis of macrophages can have profoundly deleterious consequences. The release of cytokines from dying cells may be important, as originally established for Shigella infection (365), and apoptotic cells stimulate infiltration of neutrophils to engulf cellular debris, leading to potentiation of inflammation and increased oxidative stress from oxyradicals released by activated neutrophils. Also important is the net effect of a loss of host defense with the depletion of these effector cells. The generation of polyamines is directly involved in macrophage apoptosis (Fig. 4). The production of putrescine by ODC results in the generation of spermidine and spermine by constitutive synthase enzymes. Spermine is then back-converted by the enzyme SMO to spermidine, with the by-product of this metabolism being hydrogen peroxide (H2O2) (48). SMO is upregulated by H. pylori in macrophages, and its inhibition by the pharmacologic inhibitor MDL 72527 or by RNA interference prevents the generation of H2O2 and the intrinsic pathway of apoptosis in macrophages, since both mitochondrial membrane depolarization and caspase-3 activation are abrogated (48). H. pylori also upregulates expression and nuclear translocation of c-Myc, and the binding of this transcription factor to the 5′-untranslated region (5′-UTR) of the ODC promoter mediates ODC transcription and associated apoptosis (50).
FIG. 4.
Mechanism of macrophage apoptosis caused by H. pylori. This pathway is dependent on the activities of the enzymes arginase II, ODC, and SMO. Induction of arginase II enhances synthesis of l-ornithine, which is converted into polyamines by ODC via a process that requires both H. pylori activation of the ODC promoter and c-Myc as a transcriptional enhancer. Production of the polyamine spermine provides a substrate for SMO, which is also upregulated by H. pylori. SMO generates H2O2, which causes mitochondrial membrane depolarization, cytochrome c release from mitochondria to the cytosol, and caspase-3 activation, followed by apoptosis. Induction of macrophage apoptosis leads to impairment of mucosal immunity to H. pylori, chronic inflammation, and cancer risk (48, 50, 116).
(iv) Avoidance of phagocytosis by macrophages.
Another potential factor that may contribute to the failure of the innate immune response to eliminate H. pylori is that the bacterium can evade effective phagocytosis by macrophages (9, 364). Although H. pylori can be internalized by macrophages, into phagosomes, these phagosomes fuse and form “magasomes” containing large numbers of live bacteria. Additionally, H. pylori strains containing the cag PAI and producing a functional VacA protein prevent the fusion of phagosomes with lysosomes needed for bacterial killing, and disruption of phagosome maturation is lost when cells are exposed to an isogenic vacA mutant strain (364).
DCs.
DCs represent a critical bridge between the innate and adaptive immune responses. DCs have been identified as primary responders to stimuli that include bacterial products (251), and they serve as antigen-presenting cells (APCs) (272). DCs can penetrate epithelial monolayers in vitro and the intestinal epithelial barrier in vivo, and they take up bacteria directly (51, 221, 257). Disruption of the epithelial apical-junctional complex by H. pylori (13, 93) could facilitate both luminal and subepithelial interaction of DCs with H. pylori and antigens shed by the bacterium. After activation of TLRs, DCs in turn activate T cells in different ways, being capable of inducing either a Th1 or Th2/regulatory T-cell (Treg) response by generation of IL-12 or IL-10 (30). DCs derived from human peripheral blood mononuclear cells have been shown to produce IL-12 and IL-10 when stimulated with H. pylori ex vivo (121, 162). Pulsing of human DCs with intact H. pylori and bacterial membrane preparations results in DC maturation. Coculture of activated DCs with NK cells results in secretion of TNF-α and IFN-γ, and coculture with naïve T cells results in TNF-α, IFN-γ, and IL-2 secretion, indicative of NK and Th1 effector responses (125).
H. pylori outer membrane proteins such as Omp18 and HpaA have been reported to induce the maturation and antigen presentation of DCs (324). In human blood monocyte-derived DCs, activation and maturation of DCs occur independently of the presence of the cag PAI and vacA genotype, and activation of cytokine production does not require live bacteria (163). However, IL-12 responses are attenuated with inhibition of bacterial internalization (125, 163), indicating that phagocytosis of intact H. pylori by DCs may activate intracellular receptors. DCs interact with H. pylori by binding of glycoconjugate carbohydrate structures to DC-specific ICAM-3-grabbing nonintegrin (DC-SIGN/CD209). The identification of DC-SIGN as a novel receptor for H. pylori may be critical to understanding the shifting of T-cell responses in favor of persistence of the infection (17). Specifically, Lewis antigen expression by H. pylori LPS can block Th1 responses by binding to DC-SIGN on DCs, thus representing a form of immunosuppression (17).
Stimulation of mouse bone marrow-derived DCs with H. pylori results in phagocytosis of bacteria and expression of the proinflammatory cytokines IL-1α, IL-1β, and IL-6; however, this results in only modest IL-12 expression and diminished activation of splenocyte IFN-γ secretion and cellular proliferation compared to those induced by Acinetobacter lwoffii (151), another pathogen that causes gastritis in mice (361). DCs activated with H. pylori for 48 h exhibited an attenuated ability to induce IFN-γ production upon coculture with naïve T cells compared to DCs pulsed with H. pylori for only 8 h, and there was a similar loss of response to CD40 ligation (206). This suggests that chronic exposure of DCs to H. pylori results in a loss of ability to induce a Th1 response that could contribute to the persistence of the infection.
It has been reported that DCs are recruited into the stomach as early as 6 h postinoculation (151). Recently, two-photon microscopy revealed that DCs extend dendrites into the gastric lumen after H. pylori infection of the mouse stomach (152). Ex vivo activation of DCs by H. pylori and adoptive transfer result in a skewing of the Th17/Treg balance toward Tregs, with experimental enhancement of immunity accomplished by depletion of Tregs and enhancement of the Th17 response, indicating that the DC response to H. pylori may contribute to bacterial immune evasion (152) and thus lead to chronic inflammation and cancer risk.
T cells.
Early studies established the concepts that gastric lymphocytes from H. pylori-infected patients have increased numbers of IFN-γ-producing T cells, consistent with a Th1 cytokine response (29, 155), and that H. pylori-specific T-cell clones derived from gastric mucosa exhibit a Th1 profile in patients with peptic ulcer disease (70). Mucosal T cells in H. pylori infection produce abundant levels of the Th1 cytokines IFN-γ and IL-2 and low levels of the Th2 cytokines IL-4 and IL-5 (29, 155). IL-12 production derived from monocytes, macrophages, or DCs is important in the induction of Th1 lymphocyte responses (121, 124, 125, 162, 201, 202), and the role of gastric epithelial cells as antigen-presenting cells in the activation of CD4+ Th cells has also been demonstrated (359).
IFN-γ−/− mice have decreased levels of gastric inflammation (3), and SCID mice lacking T and B cells infected with H. pylori require adoptive transfer of CD4+ T cells for the development of gastritis (77); such studies have shown that an insufficient Th1 response is associated with increased bacterial colonization (3, 77). These studies suggest that the development of a strong Th1 response can lead to an attenuation of H. pylori colonization (77, 78). However, there is also evidence that adoptive transfer into SCID mice of CD4+ T cells from T-bet−/− mice, which do not exhibit IFN-γ production and Th1 differentiation, still results in gastritis (76).
IL-17 has been linked to chemokine-mediated neutrophil infiltration (97), and IL-17 levels are increased in infected human (183) and mouse (8a) gastric tissues. Immunization of mice with H. pylori lysate enhances IL-17 expression in the gastric mucosa and in CD4+ T cells isolated from spleens and cocultured with H. pylori-pulsed DCs or macrophages; these findings were associated with increased gastric inflammation and decreased colonization (71). These data suggest that a defective IL-17/Th17 response contributes to chronic persistence of the bacterium. Another mechanism of immune dysfunction has been demonstrated by the recent report that VacA can exert immunosuppressive effects on T cells by binding to the β2 integrin receptor subunit (CD18) and utilizing integrin receptors to cause cellular vacuolization (284).
Additional investigations have implicated Tregs in the pathogenesis of H. pylori infection. Circulating memory T cells from H. pylori-infected humans have less proliferation and IFN-γ production in response to H. pylori-pulsed DCs than do T cells from naïve donors, and this defect can be abrogated by depletion of CD4+ CD25high regulatory T cells, indicating that H. pylori-specific Tregs suppress memory T-cell responses and contribute to the persistence of the infection (182). H. pylori-infected individuals have increased levels of CD4+ CD25high T cells in the gastric and duodenal mucosa that express FOXP3, a gene involved in the development of Tregs, and high levels of the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) protein (181).
In athymic nude mice lacking T cells, reconstitution with lymph node cells depleted of CD25+ T cells resulted in a significant reduction in H. pylori colonization and in increased gastritis, infiltrating CD4+ T cells, and production of IFN-γ compared to those in mice receiving nondepleted lymph node T cells (254). BALB/c mice infected with H. pylori or H. felis and treated with anti-CD25 antibody to deplete Tregs developed a Th2 response characterized by serum IgG1 antibodies and production of IL-4 and IL-5 in paragastric lymph node-derived T cells (153). These data suggest that the Treg response can potentially act to enhance Th1 commitment, but this study did not detect any difference in colonization or severity of gastritis (153). The anti-CD25 monoclonal antibody PC61 depletes Foxp3+ Treg cells and resulted in increased H. pylori gastritis severity, gastric cytokine levels, and serum IgG1 and IgG2c levels and a concomitant decrease in bacterial colonization in C57BL/6 mice (253). In conjunction with findings that H. pylori-infected patients express increased levels of FOXP3 mRNA and protein in gastric lymphocytes (253), this study suggests that the induction of a Treg response contributes to establishing an equilibrium between host and bacterium, allowing H. pylori to survive, but also preventing the risk of destructive inflammation.
Activation of T cells by specific antigens involves expression of costimulatory molecules, and CTLA-4 inhibits this process. In the case of H. pylori infection, functional inactivation of CD4+ T cells recruited to the gastric mucosa may be related to expression of CTLA-4 on the T-cell surface and prevention of costimulation when APCs engage T-cell receptors (16). Blockade of CTLA-4 resulted in increased T-cell activation in vitro and in vivo and in decreased colonization in H. pylori-infected mice, suggesting that there is an induction of anergy in CD4+ gastric T cells (16). H. pylori can inhibit lymphocyte proliferation (111, 202), an effect attributed to a downregulatory effect of H. pylori VacA on the activation and nuclear translocation of the transcription factor nuclear factor of activated T cells (NFAT) (35, 111). VacA has also been shown to activate MAP kinase signaling that results in activation of the GTPase Rac, leading to disruption of the cytoskeleton due to actin rearrangement (35).
Collectively, there are numerous features of T-cell function that are altered in a deleterious manner by H. pylori infection. However, exciting developments related to understanding the role of Th17 cells and Tregs may lead to new therapeutic avenues to reduce chronic inflammation and cancer risk.
B cells.
There are ample data indicating that B cells also contribute to H. pylori pathogenesis. In B-cell-deficient (μMT) mice infected with H. pylori, a 2-log reduction in H. pylori colonization was reported that was associated with increased gastric inflammation and infiltration of CD4+ T cells (4). While IgG and IgA responses to H. pylori in the serum and gastric mucosa may be involved in protective immunity, this study and another (5) suggest that B-cell-mediated antibody responses may be counterproductive. A major focus of investigation has been related to the development of gastric MALT lymphoma, which arises from activated B cells. Naïve mouse splenocytes exposed to H. pylori are protected from spontaneous apoptosis and exhibit proliferation in response to low, but not high, multiplicities of infection, and the responding cells are derived from B-cell populations (41). Furthermore, chronic infection with H. pylori protects splenic B cells from apoptosis, indicating a B-cell activation/survival phenotype that may have implications for MALT lymphoma (41). In addition to producing antigen-specific antibodies, B cells have also been shown to produce autoreactive antibodies that may be pathogenic (351). The role of T-cell-B-cell interactions in the pathology of the immune response is a fertile area of future investigation.
Inflammation-mediated migration of peripheral cells.
Chronic inflammation that develops in response to H. pylori undoubtedly contributes to transformation. Studies of mice infected with H. felis have demonstrated that bone marrow-derived cells (BMDC) home to and engraft in sites of chronic inflammation, particularly within foci where tissue injury induces excessive apoptosis, which overwhelms the population of endogenous stem cells (140). Within the inflammatory environment of the infected stomach, BMDC degenerate into adenocarcinoma, suggesting that gastric epithelial carcinomas can originate from bone marrow-derived sources (140).
Apical-Junctional Complexes
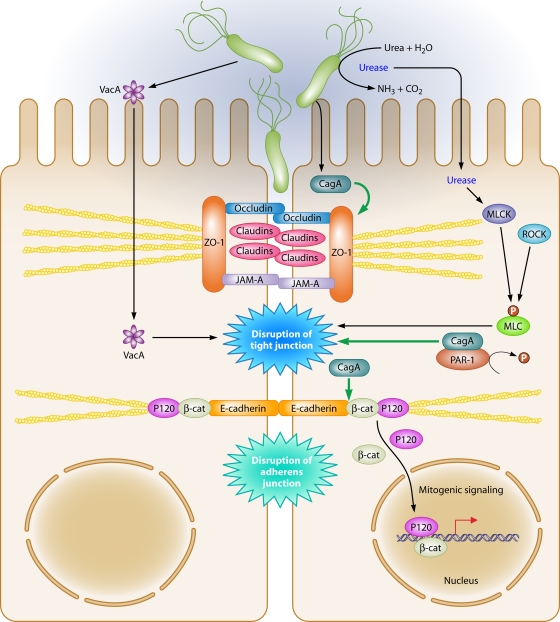
Gastric mucosal barrier function is essential for preventing potentially harmful elements present in the gastric lumen from gaining access to the gastric mucosa. Barrier function is compromised in H. pylori-induced gastritis (298) and in intestinal epithelial cells infected with H. pylori (93). The apical-junctional complex, which controls barrier function, is composed of tight junctions, which are located at the most apical region of cells, and adherens junctions, which are located immediately below (Fig. 5).
FIG. 5.
Dysregulation of the apical-junctional complex by H. pylori. H. pylori preferentially adheres to the apical-junctional complex of epithelial cells and alters localization of apical-junctional component proteins, disrupts epithelial barrier function, cell adhesion, and cell polarity, and induces an invasive phenotype. Translocated CagA interacts with PAR1, preventing phosphorylation of PAR1 by blocking PAR1 kinase activity, which culminates in disruption of the tight junction. In addition, functional urease activity can disrupt the tight junction via a mechanism involving MLC phosphorylation, which can be regulated by MLCK and Rho kinase. VacA can also increase tight junction permeability to low-molecular-weight molecules and ions.
Tight junctions.
Tight junction complexes are dynamic structures composed of integral membrane proteins, such as occludin, junctional adhesion molecules (JAMs), and the claudins, which are a family of 24 tetraspan transmembrane proteins (318), as well as membrane-associated proteins such as ZO-1. These components play critical roles in maintenance of barrier function, cell polarity, and intercellular adhesion. Disruption of the tight junction complex is associated with a variety of human diseases, including cancers of the gastrointestinal tract (318), and specific H. pylori constituents have also been reported to be involved in dysregulation of tight junctions.
The majority of H. pylori organisms are thought to be free-living within the protective mucous layer of the stomach; however, approximately 20% of bacteria attach to gastric epithelial cells. H. pylori preferentially adheres to gastric epithelial cells in close proximity to tight junctions (13, 129) and can alter localization of the component proteins that constitute apical-junctional complexes (13, 93, 164, 345). H. pylori has been detected within intraepithelial intercellular spaces directly beneath tight junctions, leading investigators to hypothesize that the tight junction may be a portal of entry for the bacterium (218), which allows the bacterium access to essential nutrients from epithelial cells to support its growth (307). Recently, viable H. pylori cells have also been identified within the lamina propria, gastric lymph nodes, and intracellular canaliculi of parietal cells (21, 144).
A variant of H. pylori that was cell adapted for increased adhesion to MDCK cells and that more efficiently translocated CagA into MDCK cells was found to modify ZO-1 expression at the tight junction in polarized MDCK cells and to recruit ZO-1 away from the tight junction to sites of bacterial attachment, which requires translocation of CagA (13). Ectopic expression of CagA in polarized MDCK cells causes a loss of apicobasal polarity, which is associated with an invasive cellular phenotype, and increased tight junction permeability (27). Similar to the case in MDCK cells, coculture of primary human epithelial cells with H. pylori altered the distribution of ZO-1 and induced an accumulation of ZO-1 in the cytoplasm that colocalized with CagA. However, the precise role of CagA in this model remains to be determined fully (164).
H. pylori replicates on the surfaces of MDCK cells, and cell polarity is an important factor for bacterial survival. CagA is required for H. pylori to disrupt MDCK cell polarity (307), and this allows H. pylori to potentially gain access to nutrients on the basolateral membrane that are required for replication. However, CagA is not required for replication on nonpolarized cells, where free mixing of molecules from the apical and lateral membranes occurs, and therefore no requirement exists for bacteria to acquire nutrients from within cells (307).
CagA specifically associates with PAR1b/MARK2, one of the four members of the PAR1 family of kinases (269). PAR1b is located on the basolateral surface membrane and has an essential role in maintaining epithelial cell polarity (269, 362). In a tyrosine phosphorylation-independent manner, CagA inhibits PAR1 kinase activity, and this prevents phosphorylation of PAR1, leading to its dissociation from the membrane, which causes defects in epithelial cell polarity and disruption of tight junctions (269) (Fig. 5). The PAR1b-binding region of CagA has been identified as the 16-amino-acid CagA sequence also known as the CagA multimerization (CM) sequence, which is involved in CagA dimerization (256). Interestingly, the CM sequence of CagA proteins isolated from East Asian H. pylori strains binds PAR1b more strongly than the CM sequence of proteins isolated from Western strains of H. pylori (180). Within Western H. pylori strains, the number of CM repeats is directly proportional to the ability of CagA to bind to PAR1b (180). The magnitude of tight junction disruption is proportional to the level of PAR1b-binding activity of CagA (180). CagA-mediated inhibition of PAR1 kinase also contributes to the hummingbird phenotype by perturbing microtubules and nonmuscle myosin II (179). The mechanism by which CagA inhibits PAR1/MARK enzymes was recently determined using crystallography. CagA uses the initial 14 amino acids of the CM motif to bind to the MARK2 substrate binding site, thereby mimicking a host cell substrate that can inhibit a kinase and exert biological effects on the host cell (219).
The ability of CagA to disrupt tight junctions has not been demonstrated in all studies, which may be due to the use of different bacterial strains and/or model systems. Although H. pylori strain SS1 is CagA+, it lacks a functional type IV secretion system and cannot inject CagA into epithelial cells (65). Interestingly, this strain induces an inflammatory reaction and disrupts barrier function in the gastric mucosa (93, 301). In a CagA- and VacA-independent manner, H. pylori has also been shown to decrease expression of claudin-4 and claudin-5 and to activate myosin light chain kinase (MLCK), which subsequently disrupts barrier function (93).
Occludin is also implicated in regulation of gastric barrier function (49) and is linked directly to the actin cytoskeleton via its C terminus (340), and indirectly through its interactions with ZO-1 (89). Expression of occludin at the level of the tight junction is disrupted by H. pylori (93, 301, 345), which leads to gastric epithelial barrier dysfunction. This requires functional urease activity and is independent of CagA, VacA, or a functional cag PAI. Disruption of barrier function also involves MLCK-dependent phosphorylation of the myosin II regulatory light chain (MLC) (345). Ammonium produced by H. pylori similarly disrupts barrier function and leads to the generation of a 42-kDa truncated form of occludin in Caco-2 cells (184). In a mechanism independent of CagA, the addition of purified VacA to MDCK cells increases tight junction permeability to low-molecular-weight molecules and ions (Fig. 5). Interestingly, this increase in permeability is not associated with a redistribution or alteration in the total amount of the tight junction proteins ZO-1 and occludin (238).
Adherens junctions.
Adherens junctions are required for cell-to-cell adhesion and are composed of cadherins, p120 catenin (p120), β-catenin, and α-catenin. The cadherins are a family of transmembrane proteins that form homodimers with cadherin molecules on adjacent cells. E-cadherin acts as a cell-cell adhesion molecule in epithelial tissues, and its turnover is regulated by binding of p120 to the cadherin juxtamembrane domain. The cytoplasmic domain of E-cadherin interacts directly with β-catenin and p120, and these molecules, in turn, interact with the actin cytoskeleton. Loss of E-cadherin function is associated with gastric cancer, and H. pylori infection induces E-cadherin gene promoter methylation, leading to a reduction in E-cadherin expression (44, 172, 246); also, eradication of H. pylori reduces E-cadherin promoter hypermethylation (45, 172, 246). Translocated CagA physically interacts with E-cadherin, leading to the destabilization of the E-cadherin-β-catenin complex and to accumulation of cytoplasmic and nuclear β-catenin, which then transactivates β-catenin-dependent genes that may promote carcinogenesis (212, 233). While H. pylori disrupts the apical junction, it does not alter expression of E-cadherin but, instead, induces translocation of E-cadherin, β-catenin, and p120 from the membrane into the cytoplasm of epithelial cells (54, 227, 303, 333) (Fig. 5). Recent work suggests that CagA directly interacts with E-cadherin and p120 and forms a complex composed of c-Met, E-cadherin, and p120, which prevents tyrosine phosphorylation of c-Met and p120 and suppresses cell-invasive phenotypes (233).
In addition to the membrane, β-catenin is also present within the cytoplasm, where it is phosphorylated by GSK-3β, within a multiprotein inhibitory complex that includes the adenomatous polyposis coli tumor suppressor protein. This complex targets β-catenin for ubiquitination and subsequent degradation (309). Increased expression of β-catenin, mutations within the adenomatous polyposis coli protein, and inhibition of GSK-3β are frequently observed in gastric cancer specimens and function to stabilize β-catenin in the cytoplasm (314). H. pylori can activate β-catenin through PI3K-dependent inactivation of GSK-3β (216, 302) and can also interact with membrane-associated β-catenin via CagA to activate β-catenin signaling and promote mitogenic signaling (168, 212). In a gerbil model of infection, a carcinogenic strain of H. pylori increased nuclear accumulation of β-catenin, which is consistent with observations in human gastric biopsy specimens, where increased nuclear β-catenin was seen for patients infected with H. pylori cag+ strains compared to that for persons infected with H. pylori cag-deficient mutant strains or in uninfected gastric biopsy specimens (101).
In addition to downregulation of E-cadherin, reduced membrane expression or aberrant localization of p120 to the cytosol or nucleus is frequently observed in gastric cancer (145, 154). Loss of E-cadherin or overexpression of p120 in vitro results in mislocalization of p120 to the cytoplasm (258), where it can promote motility and metastasis via interactions between p120 and Rho GTPases (14, 120, 223). In normal cells, p120 expression is typically low in cell nuclei; however, expression is increased within the nuclei of tumor cells (197, 273, 335). Recently, H. pylori infection has been associated with mislocalization of p120 to the nucleus in human gastric epithelia and infected murine primary gastric epithelial cells (Fig. 5) (164, 227). Mechanistically, nuclear p120 relieves transcriptional repression of mmp-7 via interacting with Kaiso. MMP-7 is a matrix metalloproteinase implicated in gastric tumorigenesis (discussed in detail below), and aberrant activation of this molecule likely represents another mechanism through which H. pylori may lower the threshold for developing gastric cancer (227).
Alterations in Cellular Turnover That Predispose Individuals to H. pylori-Induced Malignant Transformation
Maintenance of tissue integrity requires that enhanced cell proliferation be accompanied by increased rates of cell loss. In chronic H. pylori infection, there is a lack of epithelial necrosis (210), suggesting that other forms of cellular demise may be affected. Apoptosis is a form of programmed cell death consisting of a tightly regulated series of energy-dependent molecular events (322), and H. pylori stimulates apoptosis in vitro via multiple mechanisms (63, 88, 138, 139, 147, 266, 327, 330). Transient expression of VacA in HeLa cells induces the release of cytochrome c from mitochondria, leading to activation of caspase-3 and induction of apoptosis. These findings were confirmed by studies using wild-type and vacA mutant H. pylori strains (63, 166), as s1/m1 types of VacA induced higher levels of apoptosis than s2/m1 toxins or VacA mutants lacking the hydrophobic amino-terminal region.
In contrast to the case for cell culture, however, H. pylori has been associated with increased (148, 192, 210, 266) or decreased (136) levels of apoptosis in vivo, and within a particular patient population, substantial heterogeneity exists among apoptosis scores (148, 192, 210, 266). One explanation for these discordant results is that differences in levels of apoptosis among H. pylori-infected persons may result from strain-dependent variations in VacA structure. However, H. pylori was recently shown to inhibit gastric epithelial cell apoptosis in Mongolian gerbils (204), and this was associated with epithelial cell hyperplasia and persistent bacterial colonization of the stomach. Suppression of apoptosis in vivo was mediated by CagA, which stimulated the prosurvival MAP kinase ERK1/2 and the antiapoptotic protein MCL1 within gastric pits. Thus, CagA may counteract the effects of VacA and activate cell survival and antiapoptotic pathways to overcome self-renewal of the gastric epithelium and to help sustain H. pylori infection (204).
Another possibility for observed differences in the degree of cell death within inflamed gastric tissue is that certain H. pylori-induced host effectors may differentially regulate apoptosis depending on the particular microenvironment. MMP-7 is a matrix metalloproteinase with matrix-degrading and tumor-initiating properties that is expressed and secreted by epithelial cells (57). Overexpression of MMP-7 occurs in premalignant and malignant lesions in the stomach (2, 135, 137, 199, 270, 283, 355), and genetic polymorphisms linked to increased MMP-7 expression are associated with H. pylori infection status, gastric ulceration (a precursor for gastric cancer), and tumor-related survival among gastric cancer patients (130, 165). In mice, overexpression of MMP-7 leads to hyperproliferation and increased cancer susceptibility (267), while cell lines that overexpress MMP-7 develop enhanced tumorigenic potential (341). Conversely, MMP-7 deficiency attenuates tumorigenesis in mice with a genetic predisposition for intestinal adenocarcinoma (338).
Colonization by H. pylori cag+ strains leads to enhanced levels of MMP-7 within human gastric mucosa (69), and in vitro, cag+ isolates selectively induce MMP-7 in epithelial cells, dependent upon activation of ERK1/2 by specific components within the cag island (69). In addition to matrix degradation, MMP-7 alters epithelial proliferation and apoptosis. MMP-7-treated myofibroblasts increase gastric epithelial cell proliferation in vitro via insulin growth factor II (IGF-II) (198, 344). Short-term treatment of epithelial cells with MMP-7 cleaves membrane-bound FasL, which then induces apoptosis (95, 250). However, chronic solubilization of FasL by MMP-7 selects for a population of cells with reduced sensitivity to apoptotic stimuli (95).
A specific mechanism through which MMP-7 inhibits apoptosis is by cleaving proheparin-binding epidermal growth factor (pro-HB-EGF) to active HB-EGF, which promotes cell survival by EGFR (360). Consistent with these findings, H. pylori transactivates EGFR via activation of HB-EGF (158). H. pylori infection, gastric epithelial hyperplasia, and gastric atrophy are associated with dysregulation of EGFR and/or cognate ligands in human, animal, and cell culture models (262, 268, 328, 343, 358). Also, H. pylori-mediated activation of EGFR is dependent upon genes within the H. pylori cag pathogenicity island. Thus, chronic stimulation of MMP-7 by H. pylori in vivo may select for a population of hyperproliferating cells with reduced sensitivity to apoptosis, thereby contributing to increased carcinogenic risk.
ENVIRONMENTAL FACTORS
Role of Salt as a Risk Factor for Gastric Adenocarcinoma
The risk of gastric carcinoma is influenced not only by H. pylori strain characteristics and host genetic determinants but also by environmental elements. One factor that has uniformly been associated with an increased risk of gastric cancer is high dietary salt intake (56, 104, 313). This association has been detected in prospective studies, case-control studies, and a study that compared urinary salt excretion with gastric morbidity rates. A prospective study of a Japanese population and a case-control study in South Korea each reported that H. pylori-infected subjects consuming a high-salt diet had an increased risk of gastric cancer compared to H. pylori-infected subjects who consumed lower levels of salt (169, 286), while another study reported a positive correlation between the prevalence of H. pylori infection and levels of dietary salt intake (32).
Investigations using Mongolian gerbils have shown that the presence of H. pylori and use of a high-salt diet exert synergistic effects on the development of premalignant lesions or gastric cancer (108, 156, 297, 363). Specifically, Gamboa-Dominguez et al. reported that H. pylori-infected gerbils fed a high-salt diet developed more severe gastric inflammation and higher levels of gastric epithelial proliferation than those in H. pylori-infected gerbils consuming a regular diet (108). Similarly, Kato et al. and Nozaki et al. studied H. pylori-infected gerbils treated with a carcinogen (N-methyl-N-nitrosourea [MNU]) and reported that animals fed a high-salt diet had a higher incidence of gastric cancer than animals fed a regular diet (156, 224). Sun et al. reported that levels of three proinflammatory cytokines (IL-1, IL-6, and TNF-α) were higher in H. pylori-infected gerbils fed a high-salt diet than in infected gerbils fed a regular diet (297). Similarly, hyperosmotic stress has been reported to enhance H. pylori-induced expression of IL-1β by gastric epithelial cells in vitro (363).
The potential synergistic effects of salt and H. pylori infection on gastric inflammation and damage have also been studied in mice, although not to the same extent as in gerbils. One study reported that in comparison to a low-salt diet, high salt intake enhanced H. pylori colonization in C57BL/6 mice and resulted in increased gastric epithelial cell proliferation (99). However, other studies have not detected any effects of a high-salt diet in various mouse models (171, 261, 312).
The mechanisms by which salt increases the risk of gastric cancer in humans are incompletely understood. One possibility is that salt may have direct effects on the gastric epithelium that lower the threshold for malignant transformation. Another possibility is that salt damages the gastric mucosa, thereby allowing entry of carcinogens into gastric tissue. Upregulated production of cytokines in response to a high-salt diet may also be contributory. Finally, an intriguing possibility raised by recent observations is that high salt concentrations could potentially modulate gene expression in H. pylori. Consistent with this hypothesis, two groups have now reported that high salt concentrations result in increased expression of H. pylori virulence factors, including CagA (109, 177), providing important insights into mechanisms through which high-salt diets increase the risk for gastric cancer among subjects colonized with disease-associated strains.
Helminth Infection
Animal models and small human studies have suggested that coinfection with helminths may have an impact on the outcome of H. pylori infection. Investigations using mice have shown that concurrent infection with helminths can reduce the severity of Helicobacter-induced gastritis. This was coupled with a reduced Th1 response concomitant with higher levels of Th2 cytokines (98). One study of a Chinese population reported that concurrent helminth infections modified serological IgG responses to H. pylori and that this was associated with a decreased risk of developing H. pylori-induced atrophy, as determined by the pepsinogen I/II ratio (75). Similar results were reported in a study of Colombian children, where a higher Th2-associated IgG1 response was reported for children infected with both helminths and H. pylori and living in a coastal region where the incidence of gastric cancer is low (334). This represents an intriguing area of research with potential for future studies focused on H. pylori pathogenesis.
Dietary Antioxidants
There has been extensive research conducted into the protective role of antioxidants found in food against the development of gastric cancer (291). However, less is known about interactions between H. pylori infection and dietary factors in gastric cancer. A randomized trial conducted on a population at high risk of developing gastric cancer suggested that eradication of H. pylori in conjunction with dietary supplementation with vitamin C and β-carotene increased the regression of preneoplastic lesions at 6 years of follow-up (58). However, following a further 6 years without dietary supplementation, the preventative effects of vitamin C and β-carotene disappeared (200). A population-based case-control study in Sweden suggested that a high intake of dietary vitamin C and β-carotene may lower the risk for developing gastric cancer in H. pylori-infected individuals (80). Concordant with the work of Ekstrom et al. (80), a case-control study in Hawaii suggested that vegetable intake among individuals infected with H. pylori provided some protection against developing gastric cancer (84). Conversely, a prospective study involving 10 European countries suggested that there was no significant interaction between H. pylori infection, plasma vitamin C levels, and the risk of developing gastric cancer (146). Further studies will be needed to determine whether antioxidants are protective against gastric cancer among H. pylori-infected patients.
Cigarette Smoking
Several studies have demonstrated that cigarette smoking increases the H. pylori-associated risk of developing gastric cancer. A population-based prospective study of Japanese males suggested that cigarette smoking and H. pylori are risk factors for gastric cancer and that the combination of both factors further increased the risk of developing gastric cancer (285), and similar results were obtained in a Swedish study (288). Cigarette smoking in combination with infection by H. pylori CagA+ strains was also found to increase the risk of developing gastric cancer in a German population-based case-control study (39). However, while a case-control study in Japan reported that H. pylori and cigarette smoking were individually associated with gastric cancer, there was no significant association between H. pylori and cigarette smoking (186). A population-based case-control study in Los Angeles County also reported no statistically significant association between gastric cancer risk and H. pylori-infected smokers, although a trend toward increased risk in current smokers was observed (346). Taking these data together, it appears that there is a positive association between H. pylori infection and smoking for the risk of developing gastric cancer, and it is likely that the combined effects of all environmental factors play a role in the risk of developing gastric cancer among H. pylori-infected individuals.
CONCLUSIONS
Gastric cancer is a highly lethal disease, and establishment of H. pylori as a risk factor for this malignancy permits an approach to identify persons at increased risk; however, infection with this organism is extremely common, and most colonized persons never develop cancer. Thus, techniques to identify high-risk subpopulations must utilize other biological markers. It is apparent from recent studies that cancer risk is the summation of the polymorphic nature of the bacterial population in the host, the host genotype, and environmental exposures, each affecting the level of long-term interactions between H. pylori and humans. Analytical tools now exist, however, including genome sequences (H. pylori and human), measurable phenotypes (CagA phosphorylation), and practical animal models, and may be used to discern the fundamental biological basis of H. pylori-associated neoplasia, which should have direct clinical applications. For example, persons with polymorphisms associated with high levels of IL-1β expression and who are colonized by cag+ strains may be most likely to derive benefit from H. pylori eradication, as such treatment could result in a substantially reduced cancer risk. It is important to gain more insight into the pathogenesis of H. pylori-induced gastric adenocarcinoma, not only to develop more effective treatments for this common cancer but also because it might serve as a paradigm for the role of chronic inflammation in the genesis of other malignancies that arise within the gastrointestinal tract.
Acknowledgments
This work was supported by National Institutes of Health grants DK053620 (K.T.W.), AT004821 (K.T.W.), CA116087 (R.M.P. and K.T.W.), CA028842 (K.T.W.), DK058404 (R.M.P.), DK58587 (R.M.P.), and DK77955 (R.M.P.), by a Merit Review grant from the Office of Medical Research, Department of Veterans Affairs (K.T.W.), and by The Vanderbilt Digestive Diseases Research Center (DK058405).
Biography
 Lydia E. Wroblewski is a postdoctoral research fellow at Vanderbilt University in the Department of Medicine, Division of Gastroenterology, Hepatology and Nutrition. She received her Ph.D. from The University of Liverpool, England. Dr. Wroblewski's research interests include the interactions between Helicobacter pylori and the host, with a particular emphasis on the host and bacterial factors involved in disruption of the apical-junctional complex that may promote the risk of gastric carcinogenesis.
Lydia E. Wroblewski is a postdoctoral research fellow at Vanderbilt University in the Department of Medicine, Division of Gastroenterology, Hepatology and Nutrition. She received her Ph.D. from The University of Liverpool, England. Dr. Wroblewski's research interests include the interactions between Helicobacter pylori and the host, with a particular emphasis on the host and bacterial factors involved in disruption of the apical-junctional complex that may promote the risk of gastric carcinogenesis.
 Richard M. Peek, Jr., is the Mina Cobb Wallace Professor of Medicine and Cancer Biology and Director of the Division of Gastroenterology, Hepatology and Nutrition at Vanderbilt University. He graduated from Davidson College and then attended medical school at the University of North Carolina. He completed an internal medicine residency at the University of Alabama at Birmingham and then a fellowship in gastroenterology at Vanderbilt University. His research is focused on the molecular pathogenesis of Helicobacter pylori infections and microbial constituents that augment the risk for gastric cancer.
Richard M. Peek, Jr., is the Mina Cobb Wallace Professor of Medicine and Cancer Biology and Director of the Division of Gastroenterology, Hepatology and Nutrition at Vanderbilt University. He graduated from Davidson College and then attended medical school at the University of North Carolina. He completed an internal medicine residency at the University of Alabama at Birmingham and then a fellowship in gastroenterology at Vanderbilt University. His research is focused on the molecular pathogenesis of Helicobacter pylori infections and microbial constituents that augment the risk for gastric cancer.
 Keith T. Wilson attended Cornell University, where he was a College Scholar. He received his M.D. from Harvard Medical School. He completed a residency in internal medicine at Case Western Reserve University Hospitals of Cleveland and a fellowship in gastroenterology at The University of Chicago Medical Center. During the latter, he trained in intestinal cell biology. He then joined the faculty at the University of Maryland School of Medicine, where he established a laboratory focused on mucosal immunology and carcinogenesis. In 2005, he moved to Vanderbilt University School of Medicine as Professor of Medicine and Cancer Biology, Director of Research, and Director of Fellowship Training in the Division of Gastroenterology. His laboratory is supported by grants from the National Institutes of Health and the Department of Veterans Affairs and is investigating the immunopathogenesis of H. pylori infection and inflammatory bowel disease.
Keith T. Wilson attended Cornell University, where he was a College Scholar. He received his M.D. from Harvard Medical School. He completed a residency in internal medicine at Case Western Reserve University Hospitals of Cleveland and a fellowship in gastroenterology at The University of Chicago Medical Center. During the latter, he trained in intestinal cell biology. He then joined the faculty at the University of Maryland School of Medicine, where he established a laboratory focused on mucosal immunology and carcinogenesis. In 2005, he moved to Vanderbilt University School of Medicine as Professor of Medicine and Cancer Biology, Director of Research, and Director of Fellowship Training in the Division of Gastroenterology. His laboratory is supported by grants from the National Institutes of Health and the Department of Veterans Affairs and is investigating the immunopathogenesis of H. pylori infection and inflammatory bowel disease.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, Y., F. Itoh, H. Yamamoto, K. Matsuno, Y. Arimura, M. Kusano, T. Endoh, Y. Hinoda, M. Oohara, M. Hosokawa, and K. Imai. 1998. Matrix metalloproteinase matrilysin (MMP-7) participates in the progression of human gastric and esophageal cancers. Int. J. Oncol. 13:1031-1035. [DOI] [PubMed] [Google Scholar]
- 3.Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. [DOI] [PubMed] [Google Scholar]
- 4.Akhiani, A. A., K. Schon, L. E. Franzen, J. Pappo, and N. Lycke. 2004. Helicobacter pylori-specific antibodies impair the development of gastritis, facilitate bacterial colonization, and counteract resistance against infection. J. Immunol. 172:5024-5033. [DOI] [PubMed] [Google Scholar]
- 5.Akhiani, A. A., A. Stensson, K. Schon, and N. Y. Lycke. 2005. IgA antibodies impair resistance against Helicobacter pylori infection: studies on immune evasion in IL-10-deficient mice. J. Immunol. 174:8144-8153. [DOI] [PubMed] [Google Scholar]
- 6.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 7.Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28:37-53. [DOI] [PubMed] [Google Scholar]
- 8.Akre, K., A. M. Ekstrom, L. B. Signorello, L. E. Hansson, and O. Nyren. 2001. Aspirin and risk for gastric cancer: a population-based case-control study in Sweden. Br. J. Cancer 84:965-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Algood, H. M., J. Gallo-Romero, K. T. Wilson, R. M. Peek, Jr., and T. L. Cover. 2007. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol. Med. Microbiol. 51:577-586. [DOI] [PubMed] [Google Scholar]
- 9.Allen, L. A., L. S. Schlesinger, and B. Kang. 2000. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 191:115-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 11.Amedei, A., A. Cappon, G. Codolo, A. Cabrelle, A. Polenghi, M. Benagiano, E. Tasca, A. Azzurri, M. M. D'Elios, G. Del Prete, and M. de Bernard. 2006. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J. Clin. Invest. 116:1092-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amieva, M. R., N. R. Salama, L. S. Tompkins, and S. Falkow. 2002. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell. Microbiol. 4:677-690. [DOI] [PubMed] [Google Scholar]
- 13.Amieva, M. R., R. Vogelmann, A. Covacci, L. S. Tompkins, W. J. Nelson, and S. Falkow. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anastasiadis, P. Z., and A. B. Reynolds. 2001. Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13:604-610. [DOI] [PubMed] [Google Scholar]
- 15.Andersen-Nissen, E., K. D. Smith, K. L. Strobe, S. L. Barrett, B. T. Cookson, S. M. Logan, and A. Aderem. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl. Acad. Sci. U. S. A. 102:9247-9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson, K. M., S. J. Czinn, R. W. Redline, and T. G. Blanchard. 2006. Induction of CTLA-4-mediated anergy contributes to persistent colonization in the murine model of gastric Helicobacter pylori infection. J. Immunol. 176:5306-5313. [DOI] [PubMed] [Google Scholar]
- 17.Appelmelk, B. J., I. van Die, S. J. van Vliet, C. M. Vandenbroucke-Grauls, T. B. Geijtenbeek, and Y. van Kooyk. 2003. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170:1635-1639. [DOI] [PubMed] [Google Scholar]
- 18.Argent, R. H., A. Burette, V. Y. Miendje Deyi, and J. C. Atherton. 2007. The presence of dupA in Helicobacter pylori is not significantly associated with duodenal ulceration in Belgium, South Africa, China, or North America. Clin. Infect. Dis. 45:1204-1206. [DOI] [PubMed] [Google Scholar]
- 19.Argent, R. H., J. L. Hale, E. M. El-Omar, and J. C. Atherton. 2008. Differences in Helicobacter pylori CagA tyrosine phosphorylation motif patterns between Western and East Asian strains, and influences on interleukin-8 secretion. J. Med. Microbiol. 57:1062-1067. [DOI] [PubMed] [Google Scholar]
- 20.Asahi, M., T. Azuma, S. Ito, Y. Ito, H. Suto, Y. Nagai, M. Tsubokawa, Y. Tohyama, S. Maeda, M. Omata, T. Suzuki, and C. Sasakawa. 2000. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J. Exp. Med. 191:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aspholm, M., F. O. Olfat, J. Norden, B. Sonden, C. Lundberg, R. Sjostrom, S. Altraja, S. Odenbreit, R. Haas, T. Wadstrom, L. Engstrand, C. Semino-Mora, H. Liu, A. Dubois, S. Teneberg, A. Arnqvist, and T. Boren. 2006. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog. 2:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aspholm-Hurtig, M., G. Dailide, M. Lahmann, A. Kalia, D. Ilver, N. Roche, S. Vikstrom, R. Sjostrom, S. Linden, A. Backstrom, C. Lundberg, A. Arnqvist, J. Mahdavi, U. J. Nilsson, B. Velapatino, R. H. Gilman, M. Gerhard, T. Alarcon, M. Lopez-Brea, T. Nakazawa, J. G. Fox, P. Correa, M. G. Dominguez-Bello, G. I. Perez-Perez, M. J. Blaser, S. Normark, I. Carlstedt, S. Oscarson, S. Teneberg, D. E. Berg, and T. Boren. 2004. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305:519-522. [DOI] [PubMed] [Google Scholar]
- 23.Atherton, J. C. 2006. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. 1:63-96. [DOI] [PubMed] [Google Scholar]
- 24.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 25.Atherton, J. C., R. M. Peek, Jr., K. T. Tham, T. L. Cover, and M. J. Blaser. 1997. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 112:92-99. [DOI] [PubMed] [Google Scholar]
- 26.Azevedo, M., S. Eriksson, N. Mendes, J. Serpa, C. Figueiredo, L. P. Resende, N. Ruvoen-Clouet, R. Haas, T. Boren, J. Le Pendu, and L. David. 2008. Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J. Pathol. 215:308-316. [DOI] [PubMed] [Google Scholar]
- 27.Bagnoli, F., L. Buti, L. Tompkins, A. Covacci, and M. R. Amieva. 2005. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc. Natl. Acad. Sci. U. S. A. 102:16339-16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baik, S. C., H. S. Youn, M. H. Chung, W. K. Lee, M. J. Cho, G. H. Ko, C. K. Park, H. Kasai, and K. H. Rhee. 1996. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 56:1279-1282. [PubMed] [Google Scholar]
- 29.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 30.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 31.Basso, D., C. F. Zambon, D. P. Letley, A. Stranges, A. Marchet, J. L. Rhead, S. Schiavon, G. Guariso, M. Ceroti, D. Nitti, M. Rugge, M. Plebani, and J. C. Atherton. 2008. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology 135:91-99. [DOI] [PubMed] [Google Scholar]
- 32.Beevers, D. G., G. Y. Lip, and A. D. Blann. 2004. Salt intake and Helicobacter pylori infection. J. Hypertens. 22:1475-1477. [DOI] [PubMed] [Google Scholar]
- 33.Blaser, M. J., and D. E. Berg. 2001. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Invest. 107:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaser, M. J., P. H. Chyou, and A. Nomura. 1995. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 55:562-565. [PubMed] [Google Scholar]
- 35.Boncristiano, M., S. R. Paccani, S. Barone, C. Ulivieri, L. Patrussi, D. Ilver, A. Amedei, M. M. D'Elios, J. L. Telford, and C. T. Baldari. 2003. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J. Exp. Med. 198:1887-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boren, T., P. Falk, K. A. Roth, G. Larson, and S. Normark. 1993. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science 262:1892-1895. [DOI] [PubMed] [Google Scholar]
- 37.Boughan, P. K., R. H. Argent, M. Body-Malapel, J. H. Park, K. E. Ewings, A. G. Bowie, S. J. Ong, S. J. Cook, O. E. Sorensen, B. A. Manzo, N. Inohara, N. J. Klein, G. Nunez, J. C. Atherton, and M. Bajaj-Elliott. 2006. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: critical regulators of beta-defensins during Helicobacter pylori infection. J. Biol. Chem. 281:11637-11648. [DOI] [PubMed] [Google Scholar]
- 38.Bourzac, K. M., C. M. Botham, and K. Guillemin. 2007. Helicobacter pylori CagA induces AGS cell elongation through a cell retraction defect that is independent of Cdc42, Rac1, and Arp2/3. Infect. Immun. 75:1203-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenner, H., V. Arndt, G. Bode, C. Stegmaier, H. Ziegler, and T. Stumer. 2002. Risk of gastric cancer among smokers infected with Helicobacter pylori. Int. J. Cancer 98:446-449. [DOI] [PubMed] [Google Scholar]
- 40.Bunt, S. K., L. Yang, P. Sinha, V. K. Clements, J. Leips, and S. Ostrand-Rosenberg. 2007. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 67:10019-10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bussiere, F. I., R. Chaturvedi, M. Asim, K. L. Hoek, Y. Cheng, J. Gainor, A. Scholz, W. N. Khan, and K. T. Wilson. 2006. Low multiplicity of infection of Helicobacter pylori suppresses apoptosis of B lymphocytes. Cancer Res. 66:6834-6842. [DOI] [PubMed] [Google Scholar]
- 42.Bussiere, F. I., R. Chaturvedi, Y. Cheng, A. P. Gobert, M. Asim, D. R. Blumberg, H. Xu, P. Y. Kim, A. Hacker, R. A. Casero, Jr., and K. T. Wilson. 2005. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J. Biol. Chem. 280:2409-2412. [DOI] [PubMed] [Google Scholar]
- 43.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. U. S. A. 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan, A. O., S. K. Lam, B. C. Wong, W. M. Wong, M. F. Yuen, Y. H. Yeung, W. M. Hui, A. Rashid, and Y. L. Kwong. 2003. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut 52:502-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan, A. O., J. Z. Peng, S. K. Lam, K. C. Lai, M. F. Yuen, H. K. Cheung, Y. L. Kwong, A. Rashid, C. K. Chan, and B. C. Wong. 2006. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut 55:463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaturvedi, R., M. Asim, N. D. Lewis, H. M. Algood, T. L. Cover, P. Y. Kim, and K. T. Wilson. 2007. l-Arginine availability regulates inducible nitric oxide synthase-dependent host defense against Helicobacter pylori. Infect. Immun. 75:4305-4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaturvedi, R., M. Asim, N. D. Lewis, F. I. Bussiere, and K. T. Wilson. 2006. Arginine availability is critical to the innate immune response to Helicobacter pylori by regulation of iNOS translation. Nitric Oxide 14:A8. [Google Scholar]
- 48.Chaturvedi, R., Y. Cheng, M. Asim, F. I. Bussiere, H. Xu, A. P. Gobert, A. Hacker, R. A. Casero, Jr., and K. T. Wilson. 2004. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J. Biol. Chem. 279:40161-40173. [DOI] [PubMed] [Google Scholar]
- 49.Chen, Y., C. Merzdorf, D. L. Paul, and D. A. Goodenough. 1997. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J. Cell Biol. 138:891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng, Y., R. Chaturvedi, M. Asim, F. I. Bussiere, H. Xu, R. A. Casero, Jr., and K. T. Wilson. 2005. Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J. Biol. Chem. 280:22492-22496. [DOI] [PubMed] [Google Scholar]
- 51.Chieppa, M., M. Rescigno, A. Y. Huang, and R. N. Germain. 2006. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 203:2841-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chomvarin, C., W. Namwat, K. Chaicumpar, P. Mairiang, A. Sangchan, B. Sripa, S. Tor-Udom, and R. K. Vilaichone. 2008. Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int. J. Infect. Dis. 12:30-36. [DOI] [PubMed] [Google Scholar]
- 53.Churin, Y., L. Al-Ghoul, O. Kepp, T. F. Meyer, W. Birchmeier, and M. Naumann. 2003. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J. Cell Biol. 161:249-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conlin, V. S., S. B. Curtis, Y. Zhao, E. D. Moore, V. C. Smith, R. M. Meloche, B. B. Finlay, and A. M. Buchan. 2004. Helicobacter pylori infection targets adherens junction regulatory proteins and results in increased rates of migration in human gastric epithelial cells. Infect. Immun. 72:5181-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Correa, P. 1996. Helicobacter pylori and gastric cancer: state of the art. Cancer Epidemiol. Biomarkers Prev. 5:477-481. [PubMed] [Google Scholar]
- 56.Correa, P. 1992. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 52:6735-6740. [PubMed] [Google Scholar]
- 57.Correa, P. 2004. Is gastric cancer preventable? Gut 53:1217-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Correa, P., E. T. Fontham, J. C. Bravo, L. E. Bravo, B. Ruiz, G. Zarama, J. L. Realpe, G. T. Malcom, D. Li, W. D. Johnson, and R. Mera. 2000. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J. Natl. Cancer Inst. 92:1881-1888. [DOI] [PubMed] [Google Scholar]
- 59.Correa, P., and J. Houghton. 2007. Carcinogenesis of Helicobacter pylori. Gastroenterology 133:659-672. [DOI] [PubMed] [Google Scholar]
- 60.Covacci, A., and R. Rappuoli. 2000. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J. Exp. Med. 191:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cover, T. L., and M. J. Blaser. 1992. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267:10570-10575. [PubMed] [Google Scholar]
- 62.Cover, T. L., C. P. Dooley, and M. J. Blaser. 1990. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect. Immun. 58:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cover, T. L., U. S. Krishna, D. A. Israel, and R. M. Peek, Jr. 2003. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 63:951-957. [PubMed] [Google Scholar]
- 64.Cover, T. L., M. K. Tummuru, P. Cao, S. A. Thompson, and M. J. Blaser. 1994. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J. Biol. Chem. 269:10566-10573. [PubMed] [Google Scholar]
- 65.Crabtree, J. E., R. L. Ferrero, and J. G. Kusters. 2002. The mouse colonizing Helicobacter pylori strain SS1 may lack a functional cag pathogenicity island. Helicobacter 7:139-140. [DOI] [PubMed] [Google Scholar]
- 66.Crabtree, J. E., T. M. Shallcross, R. V. Heatley, and J. I. Wyatt. 1991. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 32:1473-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crabtree, J. E., J. D. Taylor, J. I. Wyatt, R. V. Heatley, T. M. Shallcross, D. S. Tompkins, and B. J. Rathbone. 1991. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet 338:332-335. [DOI] [PubMed] [Google Scholar]
- 68.Crabtree, J. E., J. I. Wyatt, G. M. Sobala, G. Miller, D. S. Tompkins, J. N. Primrose, and A. G. Morgan. 1993. Systemic and mucosal humoral responses to Helicobacter pylori in gastric cancer. Gut 34:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crawford, H. C., U. S. Krishna, D. A. Israel, L. M. Matrisian, M. K. Washington, and R. M. Peek, Jr. 2003. Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology 125:1125-1136. [DOI] [PubMed] [Google Scholar]
- 70.D'Elios, M. M., M. Manghetti, F. Almerigogna, A. Amedei, F. Costa, D. Burroni, C. T. Baldari, S. Romagnani, J. L. Telford, and G. Del Prete. 1997. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur. J. Immunol. 27:1751-1755. [DOI] [PubMed] [Google Scholar]
- 71.DeLyria, E. S., R. W. Redline, and T. G. Blanchard. 2009. Vaccination of mice against H. pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology 136:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding, S. Z., Y. Minohara, X. J. Fan, J. Wang, V. E. Reyes, J. Patel, B. Dirden-Kramer, I. Boldogh, P. B. Ernst, and S. E. Crowe. 2007. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect. Immun. 75:4030-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dossumbekova, A., C. Prinz, M. Gerhard, L. Brenner, S. Backert, J. G. Kusters, R. M. Schmid, and R. Rad. 2006. Helicobacter pylori outer membrane proteins and gastric inflammation. Gut 55:1360-1361. [PMC free article] [PubMed] [Google Scholar]
- 74.Douraghi, M., Y. Talebkhan, H. Zeraati, F. Ebrahimzadeh, A. Nahvijoo, A. Morakabati, M. Ghafarpour, M. Esmaili, M. Bababeik, A. Oghalaie, N. Rakhshani, M. E. Hosseini, M. A. Mohagheghi, and M. Mohammadi. 2009. Multiple gene status in Helicobacter pylori strains and risk of gastric cancer development. Digestion 80:200-207. [DOI] [PubMed] [Google Scholar]
- 75.Du, Y., A. Agnew, X. P. Ye, P. A. Robinson, D. Forman, and J. E. Crabtree. 2006. Helicobacter pylori and Schistosoma japonicum co-infection in a Chinese population: helminth infection alters humoral responses to H. pylori and serum pepsinogen I/II ratio. Microbes Infect. 8:52-60. [DOI] [PubMed] [Google Scholar]
- 76.Eaton, K. A., L. H. Benson, J. Haeger, and B. M. Gray. 2006. Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice. Infect. Immun. 74:4673-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eaton, K. A., M. Mefford, and T. Thevenot. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456-7461. [DOI] [PubMed] [Google Scholar]
- 78.Eaton, K. A., and M. E. Mefford. 2001. Cure of Helicobacter pylori infection and resolution of gastritis by adoptive transfer of splenocytes in mice. Infect. Immun. 69:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eaton, K. A., S. Suerbaum, C. Josenhans, and S. Krakowka. 1996. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect. Immun. 64:2445-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ekstrom, A. M., M. Serafini, O. Nyren, L. E. Hansson, W. Ye, and A. Wolk. 2000. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: a population-based case-control study in Sweden. Int. J. Cancer 87:133-140. [PubMed] [Google Scholar]
- 81.El-Omar, E. M. 2001. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut 48:743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Omar, E. M., M. Carrington, W. H. Chow, K. E. McColl, J. H. Bream, H. A. Young, J. Herrera, J. Lissowska, C. C. Yuan, N. Rothman, G. Lanyon, M. Martin, J. F. Fraumeni, Jr., and C. S. Rabkin. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398-402. [DOI] [PubMed] [Google Scholar]
- 83.El-Omar, E. M., C. S. Rabkin, M. D. Gammon, T. L. Vaughan, H. A. Risch, J. B. Schoenberg, J. L. Stanford, S. T. Mayne, J. Goedert, W. J. Blot, J. F. Fraumeni, Jr., and W. H. Chow. 2003. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 124:1193-1201. [DOI] [PubMed] [Google Scholar]
- 84.Epplein, M., A. M. Nomura, J. H. Hankin, M. J. Blaser, G. Perez-Perez, G. N. Stemmermann, L. R. Wilkens, and L. N. Kolonel. 2008. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer Causes Control 19:869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615-640. [DOI] [PubMed] [Google Scholar]
- 86.Erzin, Y., V. Koksal, S. Altun, A. Dobrucali, M. Aslan, S. Erdamar, S. Goksel, A. Dirican, and B. Kocazeybek. 2008. Role of host interleukin 1beta gene (IL-1B) and interleukin 1 receptor antagonist gene (IL-1RN) polymorphisms in clinical outcomes in Helicobacter pylori-positive Turkish patients with dyspepsia. J. Gastroenterol. 43:705-710. [DOI] [PubMed] [Google Scholar]
- 87.Everhart, J. E. 2000. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol. Clin. North Am. 29:559-578. [DOI] [PubMed] [Google Scholar]
- 88.Fan, X., H. Gunasena, Z. Cheng, R. Espejo, S. E. Crowe, P. B. Ernst, and V. E. Reyes. 2000. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J. Immunol. 165:1918-1924. [DOI] [PubMed] [Google Scholar]
- 89.Fanning, A. S., B. J. Jameson, L. A. Jesaitis, and J. M. Anderson. 1998. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 273:29745-29753. [DOI] [PubMed] [Google Scholar]
- 90.Farinati, F., R. Cardin, P. Degan, M. Rugge, F. D. Mario, P. Bonvicini, and R. Naccarato. 1998. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut 42:351-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Farrar, J. D., H. Asnagli, and K. M. Murphy. 2002. T helper subset development: roles of instruction, selection, and transcription. J. Clin. Invest. 109:431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farrow, D. C., T. L. Vaughan, P. D. Hansten, J. L. Stanford, H. A. Risch, M. D. Gammon, W. H. Chow, R. Dubrow, H. Ahsan, S. T. Mayne, J. B. Schoenberg, A. B. West, H. Rotterdam, J. F. Fraumeni, Jr., and W. J. Blot. 1998. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol. Biomarkers Prev. 7:97-102. [PubMed] [Google Scholar]
- 93.Fedwick, J. P., T. K. Lapointe, J. B. Meddings, P. M. Sherman, and A. G. Buret. 2005. Helicobacter pylori activates myosin light-chain kinase to disrupt claudin-4 and claudin-5 and increase epithelial permeability. Infect. Immun. 73:7844-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Figueiredo, C., J. C. Machado, P. Pharoah, R. Seruca, S. Sousa, R. Carvalho, A. F. Capelinha, W. Quint, C. Caldas, L. J. van Doorn, F. Carneiro, and M. Sobrinho-Simoes. 2002. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J. Natl. Cancer Inst. 94:1680-1687. [DOI] [PubMed] [Google Scholar]
- 95.Fingleton, B., T. Vargo-Gogola, H. C. Crawford, and L. M. Matrisian. 2001. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia 3:459-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Forte, J. G., and X. Yao. 1996. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol. 6:45-48. [DOI] [PubMed] [Google Scholar]
- 97.Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, J. J. Pin, P. Garrone, E. Garcia, S. Saeland, D. Blanchard, C. Gaillard, B. Das Mahapatra, E. Rouvier, P. Golstein, J. Banchereau, and S. Lebecque. 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces Helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 99.Fox, J. G., C. A. Dangler, N. S. Taylor, A. King, T. J. Koh, and T. C. Wang. 1999. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 59:4823-4828. [PubMed] [Google Scholar]
- 100.Franco, A. T., D. B. Friedman, T. A. Nagy, J. Romero-Gallo, U. Krishna, A. Kendall, D. A. Israel, N. Tegtmeyer, M. K. Washington, and R. M. Peek, Jr. 2009. Delineation of a carcinogenic Helicobacter pylori proteome. Mol. Cell. Proteomics 8:1947-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Franco, A. T., D. A. Israel, M. K. Washington, U. Krishna, J. G. Fox, A. B. Rogers, A. S. Neish, L. Collier-Hyams, G. I. Perez-Perez, M. Hatakeyama, R. Whitehead, K. Gaus, D. P. O'Brien, J. Romero-Gallo, and R. M. Peek, Jr. 2005. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 102:10646-10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Franco, A. T., E. Johnston, U. Krishna, Y. Yamaoka, D. A. Israel, T. A. Nagy, L. E. Wroblewski, M. B. Piazuelo, P. Correa, and R. M. Peek, Jr. 2008. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 68:379-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu, S., K. S. Ramanujam, A. Wong, G. T. Fantry, C. B. Drachenberg, S. P. James, S. J. Meltzer, and K. T. Wilson. 1999. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 116:1319-1329. [DOI] [PubMed] [Google Scholar]
- 104.Fuchs, C. S., and R. J. Mayer. 1995. Gastric carcinoma. N. Engl. J. Med. 333:32-41. [DOI] [PubMed] [Google Scholar]
- 105.Fujikawa, A., D. Shirasaka, S. Yamamoto, H. Ota, K. Yahiro, M. Fukada, T. Shintani, A. Wada, N. Aoyama, T. Hirayama, H. Fukamachi, and M. Noda. 2003. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat. Genet. 33:375-381. [DOI] [PubMed] [Google Scholar]
- 106.Furuta, T., E. M. El-Omar, F. Xiao, N. Shirai, M. Takashima, and H. Sugimura. 2002. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology 123:92-105. [DOI] [PubMed] [Google Scholar]
- 107.Galmiche, A., J. Rassow, A. Doye, S. Cagnol, J. C. Chambard, S. Contamin, V. de Thillot, I. Just, V. Ricci, E. Solcia, E. Van Obberghen, and P. Boquet. 2000. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gamboa-Dominguez, A., T. Ubbelohde, M. Saqui-Salces, L. Romano-Mazzoti, M. Cervantes, C. Dominguez-Fonseca, M. de la Luz Estreber, and G. M. Ruiz-Palacios. 2007. Salt and stress synergize H. pylori-induced gastric lesions, cell proliferation, and p21 expression in Mongolian gerbils. Dig. Dis. Sci. 52:1517-1526. [DOI] [PubMed] [Google Scholar]
- 109.Gancz, H., K. R. Jones, and D. S. Merrell. 2008. Sodium chloride affects Helicobacter pylori growth and gene expression. J. Bacteriol. 190:4100-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gatti, L. L., J. L. Modena, S. L. Payao, A. Smith Mde, Y. Fukuhara, J. L. Modena, R. B. de Oliveira, and M. Brocchi. 2006. Prevalence of Helicobacter pylori cagA, iceA and babA2 alleles in Brazilian patients with upper gastrointestinal diseases. Acta Trop. 100:232-240. [DOI] [PubMed] [Google Scholar]
- 111.Gebert, B., W. Fischer, E. Weiss, R. Hoffmann, and R. Haas. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099-1102. [DOI] [PubMed] [Google Scholar]
- 112.Geis, G., H. Leying, S. Suerbaum, U. Mai, and W. Opferkuch. 1989. Ultrastructure and chemical analysis of Campylobacter pylori flagella. J. Clin. Microbiol. 27:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gerhard, M., N. Lehn, N. Neumayer, T. Boren, R. Rad, W. Schepp, S. Miehlke, M. Classen, and C. Prinz. 1999. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. U. S. A. 96:12778-12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gewirtz, A. T., Y. Yu, U. S. Krishna, D. A. Israel, S. L. Lyons, and R. M. Peek, Jr. 2004. Helicobacter pylori flagellin evades Toll-like receptor 5-mediated innate immunity. J. Infect. Dis. 189:1914-1920. [DOI] [PubMed] [Google Scholar]
- 115.Gobert, A. P., J. C. Bambou, C. Werts, V. Balloy, M. Chignard, A. P. Moran, and R. L. Ferrero. 2003. Helicobacter pylori heat shock protein 60 mediates interleukin-6 production by macrophages via a Toll-like receptor (TLR)-2-, TLR-4- and myeloid differentiation factor 88-independent mechanism. J. Biol. Chem. 279:245-250. [DOI] [PubMed] [Google Scholar]
- 116.Gobert, A. P., Y. Cheng, J. Y. Wang, J. L. Boucher, R. K. Iyer, S. D. Cederbaum, R. A. Casero, Jr., J. C. Newton, and K. T. Wilson. 2002. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J. Immunol. 168:4692-4700. [DOI] [PubMed] [Google Scholar]
- 117.Gobert, A. P., D. J. McGee, M. Akhtar, G. L. Mendz, J. C. Newton, Y. Cheng, H. L. Mobley, and K. T. Wilson. 2001. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc. Natl. Acad. Sci. U. S. A. 98:13844-13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gobert, A. P., B. D. Mersey, Y. Cheng, D. R. Blumberg, J. C. Newton, and K. T. Wilson. 2002. Cutting edge: urease release by Helicobacter pylori stimulates macrophage inducible nitric oxide synthase. J. Immunol. 168:6002-6006. [DOI] [PubMed] [Google Scholar]
- 119.Goodwin, C. S., J. A. Armstrong, and B. J. Marshall. 1986. Campylobacter pyloridis, gastritis, and peptic ulceration. J. Clin. Pathol. 39:353-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grosheva, I., M. Shtutman, M. Elbaum, and A. D. Bershadsky. 2001. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114:695-707. [DOI] [PubMed] [Google Scholar]
- 121.Guiney, D. G., P. Hasegawa, and S. P. Cole. 2003. Helicobacter pylori preferentially induces interleukin 12 (IL-12) rather than IL-6 or IL-10 in human dendritic cells. Infect. Immun. 71:4163-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gupta, R. A., and R. N. Dubois. 2001. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer 1:11-21. [DOI] [PubMed] [Google Scholar]
- 123.Guruge, J. L., P. G. Falk, R. G. Lorenz, M. Dans, H. P. Wirth, M. J. Blaser, D. E. Berg, and J. I. Gordon. 1998. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc. Natl. Acad. Sci. U. S. A. 95:3925-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haeberle, H. A., M. Kubin, K. B. Bamford, R. Garofalo, D. Y. Graham, F. El-Zaatari, R. Karttunen, S. E. Crowe, V. E. Reyes, and P. B. Ernst. 1997. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect. Immun. 65:4229-4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hafsi, N., P. Voland, S. Schwendy, R. Rad, W. Reindl, M. Gerhard, and C. Prinz. 2004. Human dendritic cells respond to Helicobacter pylori, promoting NK cell and Th1-effector responses in vitro. J. Immunol. 173:1249-1257. [DOI] [PubMed] [Google Scholar]
- 126.Harrington, L. E., R. D. Hatton, P. R. Mangan, H. Turner, T. L. Murphy, K. M. Murphy, and C. T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123-1132. [DOI] [PubMed] [Google Scholar]
- 127.Harris, P. R., P. B. Ernst, S. Kawabata, H. Kiyono, M. F. Graham, and P. D. Smith. 1998. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J. Infect. Dis. 178:1516-1520. [DOI] [PubMed] [Google Scholar]
- 128.Hatakeyama, M. 2004. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 4:688-694. [DOI] [PubMed] [Google Scholar]
- 129.Hazell, S. L., A. Lee, L. Brady, and W. Hennessy. 1986. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J. Infect. Dis. 153:658-663. [DOI] [PubMed] [Google Scholar]
- 130.Hellmig, S., S. Ott, P. Rosenstiel, U. R. Folsch, J. Hampe, and S. Schreiber. 2006. Genetic variants in matrix metalloproteinase genes are associated with development of gastric ulcer in H. pylori infection. Am. J. Gastroenterol. 101:29-35. [DOI] [PubMed] [Google Scholar]
- 131.Hida, N., T. Shimoyama, Jr., P. Neville, M. F. Dixon, A. T. Axon, T. Shimoyama, Sr., and J. E. Crabtree. 1999. Increased expression of IL-10 and IL-12 (p40) mRNA in Helicobacter pylori infected gastric mucosa: relation to bacterial cag status and peptic ulceration. J. Clin. Pathol. 52:658-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Higashi, H., A. Nakaya, R. Tsutsumi, K. Yokoyama, Y. Fujii, S. Ishikawa, M. Higuchi, A. Takahashi, Y. Kurashima, Y. Teishikata, S. Tanaka, T. Azuma, and M. Hatakeyama. 2004. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J. Biol. Chem. 279:17205-17216. [DOI] [PubMed] [Google Scholar]
- 133.Higashi, H., R. Tsutsumi, S. Muto, T. Sugiyama, T. Azuma, M. Asaka, and M. Hatakeyama. 2002. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science 295:683-686. [DOI] [PubMed] [Google Scholar]
- 134.Higashi, H., K. Yokoyama, Y. Fujii, S. Ren, H. Yuasa, I. Saadat, N. Murata-Kamiya, T. Azuma, and M. Hatakeyama. 2005. EPIYA motif is a membrane-targeting signal of Helicobacter pylori virulence factor CagA in mammalian cells. J. Biol. Chem. 280:23130-23137. [DOI] [PubMed] [Google Scholar]
- 135.Hippo, Y., H. Taniguchi, S. Tsutsumi, N. Machida, J. M. Chong, M. Fukayama, T. Kodama, and H. Aburatani. 2002. Global gene expression analysis of gastric cancer by oligonucleotide microarrays. Cancer Res. 62:233-240. [PubMed] [Google Scholar]
- 136.Hirasawa, R., M. Tatsuta, H. Iishi, H. Yano, M. Baba, N. Uedo, and N. Sakai. 1999. Increase in apoptosis and decrease in ornithine decarboxylase activity of the gastric mucosa in patients with atrophic gastritis and gastric ulcer after successful eradication of Helicobacter pylori. Am. J. Gastroenterol. 94:2398-2402. [DOI] [PubMed] [Google Scholar]
- 137.Honda, M., M. Mori, H. Ueo, K. Sugimachi, and T. Akiyoshi. 1996. Matrix metalloproteinase-7 expression in gastric carcinoma. Gut 39:444-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Houghton, J., R. M. Korah, M. R. Condon, and K. H. Kim. 1999. Apoptosis in Helicobacter pylori-associated gastric and duodenal ulcer disease is mediated via the Fas antigen pathway. Dig. Dis. Sci. 44:465-478. [DOI] [PubMed] [Google Scholar]
- 139.Houghton, J., L. S. Macera-Bloch, L. Harrison, K. H. Kim, and R. M. Korah. 2000. Tumor necrosis factor alpha and interleukin 1beta up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect. Immun. 68:1189-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Houghton, J., C. Stoicov, S. Nomura, A. B. Rogers, J. Carlson, H. Li, X. Cai, J. G. Fox, J. R. Goldenring, and T. C. Wang. 2004. Gastric cancer originating from bone marrow-derived cells. Science 306:1568-1571. [DOI] [PubMed] [Google Scholar]
- 141.Hussein, N. R., M. Mohammadi, Y. Talebkhan, M. Doraghi, D. P. Letley, M. K. Muhammad, R. H. Argent, and J. C. Atherton. 2008. Differences in virulence markers between Helicobacter pylori strains from Iraq and those from Iran: potential importance of regional differences in H. pylori-associated disease. J. Clin. Microbiol. 46:1774-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hwang, I. R., T. Kodama, S. Kikuchi, K. Sakai, L. E. Peterson, D. Y. Graham, and Y. Yamaoka. 2002. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology 123:1793-1803. [DOI] [PubMed] [Google Scholar]
- 143.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 144.Ito, T., D. Kobayashi, K. Uchida, T. Takemura, S. Nagaoka, I. Kobayashi, T. Yokoyama, I. Ishige, Y. Ishige, N. Ishida, A. Furukawa, H. Muraoka, S. Ikeda, M. Sekine, N. Ando, Y. Suzuki, T. Yamada, T. Suzuki, and Y. Eishi. 2008. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab. Invest. 88:664-681. [DOI] [PubMed] [Google Scholar]
- 145.Jawhari, A. U., M. Noda, M. Pignatelli, and M. Farthing. 1999. Up-regulated cytoplasmic expression, with reduced membranous distribution, of the src substrate p120(ctn) in gastric carcinoma. J. Pathol. 189:180-185. [DOI] [PubMed] [Google Scholar]
- 146.Jenab, M., E. Riboli, P. Ferrari, J. Sabate, N. Slimani, T. Norat, M. Friesen, A. Tjonneland, A. Olsen, K. Overvad, M. C. Boutron-Ruault, F. Clavel-Chapelon, M. Touvier, H. Boeing, M. Schulz, J. Linseisen, G. Nagel, A. Trichopoulou, A. Naska, E. Oikonomou, V. Krogh, S. Panico, G. Masala, C. Sacerdote, R. Tumino, P. H. Peeters, M. E. Numans, H. B. Bueno-de-Mesquita, F. L. Buchner, E. Lund, G. Pera, C. N. Sanchez, M. J. Sanchez, L. Arriola, A. Barricarte, J. R. Quiros, G. Hallmans, R. Stenling, G. Berglund, S. Bingham, K. T. Khaw, T. Key, N. Allen, F. Carneiro, U. Mahlke, G. Del Giudice, D. Palli, R. Kaaks, and C. A. Gonzalez. 2006. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 27:2250-2257. [DOI] [PubMed] [Google Scholar]
- 147.Jones, N. L., A. S. Day, H. A. Jennings, and P. M. Sherman. 1999. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect. Immun. 67:4237-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jones, N. L., P. T. Shannon, E. Cutz, H. Yeger, and P. M. Sherman. 1997. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am. J. Pathol. 151:1695-1703. [PMC free article] [PubMed] [Google Scholar]
- 149.Josenhans, C., A. Labigne, and S. Suerbaum. 1995. Comparative ultrastructural and functional studies of Helicobacter pylori and Helicobacter mustelae flagellin mutants: both flagellin subunits, FlaA and FlaB, are necessary for full motility in Helicobacter species. J. Bacteriol. 177:3010-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Juttner, S., T. Cramer, S. Wessler, A. Walduck, F. Gao, F. Schmitz, C. Wunder, M. Weber, S. M. Fischer, W. E. Schmidt, B. Wiedenmann, T. F. Meyer, M. Naumann, and M. Hocker. 2003. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell. Microbiol. 5:821-834. [DOI] [PubMed] [Google Scholar]
- 151.Kao, J. Y., S. Rathinavelu, K. A. Eaton, L. Bai, Y. Zavros, M. Takami, A. Pierzchala, and J. L. Merchant. 2006. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am. J. Physiol. Gastrointest. Liver Physiol. 291:G73-G81. [DOI] [PubMed] [Google Scholar]
- 152.Kao, J. Y., M. Zhang, M. J. Miller, J. C. Mills, B. Wang, M. Liu, K. A. Eaton, W. Zou, B. E. Berndt, T. S. Cole, T. Takeuchi, S. Y. Owyang, and J. Luther. 2009. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology 138:1046-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kaparakis, M., K. L. Laurie, O. Wijburg, J. Pedersen, M. Pearse, I. R. van Driel, P. A. Gleeson, and R. A. Strugnell. 2006. CD4+ CD25+ regulatory T cells modulate the T-cell and antibody responses in Helicobacter-infected BALB/c mice. Infect. Immun. 74:3519-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Karayiannakis, A. J., K. N. Syrigos, D. Alexiou, N. Kalahanis, T. Rosenberg, E. Bastounis, and M. Pignatelli. 1999. Expression patterns of the novel catenin p120cas in gastrointestinal cancers. Anticancer Res. 19:4401-4405. [PubMed] [Google Scholar]
- 155.Karttunen, R., T. Karttunen, H. P. Ekre, and T. T. MacDonald. 1995. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 36:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kato, S., T. Tsukamoto, T. Mizoshita, H. Tanaka, T. Kumagai, H. Ota, T. Katsuyama, M. Asaka, and M. Tatematsu. 2006. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int. J. Cancer 119:1558-1566. [DOI] [PubMed] [Google Scholar]
- 157.Kavermann, H., B. P. Burns, K. Angermuller, S. Odenbreit, W. Fischer, K. Melchers, and R. Haas. 2003. Identification and characterization of Helicobacter pylori genes essential for gastric colonization. J. Exp. Med. 197:813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Keates, S., S. Sougioultzis, A. C. Keates, D. Zhao, R. M. Peek, Jr., L. M. Shaw, and C. P. Kelly. 2001. cag+ Helicobacter pylori induce transactivation of the epidermal growth factor receptor in AGS gastric epithelial cells. J. Biol. Chem. 276:48127-48134. [DOI] [PubMed] [Google Scholar]
- 159.Khader, S. A., S. L. Gaffen, and J. K. Kolls. 2009. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim, J. S., J. H. Chang, S. I. Chung, and J. S. Yum. 1999. Molecular cloning and characterization of the Helicobacter pylori fliD gene, an essential factor in flagellar structure and motility. J. Bacteriol. 181:6969-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kirschner, D. E., and M. J. Blaser. 1995. The dynamics of Helicobacter pylori infection of the human stomach. J. Theor. Biol. 176:281-290. [DOI] [PubMed] [Google Scholar]
- 162.Kranzer, K., A. Eckhardt, M. Aigner, G. Knoll, L. Deml, C. Speth, N. Lehn, M. Rehli, and W. Schneider-Brachert. 2004. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect. Immun. 72:4416-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kranzer, K., L. Sollner, M. Aigner, N. Lehn, L. Deml, M. Rehli, and W. Schneider-Brachert. 2005. Impact of Helicobacter pylori virulence factors and compounds on activation and maturation of human dendritic cells. Infect. Immun. 73:4180-4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Krueger, S., T. Hundertmark, D. Kuester, T. Kalinski, U. Peitz, and A. Roessner. 2007. Helicobacter pylori alters the distribution of ZO-1 and p120ctn in primary human gastric epithelial cells. Pathol. Res. Pract. 203:433-444. [DOI] [PubMed] [Google Scholar]
- 165.Kubben, F. J., C. F. Sier, M. J. Meijer, M. van den Berg, J. J. van der Reijden, G. Griffioen, C. J. van de Velde, C. B. Lamers, and H. W. Verspaget. 2006. Clinical impact of MMP and TIMP gene polymorphisms in gastric cancer. Br. J. Cancer 95:744-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kuck, D., B. Kolmerer, C. Iking-Konert, P. H. Krammer, W. Stremmel, and J. Rudi. 2001. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect. Immun. 69:5080-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kuipers, E. J., G. I. Perez-Perez, S. G. Meuwissen, and M. J. Blaser. 1995. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J. Natl. Cancer Inst. 87:1777-1780. [DOI] [PubMed] [Google Scholar]
- 168.Kurashima, Y., N. Murata-Kamiya, K. Kikuchi, H. Higashi, T. Azuma, S. Kondo, and M. Hatakeyama. 2008. Deregulation of beta-catenin signal by Helicobacter pylori CagA requires the CagA-multimerization sequence. Int. J. Cancer 122:823-831. [DOI] [PubMed] [Google Scholar]
- 169.Lee, S. A., D. Kang, K. N. Shim, J. W. Choe, W. S. Hong, and H. Choi. 2003. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J. Epidemiol. 13:162-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee, S. K., A. Stack, E. Katzowitsch, S. I. Aizawa, S. Suerbaum, and C. Josenhans. 2003. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 5:1345-1356. [DOI] [PubMed] [Google Scholar]
- 171.Leppilampi, M., T. J. Karttunen, J. Kivela, M. O. Gut, S. Pastorekova, J. Pastorek, and S. Parkkila. 2005. Gastric pit cell hyperplasia and glandular atrophy in carbonic anhydrase IX knockout mice: studies on two strains C57/BL6 and BALB/c. Transgenic Res. 14:655-663. [DOI] [PubMed] [Google Scholar]
- 172.Leung, W. K., E. P. Man, J. Yu, M. Y. Go, K. F. To, Y. Yamaoka, V. Y. Cheng, E. K. Ng, and J. J. Sung. 2006. Effects of Helicobacter pylori eradication on methylation status of E-cadherin gene in noncancerous stomach. Clin. Cancer Res. 12:3216-3221. [DOI] [PubMed] [Google Scholar]
- 173.Leunk, R. D., P. T. Johnson, B. C. David, W. G. Kraft, and D. R. Morgan. 1988. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J. Med. Microbiol. 26:93-99. [DOI] [PubMed] [Google Scholar]
- 174.Lewis, N. D., M. Asim, D. P. Barry, K. Singh, T. de Sablet, J. L. Boucher, A. P. Gobert, R. Chaturvedi, and K. T. Wilson. 2010. Arginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages. J. Immunol. 184:2572-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leying, H., S. Suerbaum, G. Geis, and R. Haas. 1992. Cloning and genetic characterization of a Helicobacter pylori flagellin gene. Mol. Microbiol. 6:2863-2874. [DOI] [PubMed] [Google Scholar]
- 176.Linz, B., F. Balloux, Y. Moodley, A. Manica, H. Liu, P. Roumagnac, D. Falush, C. Stamer, F. Prugnolle, S. W. van der Merwe, Y. Yamaoka, D. Y. Graham, E. Perez-Trallero, T. Wadstrom, S. Suerbaum, and M. Achtman. 2007. An African origin for the intimate association between humans and Helicobacter pylori. Nature 445:915-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Loh, J. T., V. J. Torres, and T. L. Cover. 2007. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 67:4709-4715. [DOI] [PubMed] [Google Scholar]
- 178.Lu, H., P. I. Hsu, D. Y. Graham, and Y. Yamaoka. 2005. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology 128:833-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu, H., N. Murata-Kamiya, Y. Saito, and M. Hatakeyama. 2009. Role of partitioning-defective 1/microtubule affinity-regulating kinases in the morphogenetic activity of Helicobacter pylori CagA. J. Biol. Chem. 284:23024-23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu, H. S., Y. Saito, M. Umeda, N. Murata-Kamiya, H. M. Zhang, H. Higashi, and M. Hatakeyama. 2008. Structural and functional diversity in the PAR1b/MARK2-binding region of Helicobacter pylori CagA. Cancer Sci. 99:2004-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lundgren, A., E. Stromberg, A. Sjoling, C. Lindholm, K. Enarsson, A. Edebo, E. Johnsson, E. Suri-Payer, P. Larsson, A. Rudin, A. M. Svennerholm, and B. S. Lundin. 2005. Mucosal FOXP3-expressing CD4+ CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 73:523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lundgren, A., E. Suri-Payer, K. Enarsson, A. M. Svennerholm, and B. S. Lundin. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 71:1755-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Luzza, F., T. Parrello, G. Monteleone, L. Sebkova, M. Romano, R. Zarrilli, M. Imeneo, and F. Pallone. 2000. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J. Immunol. 165:5332-5337. [DOI] [PubMed] [Google Scholar]
- 184.Lytton, S. D., W. Fischer, W. Nagel, R. Haas, and F. X. Beck. 2005. Production of ammonium by Helicobacter pylori mediates occludin processing and disruption of tight junctions in Caco-2 cells. Microbiology 151:3267-3276. [DOI] [PubMed] [Google Scholar]
- 185.Machado, J. C., P. Pharoah, S. Sousa, R. Carvalho, C. Oliveira, C. Figueiredo, A. Amorim, R. Seruca, C. Caldas, F. Carneiro, and M. Sobrinho-Simoes. 2001. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology 121:823-829. [DOI] [PubMed] [Google Scholar]
- 186.Machida-Montani, A., S. Sasazuki, M. Inoue, S. Natsukawa, K. Shaura, Y. Koizumi, Y. Kasuga, T. Hanaoka, and S. Tsugane. 2004. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer 7:46-53. [DOI] [PubMed] [Google Scholar]
- 187.Mahdavi, J., B. Sonden, M. Hurtig, F. O. Olfat, L. Forsberg, N. Roche, J. Angstrom, T. Larsson, S. Teneberg, K. A. Karlsson, S. Altraja, T. Wadstrom, D. Kersulyte, D. E. Berg, A. Dubois, C. Petersson, K. E. Magnusson, T. Norberg, F. Lindh, B. B. Lundskog, A. Arnqvist, L. Hammarstrom, and T. Boren. 2002. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mai, U. E., G. I. Perez-Perez, J. B. Allen, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1992. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 175:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mai, U. E., G. I. Perez-Perez, L. M. Wahl, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1991. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J. Clin. Invest. 87:894-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Manente, L., A. Perna, E. Buommino, L. Altucci, A. Lucariello, G. Citro, A. Baldi, G. Iaquinto, M. A. Tufano, and A. De Luca. 2008. The Helicobacter pylori's protein VacA has direct effects on the regulation of cell cycle and apoptosis in gastric epithelial cells. J. Cell. Physiol. 214:582-587. [DOI] [PubMed] [Google Scholar]
- 191.Mangan, P. R., L. E. Harrington, D. B. O'Quinn, W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231-234. [DOI] [PubMed] [Google Scholar]
- 192.Mannick, E. E., L. E. Bravo, G. Zarama, J. L. Realpe, X. J. Zhang, B. Ruiz, E. T. Fontham, R. Mera, M. J. Miller, and P. Correa. 1996. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 56:3238-3243. [PubMed] [Google Scholar]
- 193.Reference deleted.
- 194.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 195.Marcos, N. T., A. Magalhaes, B. Ferreira, M. J. Oliveira, A. S. Carvalho, N. Mendes, T. Gilmartin, S. R. Head, C. Figueiredo, L. David, F. Santos-Silva, and C. A. Reis. 2008. Helicobacter pylori induces beta3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J. Clin. Invest. 118:2325-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marshall, B. J., and J. R. Warren. 1984. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet i:1311-1315. [DOI] [PubMed] [Google Scholar]
- 197.Mayerle, J., H. Friess, M. W. Buchler, J. Schnekenburger, F. U. Weiss, K. P. Zimmer, W. Domschke, and M. M. Lerch. 2003. Up-regulation, nuclear import, and tumor growth stimulation of the adhesion protein p120 in pancreatic cancer. Gastroenterology 124:949-960. [DOI] [PubMed] [Google Scholar]
- 198.McCaig, C., C. Duval, E. Hemers, I. Steele, D. M. Pritchard, S. Przemeck, R. Dimaline, S. Ahmed, K. Bodger, D. D. Kerrigan, T. C. Wang, G. J. Dockray, and A. Varro. 2006. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology 130:1754-1763. [DOI] [PubMed] [Google Scholar]
- 199.McDonnell, S., M. Navre, R. J. Coffey, Jr., and L. M. Matrisian. 1991. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol. Carcinog. 4:527-533. [DOI] [PubMed] [Google Scholar]
- 200.Mera, R., E. T. Fontham, L. E. Bravo, J. C. Bravo, M. B. Piazuelo, M. C. Camargo, and P. Correa. 2005. Long term follow up of patients treated for Helicobacter pylori infection. Gut 54:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Meyer, F., K. S. Ramanujam, A. P. Gobert, S. P. James, and K. T. Wilson. 2003. Cutting edge: cyclooxygenase-2 activation suppresses Th1 polarization in response to Helicobacter pylori. J. Immunol. 171:3913-3917. [DOI] [PubMed] [Google Scholar]
- 202.Meyer, F., K. T. Wilson, and S. P. James. 2000. Modulation of innate cytokine responses by products of Helicobacter pylori. Infect. Immun. 68:6265-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Miehlke, S., C. Kirsch, K. Agha-Amiri, T. Gunther, N. Lehn, P. Malfertheiner, M. Stolte, G. Ehninger, and E. Bayerdorffer. 2000. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int. J. Cancer 87:322-327. [PubMed] [Google Scholar]
- 204.Mimuro, H., T. Suzuki, S. Nagai, G. Rieder, M. Suzuki, T. Nagai, Y. Fujita, K. Nagamatsu, N. Ishijima, S. Koyasu, R. Haas, and C. Sasakawa. 2007. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe 2:250-263. [DOI] [PubMed] [Google Scholar]
- 205.Mimuro, H., T. Suzuki, J. Tanaka, M. Asahi, R. Haas, and C. Sasakawa. 2002. Grb2 is a key mediator of Helicobacter pylori CagA protein activities. Mol. Cell 10:745-755. [DOI] [PubMed] [Google Scholar]
- 206.Mitchell, P., C. Germain, P. L. Fiori, W. Khamri, G. R. Foster, S. Ghosh, R. I. Lechler, K. B. Bamford, and G. Lombardi. 2007. Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect. Immun. 75:810-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Miyamoto, T., N. Ogino, S. Yamamoto, and O. Hayaishi. 1976. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J. Biol. Chem. 251:2629-2636. [PubMed] [Google Scholar]
- 208.Moese, S., M. Selbach, V. Brinkmann, A. Karlas, B. Haimovich, S. Backert, and T. F. Meyer. 2007. The Helicobacter pylori CagA protein disrupts matrix adhesion of gastric epithelial cells by dephosphorylation of vinculin. Cell. Microbiol. 9:1148-1161. [DOI] [PubMed] [Google Scholar]
- 209.Moese, S., M. Selbach, T. Kwok, V. Brinkmann, W. Konig, T. F. Meyer, and S. Backert. 2004. Helicobacter pylori induces AGS cell motility and elongation via independent signaling pathways. Infect. Immun. 72:3646-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Moss, S. F., J. Calam, B. Agarwal, S. Wang, and P. R. Holt. 1996. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut 38:498-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Murata-Kamiya, N., K. Kikuchi, T. Hayashi, H. Higashi, and M. Hatakeyama. 2010. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe 7:399-411. [DOI] [PubMed] [Google Scholar]
- 212.Murata-Kamiya, N., Y. Kurashima, Y. Teishikata, Y. Yamahashi, Y. Saito, H. Higashi, H. Aburatani, T. Akiyama, R. M. Peek, Jr., T. Azuma, and M. Hatakeyama. 2007. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 26:4617-4626. [DOI] [PubMed] [Google Scholar]
- 213.Murphy, K. M., and S. L. Reiner. 2002. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2:933-944. [DOI] [PubMed] [Google Scholar]
- 214.Nagy, T. A., M. R. Frey, F. Yan, D. A. Israel, D. B. Polk, and R. M. Peek, Jr. 2009. Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J. Infect. Dis. 199:641-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Naito, M., T. Yamazaki, R. Tsutsumi, H. Higashi, K. Onoe, S. Yamazaki, T. Azuma, and M. Hatakeyama. 2006. Influence of EPIYA-repeat polymorphism on the phosphorylation-dependent biological activity of Helicobacter pylori CagA. Gastroenterology 130:1181-1190. [DOI] [PubMed] [Google Scholar]
- 216.Nakayama, M., J. Hisatsune, E. Yamasaki, H. Isomoto, H. Kurazono, M. Hatakeyama, T. Azuma, Y. Yamaoka, K. Yahiro, J. Moss, and T. Hirayama. 2009. Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. J. Biol. Chem. 284:1612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nardone, G., E. L. Holicky, J. R. Uhl, L. Sabatino, S. Staibano, A. Rocco, V. Colantuoni, B. A. Manzo, M. Romano, G. Budillon, F. R. Cockerill III, and L. J. Miller. 2001. In vivo and in vitro studies of cytosolic phospholipase A2 expression in Helicobacter pylori infection. Infect. Immun. 69:5857-5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Necchi, V., M. E. Candusso, F. Tava, O. Luinetti, U. Ventura, R. Fiocca, V. Ricci, and E. Solcia. 2007. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology 132:1009-1023. [DOI] [PubMed] [Google Scholar]
- 219.Nesic, D., M. C. Miller, Z. T. Quinkert, M. Stein, B. T. Chait, and C. E. Stebbins. 2009. Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nat. Struct. Mol. Biol. 17:130-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nguyen, L. T., T. Uchida, Y. Tsukamoto, A. Kuroda, T. Okimoto, M. Kodama, K. Murakami, T. Fujioka, and M. M. And. 2010. Helicobacter pylori dupA gene is not associated with clinical outcomes in the Japanese population. Clin. Microbiol. Infect. 16:1264-1269. [DOI] [PubMed] [Google Scholar]
- 221.Niess, J. H., S. Brand, X. Gu, L. Landsman, S. Jung, B. A. McCormick, J. M. Vyas, M. Boes, H. L. Ploegh, J. G. Fox, D. R. Littman, and H. C. Reinecker. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254-258. [DOI] [PubMed] [Google Scholar]
- 222.Noach, L. A., N. B. Bosma, J. Jansen, F. J. Hoek, S. J. van Deventer, and G. N. Tytgat. 1994. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand. J. Gastroenterol. 29:425-429. [DOI] [PubMed] [Google Scholar]
- 223.Noren, N. K., B. P. Liu, K. Burridge, and B. Kreft. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150:567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nozaki, K., N. Shimizu, K. Inada, T. Tsukamoto, M. Inoue, T. Kumagai, A. Sugiyama, T. Mizoshita, M. Kaminishi, and M. Tatematsu. 2002. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn. J. Cancer Res. 93:1083-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Obst, B., S. Wagner, K. F. Sewing, and W. Beil. 2000. Helicobacter pylori causes DNA damage in gastric epithelial cells. Carcinogenesis 21:1111-1115. [PubMed] [Google Scholar]
- 226.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 227.Ogden, S. R., L. E. Wroblewski, C. Weydig, J. Romero-Gallo, D. P. O'Brien, D. A. Israel, U. S. Krishna, B. Fingleton, A. B. Reynolds, S. Wessler, and R. M. Peek, Jr. 2008. p120 and Kaiso regulate Helicobacter pylori-induced expression of matrix metalloproteinase-7. Mol. Biol. Cell 19:4110-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ogiwara, H., D. Y. Graham, and Y. Yamaoka. 2008. vacA i-region subtyping. Gastroenterology 134:1267. [DOI] [PubMed] [Google Scholar]
- 229.Ogiwara, H., M. Sugimoto, T. Ohno, R. K. Vilaichone, V. Mahachai, D. Y. Graham, and Y. Yamaoka. 2009. Role of deletion located between the intermediate and middle regions of the Helicobacter pylori vacA gene in cases of gastroduodenal diseases. J. Clin. Microbiol. 47:3493-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229a.Oguma, K., H. Oshima, M. Aoki, R. Uchio, K. Naka, S. Nakamura, A. Hirao, H. Saya, M. M. Taketo, and M. Oshima. 2008. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gactric tumour cells. EMBO J. 27:1671-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Oh, J. D., S. M. Karam, and J. I. Gordon. 2005. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc. Natl. Acad. Sci. U. S. A. 102:5186-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ohnishi, N., H. Yuasa, S. Tanaka, H. Sawa, M. Miura, A. Matsui, H. Higashi, M. Musashi, K. Iwabuchi, M. Suzuki, G. Yamada, T. Azuma, and M. Hatakeyama. 2008. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl. Acad. Sci. U. S. A. 105:1003-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Oldani, A., M. Cormont, V. Hofman, V. Chiozzi, O. Oregioni, A. Canonici, A. Sciullo, P. Sommi, A. Fabbri, V. Ricci, and P. Boquet. 2009. Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells. PLoS Pathog. 5:e1000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Oliveira, M. J., A. M. Costa, A. C. Costa, R. M. Ferreira, P. Sampaio, J. C. Machado, R. Seruca, M. Mareel, and C. Figueiredo. 2009. CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. J. Infect. Dis. 200:745-755. [DOI] [PubMed] [Google Scholar]
- 234.O'Toole, P. W., M. Kostrzynska, and T. J. Trust. 1994. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol. Microbiol. 14:691-703. [DOI] [PubMed] [Google Scholar]
- 235.Ottemann, K. M., and A. C. Lowenthal. 2002. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 70:1984-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Panchal, P. C., J. S. Forman, D. R. Blumberg, and K. T. Wilson. 2003. Helicobacter pylori infection: pathogenesis. Curr. Opin. Gastroenterol. 19:4-10. [DOI] [PubMed] [Google Scholar]
- 237.Papini, E., M. de Bernard, E. Milia, M. Bugnoli, M. Zerial, R. Rappuoli, and C. Montecucco. 1994. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc. Natl. Acad. Sci. U. S. A. 91:9720-9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Papini, E., B. Satin, N. Norais, M. de Bernard, J. L. Telford, R. Rappuoli, and C. Montecucco. 1998. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J. Clin. Invest. 102:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Parkin, D. M., F. Bray, J. Ferlay, and P. Pisani. 2005. Global cancer statistics, 2002. CA Cancer J. Clin. 55:74-108. [DOI] [PubMed] [Google Scholar]
- 240.Parsonnet, J., G. D. Friedman, N. Orentreich, and H. Vogelman. 1997. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Pathak, S. K., S. Basu, A. Bhattacharyya, S. Pathak, A. Banerjee, J. Basu, and M. Kundu. 2006. TLR4-dependent NF-kappaB activation and mitogen- and stress-activated protein kinase 1-triggered phosphorylation events are central to Helicobacter pylori peptidyl prolyl cis-, trans-isomerase (HP0175)-mediated induction of IL-6 release from macrophages. J. Immunol. 177:7950-7958. [DOI] [PubMed] [Google Scholar]
- 242.Peek, R. M., Jr., and M. J. Blaser. 2002. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2:28-37. [DOI] [PubMed] [Google Scholar]
- 243.Peek, R. M., Jr., M. J. Blaser, D. J. Mays, M. H. Forsyth, T. L. Cover, S. Y. Song, U. Krishna, and J. A. Pietenpol. 1999. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 59:6124-6131. [PubMed] [Google Scholar]
- 244.Peek, R. M., Jr., and J. E. Crabtree. 2006. Helicobacter infection and gastric neoplasia. J. Pathol. 208:233-248. [DOI] [PubMed] [Google Scholar]
- 245.Peek, R. M., Jr., G. G. Miller, K. T. Tham, G. I. Perez-Perez, T. L. Cover, J. C. Atherton, G. D. Dunn, and M. J. Blaser. 1995. Detection of Helicobacter pylori gene expression in human gastric mucosa. J. Clin. Microbiol. 33:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Perri, F., R. Cotugno, A. Piepoli, A. Merla, M. Quitadamo, A. Gentile, A. Pilotto, V. Annese, and A. Andriulli. 2007. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. pylori infected patients and effect of eradication. Am. J. Gastroenterol. 102:1361-1371. [DOI] [PubMed] [Google Scholar]
- 247.Phadnis, S. H., D. Ilver, L. Janzon, S. Normark, and T. U. Westblom. 1994. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect. Immun. 62:1557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pohl, M. A., J. Romero-Gallo, J. L. Guruge, D. B. Tse, J. I. Gordon, and M. J. Blaser. 2009. Host-dependent Lewis (Le) antigen expression in Helicobacter pylori cells recovered from Leb-transgenic mice. J. Exp. Med. 206:3061-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pomorski, T., T. F. Meyer, and M. Naumann. 2001. Helicobacter pylori-induced prostaglandin E(2) synthesis involves activation of cytosolic phospholipase A(2) in epithelial cells. J. Biol. Chem. 276:804-810. [DOI] [PubMed] [Google Scholar]
- 250.Powell, W. C., B. Fingleton, C. L. Wilson, M. Boothby, and L. M. Matrisian. 1999. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr. Biol. 9:1441-1447. [DOI] [PubMed] [Google Scholar]
- 251.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science 293:253-256. [DOI] [PubMed] [Google Scholar]
- 252.Queiroz, D. M., E. N. Mendes, G. A. Rocha, A. M. Oliveira, C. A. Oliveira, P. P. Magalhaes, S. B. Moura, M. M. Cabral, and A. M. Nogueira. 1998. cagA-positive Helicobacter pylori and risk for developing gastric carcinoma in Brazil. Int. J. Cancer 78:135-139. [DOI] [PubMed] [Google Scholar]
- 253.Rad, R., L. Brenner, S. Bauer, S. Schwendy, L. Layland, C. P. da Costa, W. Reindl, A. Dossumbekova, M. Friedrich, D. Saur, H. Wagner, R. M. Schmid, and C. Prinz. 2006. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 131:525-537. [DOI] [PubMed] [Google Scholar]
- 254.Raghavan, S., M. Fredriksson, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rathbone, B. J., J. I. Wyatt, B. W. Worsley, S. E. Shires, L. K. Trejdosiewicz, R. V. Heatley, and M. S. Losowsky. 1986. Systemic and local antibody responses to gastric Campylobacter pyloridis in non-ulcer dyspepsia. Gut 27:642-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ren, S., H. Higashi, H. Lu, T. Azuma, and M. Hatakeyama. 2006. Structural basis and functional consequence of Helicobacter pylori CagA multimerization in cells. J. Biol. Chem. 281:32344-32352. [DOI] [PubMed] [Google Scholar]
- 257.Rescigno, M., M. Urbano, B. Valzasina, M. Francolini, G. Rotta, R. Bonasio, F. Granucci, J. P. Kraehenbuhl, and P. Ricciardi-Castagnoli. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361-367. [DOI] [PubMed] [Google Scholar]
- 258.Reynolds, A. B., and R. H. Carnahan. 2004. Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer. Semin. Cell Dev. Biol. 15:657-663. [DOI] [PubMed] [Google Scholar]
- 259.Rhead, J. L., D. P. Letley, M. Mohammadi, N. Hussein, M. A. Mohagheghi, M. Eshagh Hosseini, and J. C. Atherton. 2007. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology 133:926-936. [DOI] [PubMed] [Google Scholar]
- 260.Ristimaki, A., N. Honkanen, H. Jankala, P. Sipponen, and M. Harkonen. 1997. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 57:1276-1280. [PubMed] [Google Scholar]
- 261.Rogers, A. B., N. S. Taylor, M. T. Whary, E. D. Stefanich, T. C. Wang, and J. G. Fox. 2005. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 65:10709-10715. [DOI] [PubMed] [Google Scholar]
- 262.Romano, M., V. Ricci, A. Di Popolo, P. Sommi, C. Del Vecchio Blanco, C. B. Bruni, U. Ventura, T. L. Cover, M. J. Blaser, R. J. Coffey, and R. Zarrilli. 1998. Helicobacter pylori upregulates expression of epidermal growth factor-related peptides, but inhibits their proliferative effect in MKN 28 gastric mucosal cells. J. Clin. Invest. 101:1604-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Romano, M., V. Ricci, A. Memoli, C. Tuccillo, A. Di Popolo, P. Sommi, A. M. Acquaviva, C. Del Vecchio Blanco, C. B. Bruni, and R. Zarrilli. 1998. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J. Biol. Chem. 273:28560-28563. [DOI] [PubMed] [Google Scholar]
- 264.Romero-Gallo, J., E. J. Harris, U. Krishna, M. K. Washington, G. I. Perez-Perez, and R. M. Peek, Jr. 2008. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab. Invest. 88:328-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Rudi, J., C. Kolb, M. Maiwald, I. Zuna, A. von Herbay, P. R. Galle, and W. Stremmel. 1997. Serum antibodies against Helicobacter pylori proteins VacA and CagA are associated with increased risk for gastric adenocarcinoma. Dig. Dis. Sci. 42:1652-1659. [DOI] [PubMed] [Google Scholar]
- 266.Rudi, J., D. Kuck, S. Strand, A. von Herbay, S. M. Mariani, P. H. Krammer, P. R. Galle, and W. Stremmel. 1998. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori-induced gastric epithelial apoptosis. J. Clin. Invest. 102:1506-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rudolph-Owen, L. A., R. Chan, W. J. Muller, and L. M. Matrisian. 1998. The matrix metalloproteinase matrilysin influences early-stage mammary tumorigenesis. Cancer Res. 58:5500-5506. [PubMed] [Google Scholar]
- 268.Ruzsovics, A., Z. Unger, B. Molnar, L. Pronai, and Z. Tulassay. 2002. Effect of Helicobacter pylori infection on epidermal growth factor receptor (EGFR) expression and cell proliferation of gastric epithelial mucosa: correlation to macroscopic and microscopic diagnosis. Int. J. Exp. Pathol. 83:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Saadat, I., H. Higashi, C. Obuse, M. Umeda, N. Murata-Kamiya, Y. Saito, H. Lu, N. Ohnishi, T. Azuma, A. Suzuki, S. Ohno, and M. Hatakeyama. 2007. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447:330-333. [DOI] [PubMed] [Google Scholar]
- 270.Saarialho-Kere, U. K., M. Vaalamo, P. Puolakkainen, K. Airola, W. C. Parks, and M. L. Karjalainen-Lindsberg. 1996. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am. J. Pathol. 148:519-526. [PMC free article] [PubMed] [Google Scholar]
- 271.Saha, A., C. E. Hammond, M. Trojanowska, and A. J. Smolka. 2008. Helicobacter pylori-induced H,K-ATPase alpha-subunit gene repression is mediated by NF-kappaB p50 homodimer promoter binding. Am. J. Physiol. Gastrointest. Liver Physiol. 294:G795-G807. [DOI] [PubMed] [Google Scholar]
- 272.Sallusto, F., and A. Lanzavecchia. 1999. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J. Exp. Med. 189:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sarrio, D., G. Moreno-Bueno, C. Sanchez-Estevez, I. Banon-Rodriguez, G. Hernandez-Cortes, D. Hardisson, and J. Palacios. 2006. Expression of cadherins and catenins correlates with distinct histologic types of ovarian carcinomas. Hum. Pathol. 37:1042-1049. [DOI] [PubMed] [Google Scholar]
- 274.Sawaoka, H., S. Kawano, S. Tsuji, M. Tsuji, W. Sun, E. S. Gunawan, and M. Hori. 1998. Helicobacter pylori infection induces cyclooxygenase-2 expression in human gastric mucosa. Prostaglandins Leukot. Essent. Fatty Acids 59:313-316. [DOI] [PubMed] [Google Scholar]
- 275.Schmidt, H. M., S. Andres, N. O. Kaakoush, L. Engstrand, L. Eriksson, K. L. Goh, K. M. Fock, I. Hilmi, S. Dhamodaran, D. Forman, and H. Mitchell. 2009. The prevalence of the duodenal ulcer promoting gene (dupA) in Helicobacter pylori isolates varies by ethnic group and is not universally associated with disease development: a case-control study. Gut Pathog. 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Schmitt, W., and R. Haas. 1994. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol. Microbiol. 12:307-319. [DOI] [PubMed] [Google Scholar]
- 277.Schubert, M. L., and D. A. Peura. 2008. Control of gastric acid secretion in health and disease. Gastroenterology 134:1842-1860. [DOI] [PubMed] [Google Scholar]
- 278.Reference deleted.
- 279.Segal, E. D., J. Cha, J. Lo, S. Falkow, and L. S. Tompkins. 1999. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 96:14559-14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Selbach, M., S. Moese, S. Backert, P. R. Jungblut, and T. F. Meyer. 2004. The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics 4:2961-2968. [DOI] [PubMed] [Google Scholar]
- 281.Selbach, M., S. Moese, R. Hurwitz, C. R. Hauck, T. F. Meyer, and S. Backert. 2003. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 22:515-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Semino-Mora, C., S. Q. Doi, A. Marty, V. Simko, I. Carlstedt, and A. Dubois. 2003. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J. Infect. Dis. 187:1165-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Senota, A., F. Itoh, H. Yamamoto, Y. Adachi, Y. Hinoda, and K. Imai. 1998. Relation of matrilysin messenger RNA expression with invasive activity in human gastric cancer. Clin. Exp. Metastasis 16:313-321. [DOI] [PubMed] [Google Scholar]
- 284.Sewald, X., B. Gebert-Vogl, S. Prassl, I. Barwig, E. Weiss, M. Fabbri, R. Osicka, M. Schiemann, D. H. Busch, M. Semmrich, B. Holzmann, P. Sebo, and R. Haas. 2008. Integrin subunit CD18 is the T-lymphocyte receptor for the Helicobacter pylori vacuolating cytotoxin. Cell Host Microbe 3:20-29. [DOI] [PubMed] [Google Scholar]
- 285.Shikata, K., Y. Doi, K. Yonemoto, H. Arima, T. Ninomiya, M. Kubo, Y. Tanizaki, T. Matsumoto, M. Iida, and Y. Kiyohara. 2008. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study. Am. J. Epidemiol. 168:1409-1415. [DOI] [PubMed] [Google Scholar]
- 286.Shikata, K., Y. Kiyohara, M. Kubo, K. Yonemoto, T. Ninomiya, T. Shirota, Y. Tanizaki, Y. Doi, K. Tanaka, Y. Oishi, T. Matsumoto, and M. Iida. 2006. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama Study. Int. J. Cancer 119:196-201. [DOI] [PubMed] [Google Scholar]
- 287.Shimoyama, T., S. Fukuda, M. Tanaka, T. Mikami, A. Munakata, and J. E. Crabtree. 1998. CagA seropositivity associated with development of gastric cancer in a Japanese population. J. Clin. Pathol. 51:225-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Siman, J. H., A. Forsgren, G. Berglund, and C. H. Floren. 2001. Tobacco smoking increases the risk for gastric adenocarcinoma among Helicobacter pylori-infected individuals. Scand. J. Gastroenterol. 36:208-213. [DOI] [PubMed] [Google Scholar]
- 289.Sipponen, P., and B. J. Marshall. 2000. Gastritis and gastric cancer. Western countries. Gastroenterol. Clin. North Am. 29:579-592. [DOI] [PubMed] [Google Scholar]
- 290.Song, X., Y. Krelin, T. Dvorkin, O. Bjorkdahl, S. Segal, C. A. Dinarello, E. Voronov, and R. N. Apte. 2005. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J. Immunol. 175:8200-8208. [DOI] [PubMed] [Google Scholar]
- 291.Stanner, S. A., J. Hughes, C. N. Kelly, and J. Buttriss. 2004. A review of the epidemiological evidence for the ‘antioxidant hypothesis.’ Public Health Nutr. 7:407-422. [DOI] [PubMed] [Google Scholar]
- 292.Stein, M., F. Bagnoli, R. Halenbeck, R. Rappuoli, W. J. Fantl, and A. Covacci. 2002. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 43:971-980. [DOI] [PubMed] [Google Scholar]
- 293.Stein, M., R. Rappuoli, and A. Covacci. 2000. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. U. S. A. 97:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Stolte, M., E. Bayerdorffer, A. Morgner, B. Alpen, T. Wundisch, C. Thiede, and A. Neubauer. 2002. Helicobacter and gastric MALT lymphoma. Gut 50(Suppl. 3):III19-III24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Suerbaum, S., C. Josenhans, and A. Labigne. 1993. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J. Bacteriol. 175:3278-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sugimoto, M., T. Ohno, D. Y. Graham, and Y. Yamaoka. 2009. Gastric mucosal interleukin-17 and -18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer Sci. 100:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sun, J., K. Aoki, J. X. Zheng, B. Z. Su, X. H. Ouyang, and J. Misumi. 2006. Effect of NaCl and Helicobacter pylori vacuolating cytotoxin on cytokine expression and viability. World J. Gastroenterol. 12:2174-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sun, Y. Q., J. D. Soderholm, F. Petersson, and K. Borch. 2004. Long-standing gastric mucosal barrier dysfunction in Helicobacter pylori-induced gastritis in Mongolian gerbils. Helicobacter 9:217-227. [DOI] [PubMed] [Google Scholar]
- 299.Sundrud, M. S., V. J. Torres, D. Unutmaz, and T. L. Cover. 2004. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc. Natl. Acad. Sci. U. S. A. 101:7727-7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sung, J. J., W. K. Leung, M. Y. Go, K. F. To, A. S. Cheng, E. K. Ng, and F. K. Chan. 2000. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am. J. Pathol. 157:729-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Suzuki, K., Y. Kokai, N. Sawada, R. Takakuwa, K. Kuwahara, E. Isogai, H. Isogai, and M. Mori. 2002. SS1 Helicobacter pylori disrupts the paracellular barrier of the gastric mucosa and leads to neutrophilic gastritis in mice. Virchows Arch. 440:318-324. [DOI] [PubMed] [Google Scholar]
- 302.Suzuki, M., H. Mimuro, K. Kiga, M. Fukumatsu, N. Ishijima, H. Morikawa, S. Nagai, S. Koyasu, R. H. Gilman, D. Kersulyte, D. E. Berg, and C. Sasakawa. 2009. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe 5:23-34. [DOI] [PubMed] [Google Scholar]
- 303.Suzuki, M., H. Mimuro, T. Suzuki, M. Park, T. Yamamoto, and C. Sasakawa. 2005. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J. Exp. Med. 202:1235-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Tabassam, F. H., D. Y. Graham, and Y. Yamaoka. 2009. Helicobacter pylori activate epidermal growth factor receptor- and phosphatidylinositol 3-OH kinase-dependent Akt and glycogen synthase kinase 3beta phosphorylation. Cell. Microbiol. 11:70-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Takashima, M., T. Furuta, H. Hanai, H. Sugimura, and E. Kaneko. 2001. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut 48:765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Takeshima, E., K. Tomimori, H. Teruya, C. Ishikawa, M. Senba, D. D'Ambrosio, F. Kinjo, H. Mimuro, C. Sasakawa, T. Hirayama, J. Fujita, and N. Mori. 2009. Helicobacter pylori-induced interleukin-12 p40 expression. Infect. Immun. 77:1337-1348. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 307.Tan, S., L. S. Tompkins, and M. R. Amieva. 2009. Helicobacter pylori usurps cell polarity to turn the cell surface into a replicative niche. PLoS Pathog. 5:e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Tegtmeyer, N., D. Zabler, D. Schmidt, R. Hartig, S. Brandt, and S. Backert. 2009. Importance of EGF receptor, HER2/Neu and Erk1/2 kinase signalling for host cell elongation and scattering induced by the Helicobacter pylori CagA protein: antagonistic effects of the vacuolating cytotoxin VacA. Cell. Microbiol. 11:488-505. [DOI] [PubMed] [Google Scholar]
- 309.Tolwinski, N. S., and E. Wieschaus. 2004. Rethinking WNT signaling. Trends Genet. 20:177-181. [DOI] [PubMed] [Google Scholar]
- 310.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 311.Torres, J., G. I. Perez-Perez, Y. Leal-Herrera, and O. Munoz. 1998. Infection with CagA+ Helicobacter pylori strains as a possible predictor of risk in the development of gastric adenocarcinoma in Mexico. Int. J. Cancer 78:298-300. [DOI] [PubMed] [Google Scholar]
- 312.Touati, E., V. Michel, J. M. Thiberge, N. Wuscher, M. Huerre, and A. Labigne. 2003. Chronic Helicobacter pylori infections induce gastric mutations in mice. Gastroenterology 124:1408-1419. [DOI] [PubMed] [Google Scholar]
- 313.Tsugane, S. 2005. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 96:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tsukashita, S., R. Kushima, M. Bamba, E. Nakamura, K. Mukaisho, H. Sugihara, and T. Hattori. 2003. Beta-catenin expression in intramucosal neoplastic lesions of the stomach. Comparative analysis of adenoma/dysplasia, adenocarcinoma and signet-ring cell carcinoma. Oncology 64:251-258. [DOI] [PubMed] [Google Scholar]
- 315.Tsutsumi, R., H. Higashi, M. Higuchi, M. Okada, and M. Hatakeyama. 2003. Attenuation of Helicobacter pylori CagA × SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J. Biol. Chem. 278:3664-3670. [DOI] [PubMed] [Google Scholar]
- 316.Tsutsumi, R., A. Takahashi, T. Azuma, H. Higashi, and M. Hatakeyama. 2006. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol. Cell. Biol. 26:261-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Tu, S., G. Bhagat, G. Cui, S. Takaishi, E. A. Kurt-Jones, B. Rickman, K. S. Betz, M. Penz-Oesterreicher, O. Bjorkdahl, J. G. Fox, and T. C. Wang. 2008. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14:408-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Turner, J. R. 2006. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am. J. Pathol. 169:1901-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido, K. Taniyama, N. Sasaki, and R. J. Schlemper. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784-789. [DOI] [PubMed] [Google Scholar]
- 320.Umeda, M., N. Murata-Kamiya, Y. Saito, Y. Ohba, M. Takahashi, and M. Hatakeyama. 2009. Helicobacter pylori CagA causes mitotic impairment and induces chromosomal instability. J. Biol. Chem. 284:22166-22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Van Doorn, L. J., C. Figueiredo, F. Megraud, S. Pena, P. Midolo, D. M. Queiroz, F. Carneiro, B. Vanderborght, M. D. Pegado, R. Sanna, W. De Boer, P. M. Schneeberger, P. Correa, E. K. Ng, J. Atherton, M. J. Blaser, and W. G. Quint. 1999. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology 116:823-830. [DOI] [PubMed] [Google Scholar]
- 322.Vaux, D. L., and A. Strasser. 1996. The molecular biology of apoptosis. Proc. Natl. Acad. Sci. U. S. A. 93:2239-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 324.Voland, P., N. Hafsi, M. Zeitner, S. Laforsch, H. Wagner, and C. Prinz. 2003. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori. Infect. Immun. 71:3837-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vorobjova, T., I. Nilsson, K. Kull, H. I. Maaroos, A. Covacci, T. Wadstrom, and R. Uibo. 1998. CagA protein seropositivity in a random sample of adult population and gastric cancer patients in Estonia. Eur. J. Gastroenterol. Hepatol. 10:41-46. [DOI] [PubMed] [Google Scholar]
- 326.Waghray, M., Y. Zavros, M. Saqui-Salces, M. El-Zaatari, C. B. Alamelumangapuram, A. Todisco, K. A. Eaton, and J. L. Merchant. 2009. Interleukin-1beta promotes gastric atrophy through suppression of sonic hedgehog. Gastroenterology 138:562-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Wagner, S., W. Beil, J. Westermann, R. P. Logan, C. T. Bock, C. Trautwein, J. S. Bleck, and M. P. Manns. 1997. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology 113:1836-1847. [DOI] [PubMed] [Google Scholar]
- 328.Wallasch, C., J. E. Crabtree, D. Bevec, P. A. Robinson, H. Wagner, and A. Ullrich. 2002. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem. Biophys. Res. Commun. 295:695-701. [DOI] [PubMed] [Google Scholar]
- 329.Wang, F., P. Xia, F. Wu, D. Wang, W. Wang, T. Ward, Y. Liu, F. Aikhionbare, Z. Guo, M. Powell, B. Liu, F. Bi, A. Shaw, Z. Zhu, A. Elmoselhi, D. Fan, T. L. Cover, X. Ding, and X. Yao. 2008. Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells. J. Biol. Chem. 283:26714-26725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wang, J., X. Fan, C. Lindholm, M. Bennett, J. O'Connoll, F. Shanahan, E. G. Brooks, V. E. Reyes, and P. B. Ernst. 2000. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect. Immun. 68:4303-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Watanabe, T., N. Asano, S. Fichtner-Feigl, P. L. Gorelick, Y. Tsuji, Y. Matsumoto, T. Chiba, I. J. Fuss, A. Kitani, and W. Strober. 2010. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J. Clin. Invest. 120:1645-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Weeks, D. L., S. Eskandari, D. R. Scott, and G. Sachs. 2000. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science 287:482-485. [DOI] [PubMed] [Google Scholar]
- 333.Weydig, C., A. Starzinski-Powitz, G. Carra, J. Lower, and S. Wessler. 2007. CagA-independent disruption of adherence junction complexes involves E-cadherin shedding and implies multiple steps in Helicobacter pylori pathogenicity. Exp. Cell Res. 313:3459-3471. [DOI] [PubMed] [Google Scholar]
- 334.Whary, M. T., N. Sundina, L. E. Bravo, P. Correa, F. Quinones, F. Caro, and J. G. Fox. 2005. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol. Biomarkers Prev. 14:1464-1469. [DOI] [PubMed] [Google Scholar]
- 335.Wijnhoven, B. P., M. Pignatelli, W. N. Dinjens, and H. W. Tilanus. 2005. Reduced p120ctn expression correlates with poor survival in patients with adenocarcinoma of the gastroesophageal junction. J. Surg. Oncol. 92:116-123. [DOI] [PubMed] [Google Scholar]
- 336.Willhite, D. C., T. L. Cover, and S. R. Blanke. 2003. Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of Helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J. Biol. Chem. 278:48204-48209. [DOI] [PubMed] [Google Scholar]
- 337.Williams, C. S., W. Smalley, and R. N. DuBois. 1997. Aspirin use and potential mechanisms for colorectal cancer prevention. J. Clin. Invest. 100:1325-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Wilson, C. L., K. J. Heppner, P. A. Labosky, B. L. Hogan, and L. M. Matrisian. 1997. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc. Natl. Acad. Sci. U. S. A. 94:1402-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Wilson, K. T., K. S. Ramanujam, H. L. T. Mobley, R. F. Musselman, S. P. James, and S. J. Meltzer. 1996. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology 111:1524-1533. [DOI] [PubMed] [Google Scholar]
- 340.Wittchen, E. S., J. Haskins, and B. R. Stevenson. 1999. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J. Biol. Chem. 274:35179-35185. [DOI] [PubMed] [Google Scholar]
- 341.Witty, J. P., S. McDonnell, K. J. Newell, P. Cannon, M. Navre, R. J. Tressler, and L. M. Matrisian. 1994. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res. 54:4805-4812. [PubMed] [Google Scholar]
- 342.Wong, B. C., S. K. Lam, W. M. Wong, J. S. Chen, T. T. Zheng, R. E. Feng, K. C. Lai, W. H. Hu, S. T. Yuen, S. Y. Leung, D. Y. Fong, J. Ho, C. K. Ching, and J. S. Chen. 2004. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291:187-194. [DOI] [PubMed] [Google Scholar]
- 343.Wong, B. C., W. P. Wang, W. H. So, V. Y. Shin, W. M. Wong, F. M. Fung, E. S. Liu, W. M. Hiu, S. K. Lam, and C. H. Cho. 2001. Epidermal growth factor and its receptor in chronic active gastritis and gastroduodenal ulcer before and after Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 15:1459-1465. [DOI] [PubMed] [Google Scholar]
- 344.Wroblewski, L. E., and R. M. Peek, Jr. 2007. Orchestration of dysregulated epithelial turnover by a manipulative pathogen. Cell Host Microbe 2:209-211. [DOI] [PubMed] [Google Scholar]
- 345.Wroblewski, L. E., L. Shen, S. Ogden, J. Romero-Gallo, L. A. Lapierre, D. A. Israel, J. R. Turner, and R. M. Peek, Jr. 2009. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136:236-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wu, I. C., and L. B. Minor. 2003. Long-term hearing outcome in patients receiving intratympanic gentamicin for Meniere's disease. Laryngoscope 113:815-820. [DOI] [PubMed] [Google Scholar]
- 347.Wu, J. Y., H. Lu, Y. Sun, D. Y. Graham, H. S. Cheung, and Y. Yamaoka. 2006. Balance between polyoma enhancing activator 3 and activator protein 1 regulates Helicobacter pylori-stimulated matrix metalloproteinase 1 expression. Cancer Res. 66:5111-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wu, S., K. J. Rhee, E. Albesiano, S. Rabizadeh, X. Wu, H. R. Yen, D. L. Huso, F. L. Brancati, E. Wick, F. McAllister, F. Housseau, D. M. Pardoll, and C. L. Sears. 2009. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wyatt, J. I., B. J. Rathbone, and R. V. Heatley. 1986. Local immune response to gastric Campylobacter in non-ulcer dyspepsia. J. Clin. Pathol. 39:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Xu, H., R. Chaturvedi, Y. Cheng, F. I. Bussiere, M. Asim, M. D. Yao, D. Potosky, S. J. Meltzer, J. G. Rhee, S. S. Kim, S. F. Moss, A. Hacker, Y. Wang, R. A. Casero, Jr., and K. T. Wilson. 2004. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 64:8521-8525. [DOI] [PubMed] [Google Scholar]
- 351.Yamanishi, S., T. Iizumi, E. Watanabe, M. Shimizu, S. Kamiya, K. Nagata, Y. Kumagai, Y. Fukunaga, and H. Takahashi. 2006. Implications for induction of autoimmunity via activation of B-1 cells by Helicobacter pylori urease. Infect. Immun. 74:248-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yamaoka, Y., T. Kudo, H. Lu, A. Casola, A. R. Brasier, and D. Y. Graham. 2004. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology 126:1030-1043. [DOI] [PubMed] [Google Scholar]
- 353.Yamaoka, Y., D. H. Kwon, and D. Y. Graham. 2000. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 97:7533-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yamaoka, Y., O. Ojo, S. Fujimoto, S. Odenbreit, R. Haas, O. Gutierrez, H. M. El-Zimaity, R. Reddy, A. Arnqvist, and D. Y. Graham. 2006. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut 55:775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Yamashita, K., I. Azumano, M. Mai, and Y. Okada. 1998. Expression and tissue localization of matrix metalloproteinase 7 (matrilysin) in human gastric carcinomas. Implications for vessel invasion and metastasis. Int. J. Cancer 79:187-194. [DOI] [PubMed] [Google Scholar]
- 356.Yang, L., L. M. DeBusk, K. Fukuda, B. Fingleton, B. Green-Jarvis, Y. Shyr, L. M. Matrisian, D. P. Carbone, and P. C. Lin. 2004. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6:409-421. [DOI] [PubMed] [Google Scholar]
- 357.Yang, L., J. Huang, X. Ren, A. E. Gorska, A. Chytil, M. Aakre, D. P. Carbone, L. M. Matrisian, A. Richmond, P. C. Lin, and H. L. Moses. 2008. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13:23-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Yao, Y. L., B. Xu, Y. G. Song, W. D. Zhang, Y. L. Zhang, and Z. S. Zhang. 2002. Effect of Helicobacter pylori infection on gastric mucosal cell proliferation in Mongolian gerbils. Di Yi Jun Yi Da Xue Xue Bao 22:348-350, 365. [PubMed] [Google Scholar]
- 359.Ye, G., C. Barrera, X. Fan, W. K. Gourley, S. E. Crowe, P. B. Ernst, and V. E. Reyes. 1997. Expression of B7-1 and B7-2 costimulatory molecules by human gastric epithelial cells: potential role in CD4+ T cell activation during Helicobacter pylori infection. J. Clin. Invest. 99:1628-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Yu, W. H., J. F. Woessner, Jr., J. D. McNeish, and I. Stamenkovic. 2002. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 16:307-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zavros, Y., G. Rieder, A. Ferguson, and J. L. Merchant. 2002. Gastritis and hypergastrinemia due to Acinetobacter lwoffii in mice. Infect. Immun. 70:2630-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Zeaiter, Z., D. Cohen, A. Musch, F. Bagnoli, A. Covacci, and M. Stein. 2008. Analysis of detergent-resistant membranes of Helicobacter pylori infected gastric adenocarcinoma cells reveals a role for MARK2/Par1b in CagA-mediated disruption of cellular polarity. Cell. Microbiol. 10:781-794. [DOI] [PubMed] [Google Scholar]
- 363.Zhang, S., A. Yanaka, M. Tauchi, H. Suzuki, T. Shibahara, H. Matsui, A. Nakahara, and N. Tanaka. 2006. Hyperosmotic stress enhances interleukin-1beta expression in Helicobacter pylori-infected murine gastric epithelial cells in vitro. J. Gastroenterol. Hepatol. 21:759-766. [DOI] [PubMed] [Google Scholar]
- 364.Zheng, P. Y., and N. L. Jones. 2003. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell. Microbiol. 5:25-40. [DOI] [PubMed] [Google Scholar]
- 365.Zychlinsky, A., C. Fitting, J. M. Cavaillon, and P. J. Sansonetti. 1994. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J. Clin. Invest. 94:1328-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]