Abstract
Adjuvant compounds are usually included in vaccinations in order to bolster total vaccine-specific responses or to tailor an immune response toward a desired endpoint, such as the production of gamma interferon or an increase in antibody titers. While most adjuvants are studied in regard to their impact on vaccine-specific responses during and just after the vaccination period, a detailed analysis of how adjuvants skew the Th1/Th2 axis at more distant time points is not often undertaken. In the current study, we present data that suggests that adjuvants differ in their relative abilities to bolster and skew immune responses in the short term compared with more distant time points. To that end, we have employed interleukin-12 (IL-12) and IL-28B as adjuvants for DNA vaccination of rhesus macaques. While both adjuvants were able to bolster Th1-biased responses, our analysis shows that this skewing was achieved through different mechanisms. Moreover, analysis 3 months after the final immunization revealed the activity of the IL-12 adjuvant to be short lived, while the IL-28B adjuvant continued to exert its influence on the immune system. Taken together, these data suggest that the scientific and medical communities would benefit from a more detailed analysis of adjuvant function, including the determination of long-term influences of administered adjuvants.
Adjuvant compounds are employed in numerous vaccination strategies as a method for augmenting antigen-specific immune responses—both cellular and humoral. The employment of cytokine adjuvants allows the user to tailor more specific immune responses to the target antigen, allowing the creation of a vaccine that drives immune responses that are believed to be important for control of the targeted pathogen. Vaccines that are meant to induce antibody responses, for instance, benefit from cytokine adjuvants that drive Th2-biased humoral responses (5, 6, 11, 15). If the desired endpoint of vaccination is a strong cellular response, Th1-biased cytokine adjuvants may be beneficial (2-4, 7, 8, 10-12, 14, 16). The usefulness of adjuvants that are employed in this way is normally gauged via observation for an increase in the desired response, such as increased gamma interferon (IFN-γ) production (1-3, 7, 9, 10, 12). However, Th1 or Th2 skewing by an adjuvant may involve not only increases in a specific Th response but also repression of the opposite end of the axis. Thus, Th1-biased cytokines may skew antigen-specific immune responses not only by increasing Th1 responses but also by repressing Th2 responses. Unfortunately, the modulation of the Th1/Th2 axis by adjuvants during vaccination is rarely analyzed. Moreover, the modulation of Th responses is most often analyzed during or just after vaccination, with little observation of more distant memory time points.
With this in mind, we have analyzed the ability of two distinct Th1-skewing cytokine adjuvants, interleukin-12 (IL-12) and IL-28B, for their effects on the Th1/Th2 axis during vaccination and 3 months after the final immunization to determine what the short-term and long-term influences of these adjuvants were. Through the use of flow cytometry, we have been able to analyze the impact of these cytokine adjuvants on the production of the prototypical Th1 and Th2 cytokines IFN-γ and IL-4, respectively. Our results suggest that while both adjuvants skew responses toward a Th1 phenotype just after the termination of the vaccination period, they achieve this endpoint via different effects on IFN-γ production or inhibition of IL-4 production. Additionally, each adjuvant has distinct influences on different T cell compartments, as IL-12 induced IFN-γ production heavily from the CD4+ T cell subset, while IL-28B induced IFN-γ production primarily from the CD8+ T cell subset. Moreover, the data suggest that while both cytokine adjuvants show strong Th1 skewing just after the termination of the vaccination period, these effects wane as the primary immune response dies down when employing IL-12 as an adjuvant, while the IL-28B adjuvant was able to maintain the Th1 bias in long-lived responses. Thus, the data suggest that different adjuvants affect the Th1/Th2 axis through unique mechanisms and that some adjuvants may be more appropriate for long-term Th1 skewing than others.
MATERIALS AND METHODS
Animals.
Rhesus macaques (Macaca mulatta) were housed at BioQual, Inc. (Rockville, MD), in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care. Animals were allowed to acclimate for at least 30 days in quarantine prior to any experimentation.
Plasmids.
Both the HIV Gag (pGag4Y) and HIV Pol (pMPol) antigen constructs were expressed using a pVAX1 plasmid backbone (Invitrogen, Carlsbad, CA). Both the Gag and Pol plasmids were constructed as consensus sequences of the respective genes from HIV-1, inclusive of clades A to D, with several modifications, including the addition of a Kozak sequence and substitution of a leader sequence and codon, as well as RNA optimization for expression in Homo sapiens. Additionally, small deletions were made in 7 different locations within the Pol construct in order to inactivate HIV protease, reverse transcriptase, RNase H, and integrase for safety measures.
The IL-28B and IL-12 adjuvant constructs have been previously described (12). Plasmids were expanded and formulated at Inovio Pharmaceuticals (The Woodlands, TX), in sterile water for injection.
Immunization.
Animals were divided to include groups of four rhesus macaques. All immunized macaques received plasmid injections consisting of 0.5 mg of each HIV Gag (pGag4Y) and HIV Pol (pMPol) with or without adjuvant intramuscularly at weeks 0, 4, and 8, followed by electroporation using a Cellectra electroporation device (Inovio Immune Biomedical Corporation, Blue Bell, PA). The groups were divided by the presence or absence of cytokine adjuvants. One group received 0.3 mg of optimized macaque IL-12, while others received 0.3 mg of optimized macaque IL-28B/IFN-λ3. All plasmids, regardless of the inclusion of adjuvant or not, were delivered to the same single site.
PBMC isolation.
Animals were bled every 2 weeks for the duration of the study, including 2 weeks prior to the initiation of immunization. Ten milliliters of blood were collected via venipuncture in tubes containing EDTA. Peripheral blood mononuclear cells (PBMC) were isolated by standard Ficoll-Hypaque centrifugation and then resuspended in complete culture medium (RPMI 1640 with 2 mM/liter l-glutamine supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 55 μM/liter β-mercaptoethanol). Red blood cells (RBC) were lysed with ammonium chloride-potassium (ACK) lysis buffer (Cambrex Bio Science, East Rutherford, NJ).
Intracellular cytokine staining antibodies.
The following antibodies were obtained from BD Biosciences (San Jose, CA): CD3 (allophycocyanin [APC-Cy7]), IFN-γ (phycoerythrin [PE]-Cy7), CD8 (APC), CD4 (peridinin chlorophyll protein [PerCP]-Cy5.5), CD14 (Pacific blue), CD16 (Pacific blue), CD19 (Pacific blue), and IL-4 (PE).
Cell stimulation and intracellular cytokine staining.
PBMC were resuspended to 1 × 106 cells/100 μl in complete RPMI and plated in 96-well plates with stimulating peptides at 1:200 dilutions in the presence of protein transport inhibitors GolgiStop and GolgiPlug (BD Pharmingen, San Jose, CA). An unstimulated sample (medium alone) and a positive control (Staphylococcus enterotoxin B, 1 μg/ml; Sigma-Aldrich, St. Louis, MO) were included in each assay. Cells were incubated for 5.5 h at 37°C. Following incubation, cells were washed three times in phosphate-buffered saline and stained with fluorophore-conjugated antibodies to cell surface antigens. The cells were then washed and fixed using a fixation and permeabilization kit (eBioscience, San Diego, CA) according to the manufacturer's instructions. Following fixation, cells were washed twice and stained with antibodies against intracellular targets. Following staining, the cells were again washed, fixed in 2% paraformaldehyde, and analyzed on a flow cytometer.
Flow cytometry.
The cells were analyzed on a modified LSR II flow cytometer (BD Immunocytometry Systems, San Jose, CA). Data analysis was performed using FlowJo, version 7.2.5 (TreeStar, San Carlos, CA). Data are reported after background correction.
Statistical analysis.
All values are reported as the mean ± standard deviation. Analysis of differences in cytokine production was completed using the Mann-Whitney U test. Bonferroni correction was used to correct for multiple comparisons. Statistical significance was assumed at a P value of ≤0.05. All statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS, Chicago, IL).
RESULTS
Study design, vaccine composition, and gating strategy.
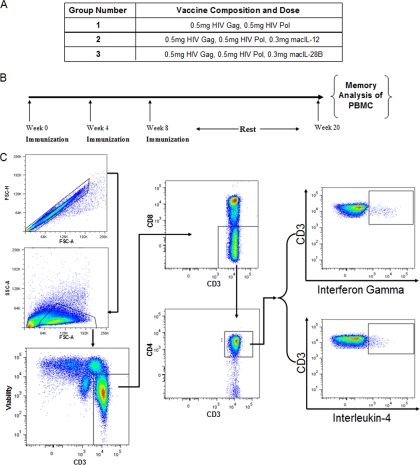
Previous studies have shown that both IL-12 and IL-28B drive strong Th1-biased cellular immune responses both in small animal models (10) and nonhuman primate models of vaccination when used as adjuvants (12). Those studies focused largely on analyzing gamma interferon (IFN-γ) production during and after the immunization period, with no significant analysis of Th2 responses, such as the production of interleukin-4 (IL-4). Thus, in the current study, we have analyzed the production of both IFN-γ and IL-4 from CD4+ and CD8+ T cells just after the termination of the immunization period and at a 3-month follow-up time point in an effort to determine the short-term and long-term influence of adjuvants on the Th1/Th2 axis. In order to accomplish this, groups of rhesus macaques (n = 4) were immunized with antigen alone, antigen in combination with the IL-12 adjuvant (designated macIL-12), or antigen in combination with the IL-28B adjuvant (designated macIL-28B) (Fig. 1 A), and analysis of IFN-γ and interleukin-4 production was performed at the indicated time points (Fig. 1B). Cytokine production was detected in PBMC via intracellular cytokine staining followed by flow cytometry using the gating strategy shown in Fig. 1C.
FIG. 1.
Group design, immunization schedule, and gating strategy. (A) Rhesus macaques were divided into 3 groups that received various immunizations consisting of HIV antigens with or without IL-12 or IL-28B/IFN-λ3 adjuvant. (B) Animals were immunized three times 1 month apart, with an initial immune analysis 2 weeks after the final vaccination. Immune analysis also occurred 3 months after the final vaccination. (C) A representative gating strategy is shown for flow cytometry-based identification of IFN-γ- and IL-4-positive lymphocytes (in this case CD4+ T cells). FSC, forward scatter; SSC, side scatter.
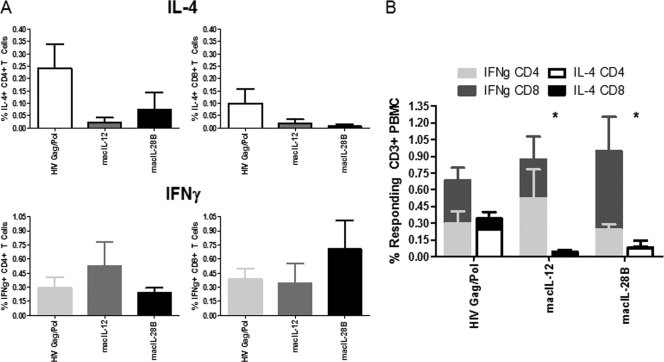
IL-12 and IL-28B adjuvants exert different influences on IL-4 and IFN-γ production two weeks postvaccination.
In order to determine if the macIL-12 and macIL-28B adjuvants were influencing cytokine production profiles, we analyzed the CD4+ and CD8+ T cell compartments 2 weeks after the final immunization. The results of this analysis show that, compared to immunization using antigen alone, both the macIL-12 and macIL-28B adjuvants actively repressed antigen-specific IL-4 production from both CD4+ and CD8+ T cells (Fig. 2 A). More specifically, the macIL-12 adjuvant was able to induce an impressive 10-fold reduction in antigen-specific IL-4 expression by CD4+ T cells, while macIL-28B repressed IL-4 expression by just over 3-fold compared with its expression following immunization using antigen alone (Fig. 2A, upper left). Repression of IL-4 production was also observed in the CD8+ T cell compartment, with the macIL-28B adjuvant showing a robust 10-fold inhibition of cytokine production, compared to a roughly 5-fold inhibition exhibited by macIL-12 (Fig. 2A, upper right).
FIG. 2.
Analysis of the effects of the IL-12 and IL-28B/IFN-λ3 adjuvants on antigen-specific IFN-γ and IL-4 production in T cells. (A) Two weeks after the final vaccination, peripheral CD4+ and CD8+ T cells were assayed for the production of IL-4 and IFN-γ. (B) Total IL-4 and IFN-γ responses are graphed to allow intragroup and intergroup comparison of responses. *, P < 0.05 for intragroup comparison of IL-4 and IFN-γ responses. IFNg, IFN-γ.
As the results of a previous small animal study have suggested possible modulation of the antibody responses by IL-12 and IL-28B (10), we also assayed for p24-specific antibodies in the plasma of immunized animals. No difference was seen in antibody titers (data not shown), suggesting that antibody responses are not significantly modulated by these adjuvants.
Upon seeing that both adjuvants were exerting a repressive effect on Th2 cytokine production, we assayed for the presence of IFN-γ, a prototypical Th1-type cytokine. Analysis of antigen-specific IFN-γ production showed that the macIL-12 adjuvant exerted a greater influence on IFN-γ production from CD4+ T cells than macIL-28B, as the animals receiving IL-12 exhibited a nearly 2-fold-greater percentage of IFN-γ-producing cells than the animals immunized with antigen alone, while animals that received the macIL-28B adjuvant did not show any difference in cytokine-positive cells (Fig. 2A, lower left). Analysis of the CD8+ T cell compartment again showed a pattern in which the macIL-28B adjuvant exerted the strongest influence, with nearly 2-fold more IFN-γ-producing cells generated than with immunization using antigen alone or antigen in combination with the macIL-12 adjuvant (Fig. 2A, lower right). Analyses of these data together with the IL-4 data show a pattern in which immunization with antigen alone drives Th1 responses to a greater extent than Th2 responses in T cells, while the addition of the macIL-12 (P < 0.05) or macIL-28B (P < 0.05) adjuvants increases Th1 cytokine synthesis and actively inhibits Th2 cytokine synthesis (Fig. 2B). Interestingly, while Th1 skewing is achieved by both adjuvants via inhibition of IL-4 synthesis and increase in IFN-γ synthesis, this phenomenon is achieved somewhat differently depending on the adjuvant used, as only macIL-12 significantly increased IFN-γ synthesis in the CD4+ T cell compartment (P < 0.05). In contrast, IL-28B increased IFN-γ synthesis largely in the CD8+ T cell compartment, but the levels of IFN-γ were significantly higher than the levels of IL-4 in the CD8 compartment both with immunization alone and with the addition of macIL-12 and macIL-28 (Fig. 2B).
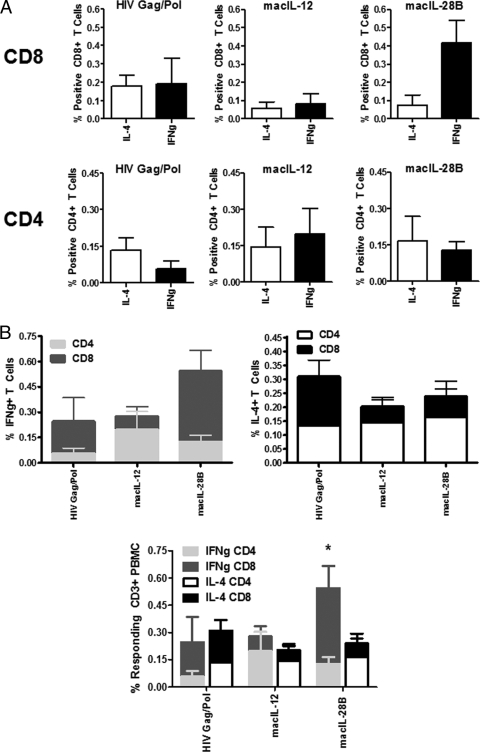
IL-28B but not IL-12 drives IFN-γ responses that persist long-term in the CD8+ T cell compartment.
Since our analysis of gamma interferon and interleukin-4 production from CD4+ and CD8+ T cells suggested that both the macIL-12 and macIL-28B adjuvants were inducing a Th1 bias 2 weeks after the termination of the immunization period, we were further interested in determining if this profile persisted in long-lived immune responses. To that end, we subjected CD4+ and CD8+ T cells to analysis of IFN-γ and IL-4 production 3 months after the immunization period had ended. The results of this analysis show that in the CD8+ T cell compartment, the total cytokine responses (IFN-γ and IL-4 together) in the macIL-12 group were lower than those seen using immunization with antigen alone (Fig. 3 A and B), while the total CD4+ responses were higher, owing to increased production of IFN-γ (Fig. 3A and B). The production of IFN-γ in this group was slightly higher than that of IL-4 in the CD4+ compartment, but not to the same extent as was seen 2 weeks after the final immunization, suggesting that the Th1-skewing influence of the macIL-12 adjuvant did not persist for prolonged periods (Fig. 3A and B). In contrast, the macIL-28B adjuvant exerted a Th1 bias even in long-lived immune responses (P < 0.05). In the CD8+ T cell compartment, the total responses were higher than those generated via immunization using antigen alone or using the macIL-12 adjuvant (Fig. 3A and B), while the responses in the CD4+ compartment were similar to what was seen using immunization with antigen alone (Fig. 3A and B). Th1 skewing occurred in the CD8+ T cell compartment via an increase in IFN-γ production compared with that seen with the macIL-12 adjuvant or immunization with antigen alone (Fig. 3A and B). These results collectively suggest that, while both the macIL-12 and macIL-28B adjuvants drive Th1 skewing, this influence is short lived when induced by macIL-12 but persists for prolonged periods when induced by macIL-28B, as evidenced by the fact that skewing was still observed 3 months after the immunization period had ended only in the macIL-28B group.
FIG. 3.
Analysis of the effects of IL-12 and IL-28B/IFN-λ3 adjuvants on long-lived responses from T cells. (A) Three months after the final vaccination, peripheral CD4+ and CD8+ T cells were again assayed for the production of IL-4 and IFN-γ. (B) Total IFN-γ production from all T cells (top left) and IL-4 production from all responding T cells (top right) are shown. Relative contributions of IFN-γ and IL-4 to the T cell immune response are shown by group (bottom). *, P < 0.05 for intragroup comparison of IL-4 and IFN-γ responses. IFNg, IFN-γ.
Differential skewing of the Th1/Th2 axis in the CD4 and CD8 T cell compartments by IL-12 and IL-28B.
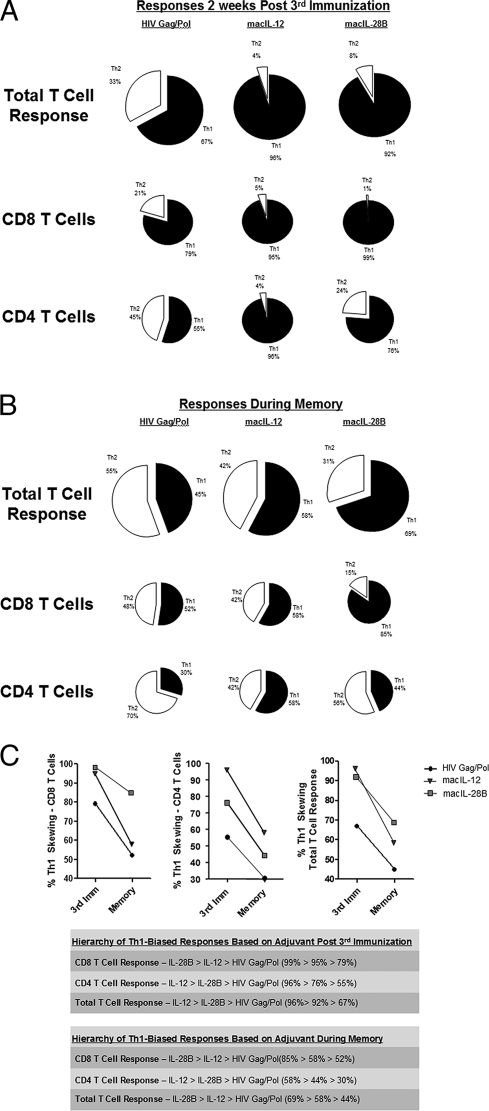
Investigation of the raw percentages of IFN-γ and IL-4 production suggested a differential ability of the macIL-12 and macIL-28B adjuvants to skew antigen-specific immune responses toward the Th1 axis during the short-term and long-term periods postvaccination. In order to have a clearer view of the balance of the Th1/Th2 axis during these periods, we performed umbrella analysis of both T cell subsets individually (CD4+ or CD8+) and taken together (total CD3+) and reported the contributions of IFN-γ production and IL-4 production as percentages of the total Th response (Fig. 4 A and B).
FIG. 4.
Cumulative overview of the ability of the IL-12 and IL-28B/IFN-λ3 adjuvants to affect the Th1/Th2 axis in the short term and long term. (A) Pie charts show relative contributions of Th1 and Th2 cytokines to the total T cell response, as well as broken down into CD8+ and CD4+ T cell responses, 2 weeks after the final immunization. (B) Pie charts show relative contributions of Th1 and Th2 cytokines to the total T cell response, as well as broken down into CD8+ and CD4+ T cell responses, 3 months after the final immunization. (C) Before-and-after plots show the Th1-skewing capabilities of the adjuvants 2 weeks after the final immunization in comparison with the Th1 levels 3 months after the final immunization in CD8+ T cells, CD4+ T cells, and total T cells. Tables display the hierarchies of Th1-biased responses driven by the adjuvants.
Analysis of the total T cell responses 2 weeks after the final vaccination shows a pattern in which both the macIL-12 and macIL-28B adjuvants were able to skew responses toward the Th1 side of the Th1/Th2 axis, as immunization using antigen only induced Th1 responses accounting for 67% of the total Th response, while the addition of macIL-12 or macIL-28B increased this response to 96% or 92%, respectively (Fig. 4A). The ability of macIL-12 and macIL-28B to shift Th responses toward the Th1 side of the axis specifically in CD8+ T cells is evidenced by the fact that these responses account for 95% and 99% of the total response, respectively (Fig. 4A). In the CD4+ T cell subset, the bias induced by macIL-12 was greater than that induced by macIL-28B, as 96% of the total response induced by macIL-12 was dedicated to the Th1 side of the axis, while the Th1 responses driven by macIL-28B accounted for 76% of the total response (Fig. 4A). These results indicate that both adjuvants induced a higher Th1-biased response than immunization alone, driving Th1 responses accounting for 79% of the total Th response in the CD8+ T cell subset and 55% for the CD4+ subset (Fig. 4A).
We next analyzed shifts in the Th1/Th2 axis 3 months after vaccination. Compared with the data reported 2 weeks after vaccination, the trends changed in regard to the influence of adjuvant in the CD4+ and CD8+ T cell subsets, as well as in regard to the total T cell response (Fig. 4B). While vaccination with antigen alone induced a Th1-biased response from antigen-specific T cells 2 weeks after vaccination (Fig. 4A), this trend shifted toward a more balanced Th1/Th2 response during the 3-month follow-up (Fig. 4B). Moreover, while the macIL-12 adjuvant drove a strong Th1-biased response from T cells early after vaccination (96%) (Fig. 4A), this influence seemed to be short lived, as it was not seen 3 months postvaccination (58% of the total response) (Fig. 4B). The macIL-28B adjuvant showed a greater sustained skewing of Th1 responses from antigen-specific T cells than any other vaccination regimen, with 69% of the total T cell response dedicated to the Th1 axis 3 months after the final vaccination. This sustained shift in the Th1/Th2 axis was predominantly due to a continued strong bias toward Th1 cytokine production in CD8+ T cells (85%) (Fig. 4B) compared with that seen for macIL-12 or immunization using antigen alone (58% and 52%, respectively) (Fig. 4B). In regard to responses in the CD4+ compartment, macIL-12 again showed the greatest bias toward the induction of Th1-related cytokine production (58%), but the difference in this bias was not as impressive as the differences seen in the CD8+ compartment. Taken together, these data suggest a profile in which both the macIL-12 and macIL-28B adjuvants induce a Th1 bias that lasts at least 2 weeks after the vaccination period, but the influence of macIL-12 wanes as time progresses, while the Th1-skewing influence of IL-28B can still be observed even 3 months after immunization has ended.
DISCUSSION
While adjuvants are often included in vaccines on the basis of being able to enhance antigen-specific immune responses just after vaccination, the data presented here suggest that, depending on the adjuvant, it is possible that this influence may be short-lived, and long-term analysis of adjuvant activity may be appropriate to ensure complete analysis of adjuvant impact.
Using the macIL-12 and macIL-28B adjuvants, we have been able to characterize two distinct long-term outcomes from adjuvants that seem to exert similar short-term effects on adaptive immune responses from peripheral T cells. Specifically, while the macIL-12 adjuvant skewed responses toward the Th1 axis shortly after the completion of the immunization schedule, this influence was lost when responses were reanalyzed after a 3-month rest period (Fig. 4C). Conversely, the macIL-28B adjuvant was able to drive Th1-biased immune responses seen 2 weeks after the termination of the immunization period that were still active after 3 months (Fig. 4C). While no boost was administered after the 3-month rest period, it is interesting to speculate as to the possible outcomes an additional immunization during this time would have had. Previous studies of IL-12 have suggested that it serves to drive effector responses that are short lived, especially in the context of CD8+ T cell function (13). Moreover, interferons have been shown to drive long-term memorylike responses quite robustly in the CD8 T cell compartment (13). It is interesting, then, to speculate that a boost with the immunization regimen containing the macIL-12 adjuvant after the rest period may have had additional short-term influences on the adaptive immune response, while a boost using the macIL-28B adjuvant may have resulted in an even greater expansion of the persisting long-term responses. Additional experimentation would be needed to verify if these outcomes would, indeed, be the case for boosting after the rest period.
Additionally, our analysis suggests that, while short-term Th1 skewing was achieved using both adjuvants, the mechanisms behind this phenomenon were based on the differential induction of IFN-γ and repression of IL-4 production in different subsets of T cells. The macIL-12 adjuvant achieved this short-term influence through the induction of IFN-γ in the CD4+ T cell compartment, while the macIL-28B adjuvant achieved this through the induction of IFN-γ in the CD8+ T cell compartment, even 3 months after the final immunization (Fig. 4C). Thus, closer examination of how adjuvants skew toward a Th1 or Th2 response may be important if correlates of immunity are known to localize to one T cell subset over another (CD4 versus CD8). The reasons behind the differences in T cell responses are currently unclear but may be related to differential expression of the IL-28 receptor complex on different subsets of macaque T cells. Indeed, comparative lymphocyte phenotyping for the IL-28 receptor complex has not yet been extensively performed in macaques and may be of significant value for studies of immunological function in the nonhuman primate model.
Understanding how adjuvants skew adaptive immunity and whether or not this skewing is maintained in long-term responses may be of critical importance in the development of novel vaccines against pathogens for which there is currently no approved protective vaccine regimen, such as HIV or hepatitis C virus. The data presented here suggest that researchers of vaccine adjuvants may find benefit in conducting additional studies over a prolonged period of time. The hierarchy of adjuvant-induced responses may be different when data are analyzed using filters for different T cell subsets or different times after immunization (Fig. 4C). Therefore, continued multiparametric analysis of immune responses generated by adjuvants is likely necessary to ensure a complete adjuvant profile.
Acknowledgments
This work was supported by NIH/NIAID/DAIDS under HVDDT contract award HHSN272200800063C to Inovio Biomedical Corporation and in part by funding from the NIH to D.B.W. and to M.P.M. from grant T32-AI070099.
We thank Jake Yalley-Ogunro and Matt Collins at BioQual for their help with animal handling and immunizations.
The laboratory of D.B.W. has grant funding and collaborations or consulting, including serving on scientific review committees for commercial entities, and in the interest of disclosure, therefore notes potential conflicts associated with this work with Pfizer, Bristol Myers Squibb, VIRxSYS, Ichor, Inovio, Merck, Althea, Aldevron, and possibly others. The remaining authors declare no competing financial interests.
Footnotes
Published ahead of print on 4 August 2010.
REFERENCES
- 1.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. U. S. A. 95:1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer, J. D., T. M. Robinson, M. A. Kutzler, R. Parkinson, S. A. Calarota, M. K. Sidhu, K. Muthumani, M. Lewis, G. Pavlakis, B. Felber, and D. Weiner. 2005. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J. Med. Primatol. 34:262-270. [DOI] [PubMed] [Google Scholar]
- 3.Chong, S. Y., M. A. Egan, M. A. Kutzler, S. Megati, A. Masood, V. Roopchard, D. Garcia-Hand, D. C. Montefiori, J. Quiroz, M. Rosati, E. B. Schadeck, J. D. Boyer, G. N. Pavlakis, D. B. Weiner, M. Sidhu, J. H. Eldridge, and Z. R. Israel. 2007. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine 25:4967-4982. [DOI] [PubMed] [Google Scholar]
- 4.Dubie, R. A., S. Maksaereekul, B. L. Shacklett, D. Lemongello, K. S. Cole, F. Villinger, S. A. Blozis, P. A. Luciw, and E. E. Sparger. 2009. Co-immunization with IL-15 enhances cellular immune responses induced by a vif-deleted simian immunodeficiency virus proviral DNA vaccine and confers partial protection against vaginal challenge with SIVmac251. Virology 386:109-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faries, M. B., E. C. Hsueh, X. Ye, M. Hoban, and D. L. Morton. 2009. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin. Cancer Res. 15:7029-7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenland, J. R., and N. L. Letvin. 2007. Chemical adjuvants for plasmid DNA vaccines. Vaccine 25:3731-3741. [DOI] [PubMed] [Google Scholar]
- 7.Hirao, L. A., L. Wu, A. S. Khan, D. A. Hokey, J. Yan, A. Dai, M. R. Betts, R. Draghia-Akli, and D. B. Weiner. 2008. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine 26:3112-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, J. J., H. C. Maguire, Jr., L. K. Nottingham, L. D. Morrison, A. Tsai, J. I. Sin, A. A. Chalian, and D. B. Weiner. 1998. Coadministration of IL-12 or IL-10 expression cassettes drives immune responses toward a Th1 phenotype. J. Interferon Cytokine Res. 18:537-547. [DOI] [PubMed] [Google Scholar]
- 9.Kraynyak, K. A., M. A. Kutzler, N. J. Cisper, D. J. Laddy, M. P. Morrow, T. A. Waldmann, and D. B. Weiner. 2009. Plasmid-encoded interleukin-15 receptor alpha enhances specific immune responses induced by a DNA vaccine in vivo. Hum. Gene Ther. 20:1143-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrow, M. P., P. Pankhong, D. J. Laddy, K. A. Schoenly, J. Yan, N. Cisper, and D. B. Weiner. 2009. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood 113:5868-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrow, M. P., and D. B. Weiner. 2008. Cytokines as adjuvants for improving anti-HIV responses. AIDS 22:333-338. [DOI] [PubMed] [Google Scholar]
- 12.Morrow, M. P., J. Yan, P. Pankhong, D. J. Shedlock, M. G. Lewis, K. Talbott, R. Toporovski, A. S. Khan, N. Y. Sardesai, and D. B. Weiner. 22 June 2010. IL-28B/IFN-lambda3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Mol. Ther. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 13.Ramos, H. J., A. M. Davis, A. G. Cole, J. D. Schatzle, J. Forman, and J. D. Farrar. 2009. Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates. Blood 113:5516-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schadeck, E. B., M. Sidhu, M. A. Egan, S. Y. Chong, P. Piacente, A. Masood, D. Garcia-Hand, S. Cappello, V. Roopchand, S. Megati, J. Quiroz, J. D. Boyer, B. K. Felber, G. N. Pavlakis, D. B. Weiner, J. H. Eldridge, and Z. R. Israel. 2006. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine 24:4677-4687. [DOI] [PubMed] [Google Scholar]
- 15.Serre, K., E. Mohr, F. Gaspal, P. J. Lane, R. Bird, A. F. Cunningham, and I. C. Maclennan. 13 April 2010. IL-4 directs both CD4 and CD8 T cells to produce Th2 cytokines in vitro, but only CD4 T cells produce these cytokines in response to alum-precipitated protein in vivo. Mol. Immunol. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 16.Sin, J. I., J. J. Kim, R. L. Arnold, K. E. Shroff, D. McCallus, C. Pachuk, S. P. McElhiney, M. W. Wolf, S. J. Pompa-de Bruin, T. J. Higgins, R. B. Ciccarelli, and D. B. Weiner. 1999. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J. Immunol. 162:2912-2921. [PubMed] [Google Scholar]