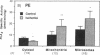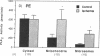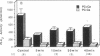Abstract
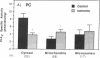
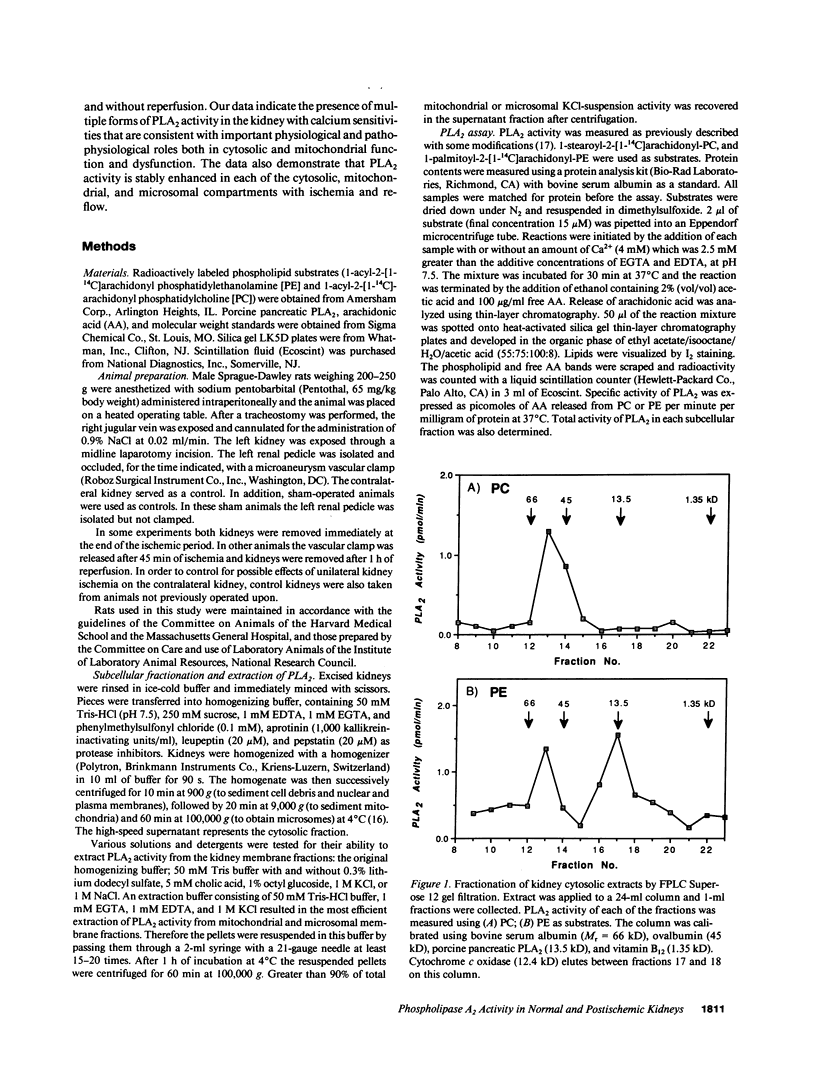
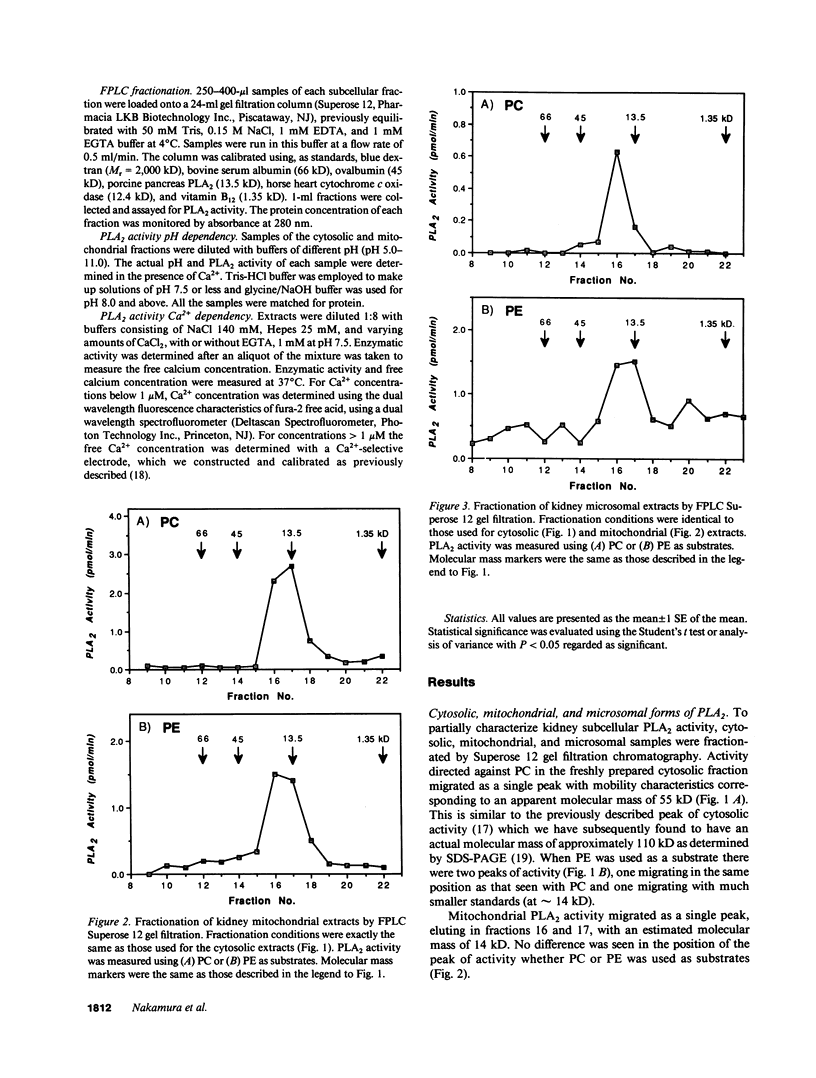
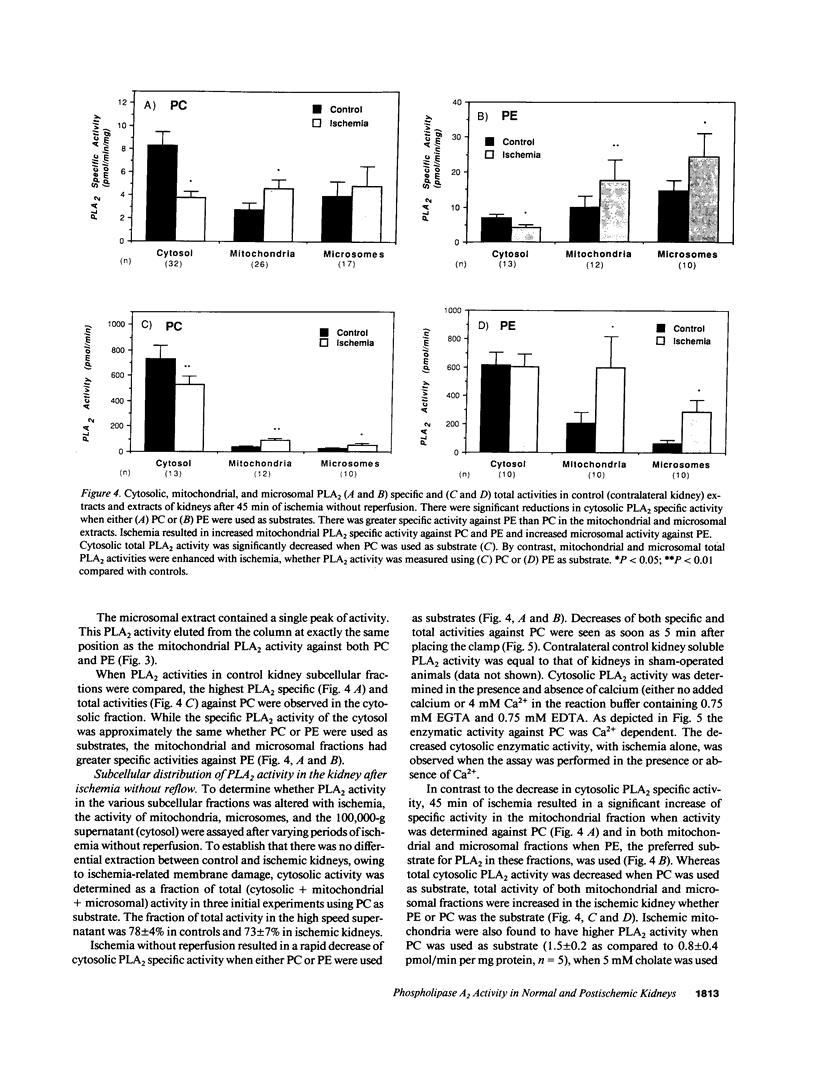
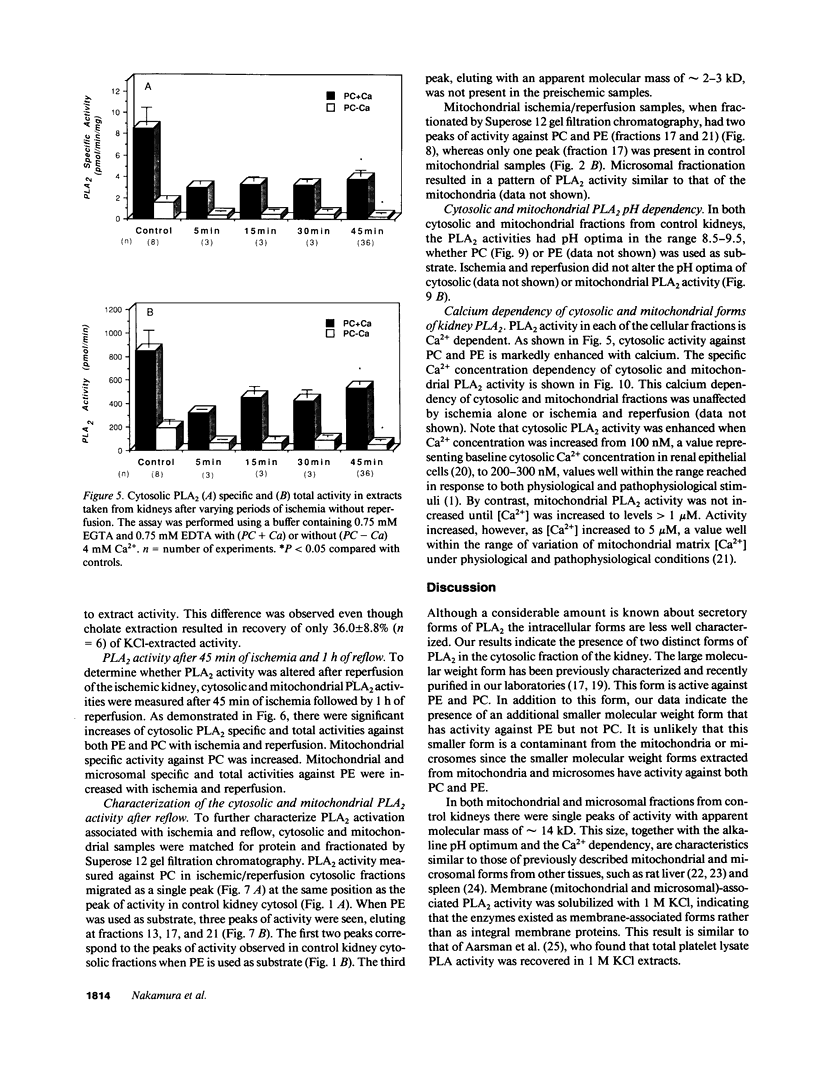
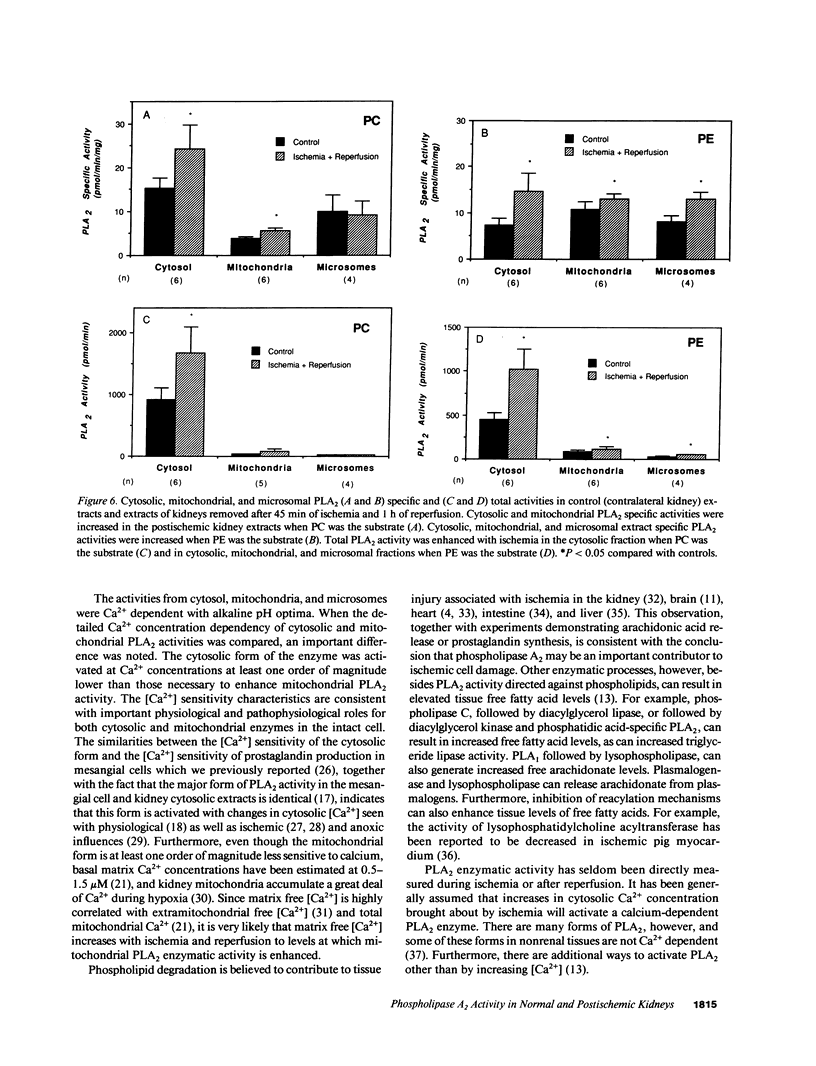
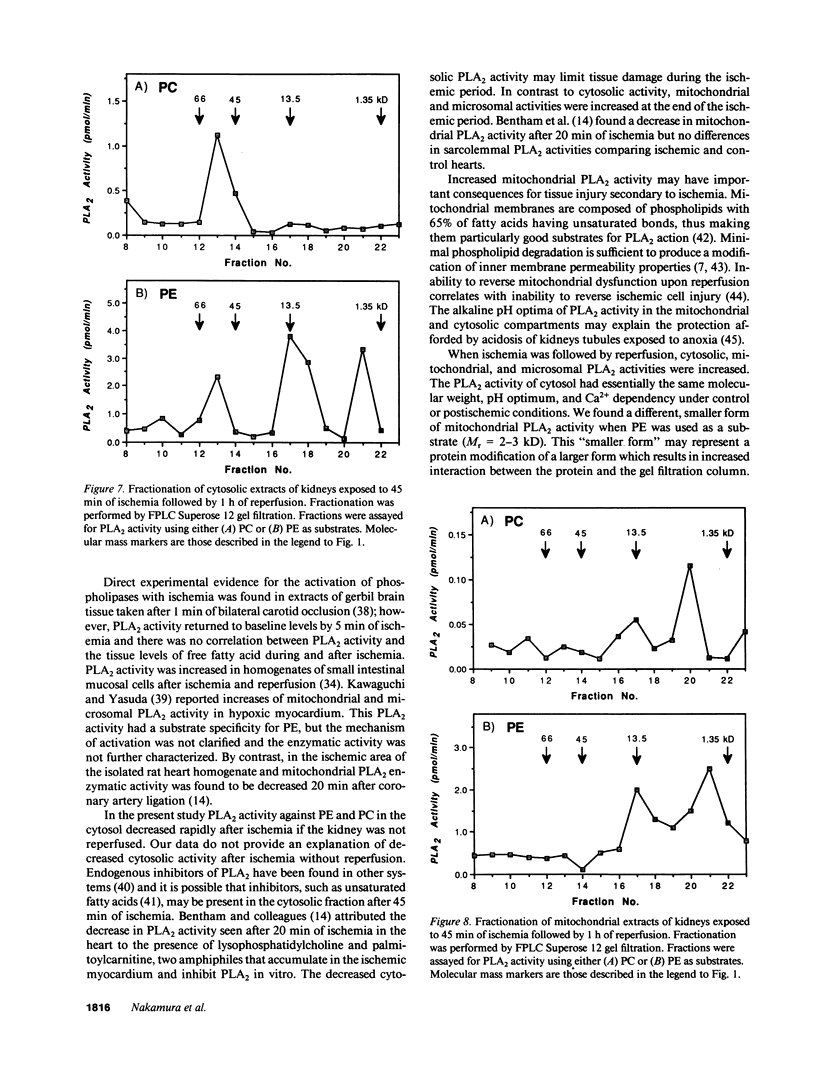
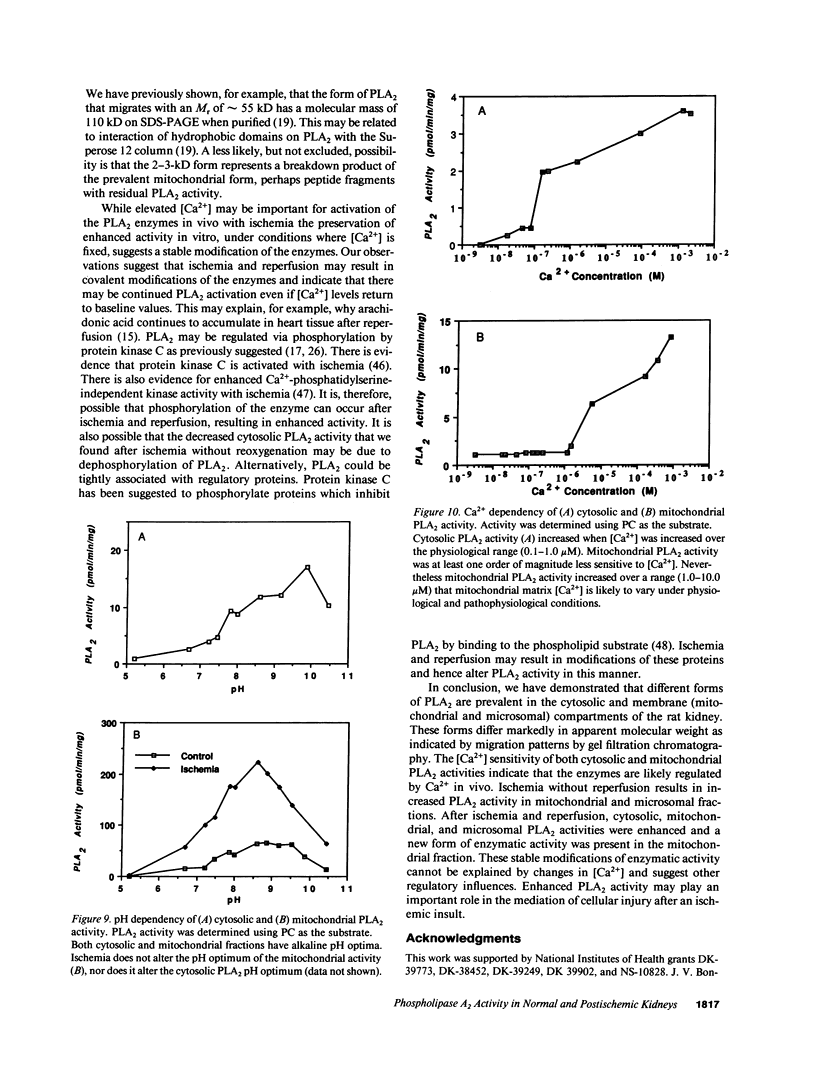
Phospholipase A2 (PLA2) activities in cytosolic, mitochondrial, and microsomal fractions of rat kidneys were characterized under control conditions, after ischemia, and subsequent to ischemia and reperfusion. Two forms of PLA2 activity were present in the cytosolic fraction: a high molecular weight form, active against phosphatidylcholine (PC), and phosphatidylethanolamine (PE), which upon purification has a molecular mass of 110 kD; and smaller form (Mr approximately 14 kD), active against PE. In mitochondrial and microsomal fractions a single form (Mr approximately 14 kD), active against both PC and PE, was dominant. Activities in each fraction were optimal at pH 8.5-9.5. Cytosolic PLA2 activity was enhanced when Ca2+ concentration [( Ca2+]) was increased over the range of 10(-7) to 10(-6) M. Mitochondrial PLA2 activity required higher [Ca2+] for activation (greater than 10(-6) M). After 45 min of ischemia cytosolic PLA2 activity was decreased, whereas mitochondrial and microsomal activities were increased. When ischemia was followed by 1 h of reperfusion, cytosolic, mitochondrial, and microsomal activities were enhanced. Ischemia alone did not change the gel filtration chromatography patterns of PLA2 activity, but ischemia and reperfusion resulted in the appearance of a new peak of activity in cytosolic and mitochondrial fractions (Mr approximately 2-3 kD). Thus, the rat kidney has multiple forms of PLA2 activity, likely representing distinct enzymes, with Ca2+ dependencies suggesting regulation by Ca2+ in vivo. Ischemia and reperfusion result in stable increases of PLA2 activity in each subcellular fraction, perhaps related to covalent modifications of PLA2's, which likely account for membrane phospholipid degradation, and increased tissue levels of unsaturated free fatty acids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarsman A. J., Leunissen-Bijvelt J., Van den Koedijk C. D., Neys F. W., Verkleij A. J., Van den Bosch H. Phospholipase A2 activity in platelets. Immuno-purification and localization of the enzyme in rat platelets. J Lipid Mediat. 1989 Jan-Feb;1(1):49–61. [PubMed] [Google Scholar]
- Aarsman A. J., de Jong J. G., Arnoldussen E., Neys F. W., van Wassenaar P. D., Van den Bosch H. Immunoaffinity purification, partial sequence, and subcellular localization of rat liver phospholipase A2. J Biol Chem. 1989 Jun 15;264(17):10008–10014. [PubMed] [Google Scholar]
- Ballou L. R., Cheung W. Y. Inhibition of human platelet phospholipase A2 activity by unsaturated fatty acids. Proc Natl Acad Sci U S A. 1985 Jan;82(2):371–375. doi: 10.1073/pnas.82.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou L. R., Cheung W. Y. Marked increase of human platelet phospholipase A2 activity in vitro and demonstration of an endogenous inhibitor. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5203–5207. doi: 10.1073/pnas.80.17.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazán N. G., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970 Oct 6;218(1):1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- Bentham J. M., Higgins A. J., Woodward B. The effects of ischaemia, lysophosphatidylcholine and palmitoylcarnitine on rat heart phospholipase A2 activity. Basic Res Cardiol. 1987;82 (Suppl 1):127–135. doi: 10.1007/978-3-662-08390-1_16. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Cheung J. Y. Cytosolic free calcium concentration in cultured renal epithelial cells. Am J Physiol. 1986 Feb;250(2 Pt 2):F329–F338. doi: 10.1152/ajprenal.1986.250.2.F329. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Cheung J. Y. Effects of metabolic acidosis on viability of cells exposed to anoxia. Am J Physiol. 1985 Jul;249(1 Pt 1):C149–C159. doi: 10.1152/ajpcell.1985.249.1.C149. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Nemenoff R. Renal tubular arachidonic acid metabolism. Kidney Int. 1991 Mar;39(3):438–449. doi: 10.1038/ki.1991.55. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Skorecki K. L., Kreisberg J. I., Cheung J. Y. Vasopressin increases cytosolic free calcium concentration in glomerular mesangial cells. Am J Physiol. 1986 Jul;251(1 Pt 2):F94–102. doi: 10.1152/ajprenal.1986.251.1.F94. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest. 1988 Jul;82(1):168–176. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiariello M., Ambrosio G., Cappelli-Bigazzi M., Nevola E., Perrone-Filardi P., Marone G., Condorelli M. Inhibition of ischemia-induced phospholipase activation by quinacrine protects jeopardized myocardium in rats with coronary artery occlusion. J Pharmacol Exp Ther. 1987 May;241(2):560–568. [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Edgar A. D., Strosznajder J., Horrocks L. A. Activation of ethanolamine phospholipase A2 in Brain during ischemia. J Neurochem. 1982 Oct;39(4):1111–1116. doi: 10.1111/j.1471-4159.1982.tb11503.x. [DOI] [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Identification and characterization of a hormonally regulated form of phospholipase A2 in rat renal mesangial cells. J Biol Chem. 1988 Nov 15;263(32):16645–16651. [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Purification of a high-molecular-mass form of phospholipase A2 from rat kidney activated at physiological calcium concentrations. Biochem J. 1990 Oct 1;271(1):37–43. doi: 10.1042/bj2710037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansford R. G., Castro F. Intramitochondrial and extramitochondrial free calcium ion concentrations of suspensions of heart mitochondria with very low, plausibly physiological, contents of total calcium. J Bioenerg Biomembr. 1982 Dec;14(5-6):361–376. doi: 10.1007/BF00743064. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H., Yasuda H. Increased phospholipase A2 activity in the kidney of spontaneously hypertensive rats. Arch Biochem Biophys. 1986 Jul;248(1):401–407. doi: 10.1016/0003-9861(86)90436-4. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H., Yasuda H. Prostacyclin biosynthesis and phospholipase activity in hypoxic rat myocardium. Circ Res. 1988 Jun;62(6):1175–1181. doi: 10.1161/01.res.62.6.1175. [DOI] [PubMed] [Google Scholar]
- Louis J. C., Magal E., Yavin E. Protein kinase C alterations in the fetal rat brain after global ischemia. J Biol Chem. 1988 Dec 25;263(36):19282–19285. [PubMed] [Google Scholar]
- Malis C. D., Bonventre J. V. Mechanism of calcium potentiation of oxygen free radical injury to renal mitochondria. A model for post-ischemic and toxic mitochondrial damage. J Biol Chem. 1986 Oct 25;261(30):14201–14208. [PubMed] [Google Scholar]
- Malis C. D., Weber P. C., Leaf A., Bonventre J. V. Incorporation of marine lipids into mitochondrial membranes increases susceptibility to damage by calcium and reactive oxygen species: evidence for enhanced activation of phospholipase A2 in mitochondria enriched with n-3 fatty acids. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8845–8849. doi: 10.1073/pnas.87.22.8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel L. J., Takano T., Soltoff S. P., Jacobs W. R., LeFurgey A., Ingram P. Multiple roles of calcium in anoxic-induced injury in renal proximal tubules. Soc Gen Physiol Ser. 1987;42:277–285. [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Kusuoka H., Porterfield J. K., Yue D. T., Chacko V. P. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts. Proc Natl Acad Sci U S A. 1987 Aug;84(16):6005–6009. doi: 10.1073/pnas.84.16.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys E., Patel Y., Kreisberg J., Stewart J. H., Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney Int. 1984 Aug;26(2):153–161. doi: 10.1038/ki.1984.149. [DOI] [PubMed] [Google Scholar]
- Nguyen V. D., Cieslinski D. A., Humes H. D. Importance of adenosine triphosphate in phospholipase A2-induced rabbit renal proximal tubule cell injury. J Clin Invest. 1988 Sep;82(3):1098–1105. doi: 10.1172/JCI113666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Shibata H., Koseki M., Nakao K., Kawashima Y., Yoshida Y., Tagawa K. Peroxidative injury of the mitochondrial respiratory chain during reperfusion of hypothermic rat liver. Biochim Biophys Acta. 1987 Jan 16;890(1):82–88. doi: 10.1016/0005-2728(87)90071-5. [DOI] [PubMed] [Google Scholar]
- Okayasu T., Curtis M. T., Farber J. L. Structural alterations of the inner mitochondrial membrane in ischemic liver cell injury. Arch Biochem Biophys. 1985 Feb 1;236(2):638–645. doi: 10.1016/0003-9861(85)90668-x. [DOI] [PubMed] [Google Scholar]
- Ono T., Tojo H., Kuramitsu S., Kagamiyama H., Okamoto M. Purification and characterization of a membrane-associated phospholipase A2 from rat spleen. Its comparison with a cytosolic phospholipase A2 S-1. J Biol Chem. 1988 Apr 25;263(12):5732–5738. [PubMed] [Google Scholar]
- Onodera H., Araki T., Kogure K. Protein kinase C activity in the rat hippocampus after forebrain ischemia: autoradiographic analysis by [3H]phorbol 12,13-dibutyrate. Brain Res. 1989 Feb 27;481(1):1–7. doi: 10.1016/0006-8993(89)90478-2. [DOI] [PubMed] [Google Scholar]
- Otamiri T., Franzén L., Lindmark D., Tagesson C. Increased phospholipase A2 and decreased lysophospholipase activity in the small intestinal mucosa after ischaemia and revascularisation. Gut. 1987 Nov;28(11):1445–1453. doi: 10.1136/gut.28.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otamiri T., Tagesson C. Role of phospholipase A2 and oxygenated free radicals in mucosal damage after small intestinal ischemia and reperfusion. Am J Surg. 1989 Jun;157(6):562–566. doi: 10.1016/0002-9610(89)90699-5. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Schmid P. C., Beatrice M. C., Schmid H. H. Intramitochondrial phospholipase activity and the effects of Ca2+ plus N-ethylmaleimide on mitochondrial function. J Biol Chem. 1979 Nov 25;254(22):11485–11494. [PubMed] [Google Scholar]
- Saxon M. E., Filippov A. K., Porotikov U. I. The possible role of phospholipase A2 in cardiac membrane destabilization under calcium overload conditions. Basic Res Cardiol. 1984 Nov-Dec;79(6):668–678. doi: 10.1007/BF01908384. [DOI] [PubMed] [Google Scholar]
- Sen A., Miller J. C., Reynolds R., Willerson J. T., Buja L. M., Chien K. R. Inhibition of the release of arachidonic acid prevents the development of sarcolemmal membrane defects in cultured rat myocardial cells during adenosine triphosphate depletion. J Clin Invest. 1988 Oct;82(4):1333–1338. doi: 10.1172/JCI113735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanian A., Kim E. Phospholipase A2 dependent release of fatty acids from peroxidized membranes. J Free Radic Biol Med. 1985;1(4):263–271. doi: 10.1016/0748-5514(85)90130-8. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Collan Y., Kahng M. W., Trump B. F. Changes in mitochondrial lipids of rat kidney during ischemia. Biochim Biophys Acta. 1980 May 28;618(2):192–201. doi: 10.1016/0005-2760(80)90025-9. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Borle A. B. Effects of low extracellular sodium on cytosolic ionized calcium. Na+-Ca2+ exchange as a major calcium influx pathway in kidney cells. J Biol Chem. 1985 Dec 5;260(28):14998–14507. [PubMed] [Google Scholar]
- Spencer T. L., See J. K., Bygrave F. L. Translocation and binding of adenine nucleotides by rat liver mitochondria partially depleted of phospholipids. Biochim Biophys Acta. 1976 Mar 12;423(3):365–373. doi: 10.1016/0005-2728(76)90193-6. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Murphy E., Levy L., London R. E. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987 May;60(5):700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- Trump B. F., Mergner W. J., Kahng M. W., Salandino A. J. Studies on the subcellular pathophysiology of ischemia. Circulation. 1976 Mar;53(3 Suppl):I17–I26. [PubMed] [Google Scholar]
- Wan B., LaNoue K. F., Cheung J. Y., Scaduto R. C., Jr Regulation of citric acid cycle by calcium. J Biol Chem. 1989 Aug 15;264(23):13430–13439. [PubMed] [Google Scholar]