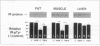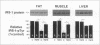Abstract
Insulin resistance is an important metabolic abnormality often associated with infections, cancer, obesity, and especially non-insulin-dependent diabetes mellitus (NIDDM). We have previously demonstrated that tumor necrosis factor-alpha produced by adipose tissue is a key mediator of insulin resistance in animal models of obesity-diabetes. However, the mechanism by which TNF-alpha interferes with insulin action is not known. Since a defective insulin receptor (IR) tyrosine kinase activity has been observed in obesity and NIDDM, we measured the IR tyrosine kinase activity in the Zucker (fa/fa) rat model of obesity and insulin resistance after neutralizing TNF-alpha with a soluble TNF receptor (TNFR)-lgG fusion protein. This neutralization resulted in a marked increase in insulin-stimulated autophosphorylation of the IR, as well as phosphorylation of insulin receptor substrate 1 (IRS-1) in muscle and fat tissues of the fa/fa rats, restoring them to near control (lean) levels. In contrast, no significant changes were observed in insulin-stimulated tyrosine phosphorylations of IR and IRS-1 in liver. The physiological significance of the improvements in IR signaling was indicated by a concurrent reduction in plasma glucose, insulin, and free fatty acid levels. These results demonstrate that TNF-alpha participates in obesity-related systemic insulin resistance by inhibiting the IR tyrosine kinase in the two tissues mainly responsible for insulin-stimulated glucose uptake: muscle and fat.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Copeland G. P., Leinster S. J., Davis J. C., Hipkin L. J. Insulin resistance in patients with colorectal cancer. Br J Surg. 1987 Nov;74(11):1031–1035. doi: 10.1002/bjs.1800741124. [DOI] [PubMed] [Google Scholar]
- Cornelius P., Lee M. D., Marlowe M., Pekala P. H. Monokine regulation of glucose transporter mRNA in L6 myotubes. Biochem Biophys Res Commun. 1989 Nov 30;165(1):429–436. doi: 10.1016/0006-291x(89)91088-7. [DOI] [PubMed] [Google Scholar]
- Feinstein R., Kanety H., Papa M. Z., Lunenfeld B., Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993 Dec 15;268(35):26055–26058. [PubMed] [Google Scholar]
- Folli F., Saad M. J., Backer J. M., Kahn C. R. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J Biol Chem. 1992 Nov 5;267(31):22171–22177. [PubMed] [Google Scholar]
- Garvey W. T., Maianu L., Huecksteadt T. P., Birnbaum M. J., Molina J. M., Ciaraldi T. P. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991 Mar;87(3):1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C., Feingold K. R. Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med. 1992 Jul 30;327(5):329–337. doi: 10.1056/NEJM199207303270506. [DOI] [PubMed] [Google Scholar]
- Heydrick S. J., Jullien D., Gautier N., Tanti J. F., Giorgetti S., Van Obberghen E., Le Marchand-Brustel Y. Defect in skeletal muscle phosphatidylinositol-3-kinase in obese insulin-resistant mice. J Clin Invest. 1993 Apr;91(4):1358–1366. doi: 10.1172/JCI116337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C., Lorenz K., Braithwaite S. S., Colca J. R., Palazuk B. J., Hotamisligil G. S., Spiegelman B. M. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994 Jan;134(1):264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. S., Murray D. L., Choy L. N., Spiegelman B. M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993 Jan 1;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang C. H., Dobrescu C., Bagby G. J. Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992 Jan;130(1):43–52. doi: 10.1210/endo.130.1.1727716. [DOI] [PubMed] [Google Scholar]
- Moller D. E., Flier J. S. Insulin resistance--mechanisms, syndromes, and implications. N Engl J Med. 1991 Sep 26;325(13):938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Jr, Backer J. M., Sun X. J., Shoelson S., Hu P., Schlessinger J., Yoakim M., Schaffhausen B., White M. F. IRS-1 activates phosphatidylinositol 3'-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10350–10354. doi: 10.1073/pnas.89.21.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O., Kahn C. R., Kahn B. B. Divergent regulation of the Glut 1 and Glut 4 glucose transporters in isolated adipocytes from Zucker rats. J Clin Invest. 1992 Jun;89(6):1964–1973. doi: 10.1172/JCI115804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M. J., Araki E., Miralpeix M., Rothenberg P. L., White M. F., Kahn C. R. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992 Nov;90(5):1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Hotamisligil G. S. Through thick and thin: wasting, obesity, and TNF alpha. Cell. 1993 May 21;73(4):625–627. doi: 10.1016/0092-8674(93)90243-j. [DOI] [PubMed] [Google Scholar]
- Stephens J. M., Pekala P. H. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem. 1991 Nov 15;266(32):21839–21845. [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Tayek J. A. A review of cancer cachexia and abnormal glucose metabolism in humans with cancer. J Am Coll Nutr. 1992 Aug;11(4):445–456. doi: 10.1080/07315724.1992.10718249. [DOI] [PubMed] [Google Scholar]
- Thies R. S., Molina J. M., Ciaraldi T. P., Freidenberg G. R., Olefsky J. M. Insulin-receptor autophosphorylation and endogenous substrate phosphorylation in human adipocytes from control, obese, and NIDDM subjects. Diabetes. 1990 Feb;39(2):250–259. doi: 10.2337/diab.39.2.250. [DOI] [PubMed] [Google Scholar]
- Wang L. M., Myers M. G., Jr, Sun X. J., Aaronson S. A., White M., Pierce J. H. IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993 Sep 17;261(5128):1591–1594. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Wilden P. A., Siddle K., Haring E., Backer J. M., White M. F., Kahn C. R. The role of insulin receptor kinase domain autophosphorylation in receptor-mediated activities. Analysis with insulin and anti-receptor antibodies. J Biol Chem. 1992 Jul 5;267(19):13719–13727. [PubMed] [Google Scholar]