Abstract
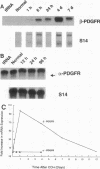
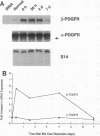
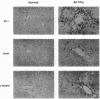
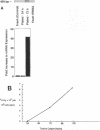
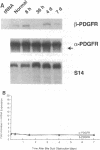
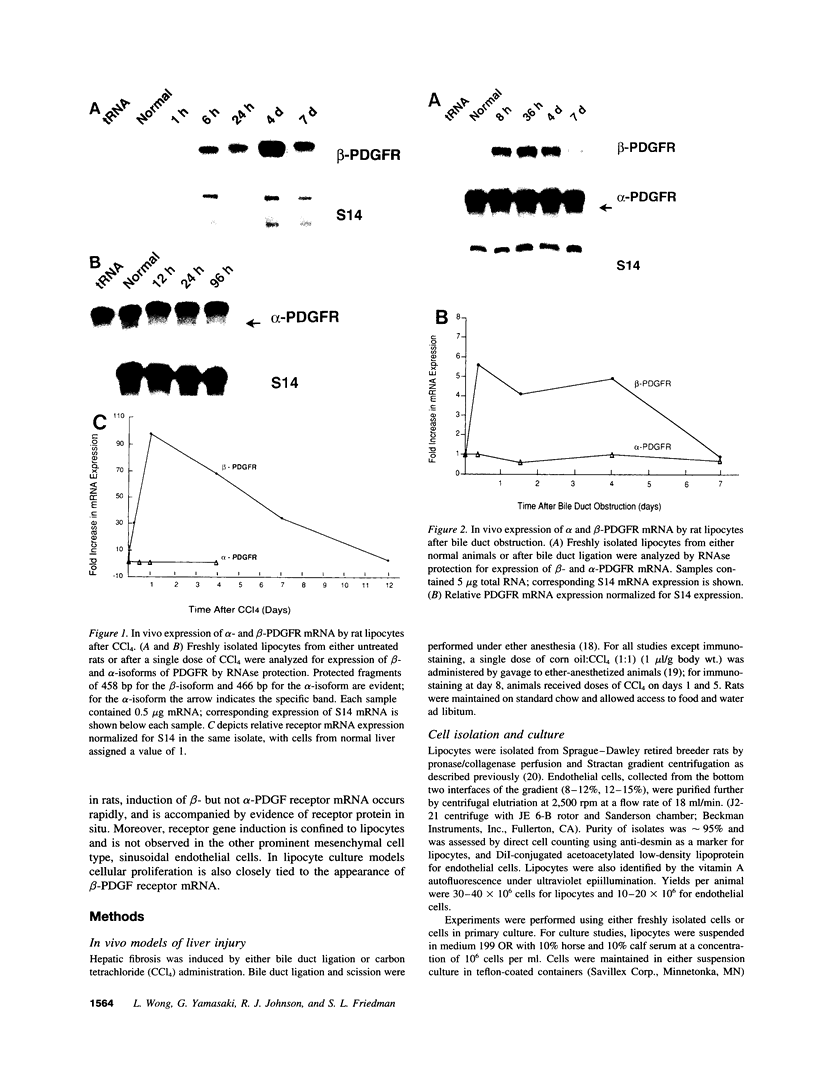
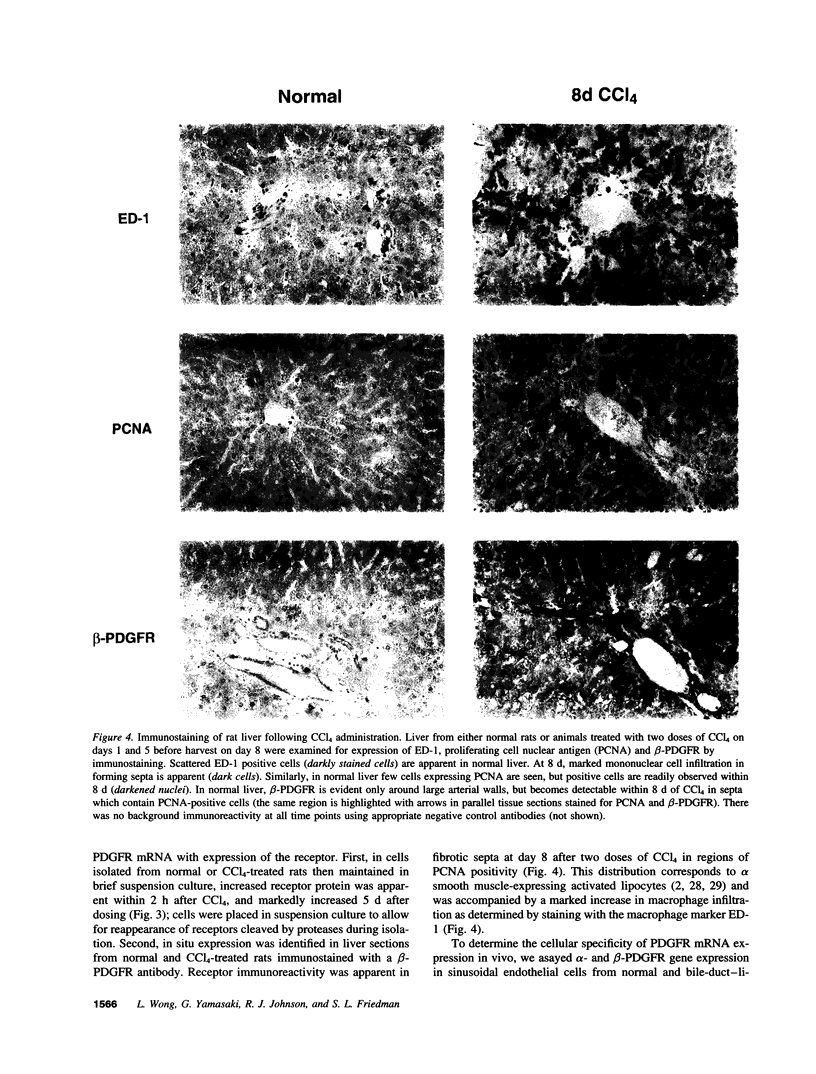
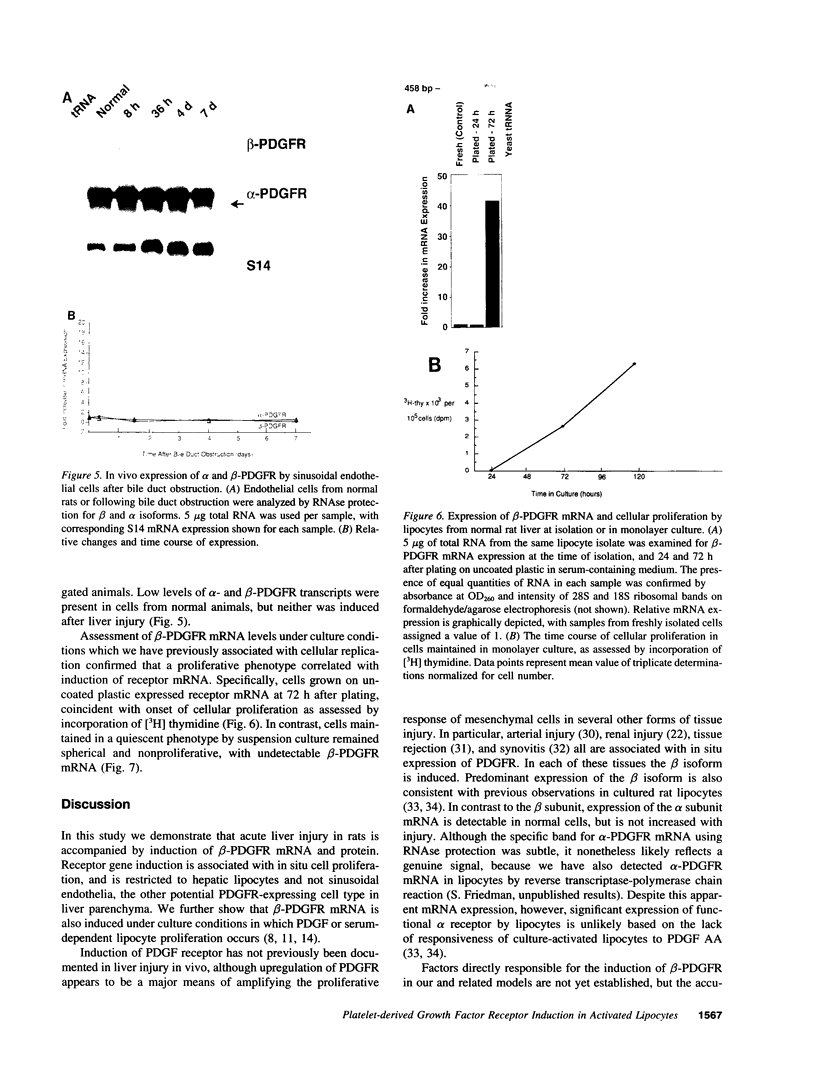
A consistent response to liver injury is the activation of resident mesenchymal cells known as lipocytes (Ito, fat-storing cells) into a proliferating cell type. In cultured lipocytes, platelet-derived growth factor (PDGF) is the most potent proliferative cytokine, but requires the activation-dependent expression of its receptor protein (Friedman, S. L., and M. J. P. Arthur. 1989. J. Clin. Invest. 84:1780-1785); the role of PDGF receptor (PDGFR) in liver injury is unknown. We have examined PDGFR gene expression in freshly isolated lipocytes during liver injury and correlated these findings with a culture model of cellular activation. Whereas lipocytes from normal rats had no detectable transcript for the beta-PDGFR subunit, this mRNA was induced within 1 h after a dose of carbon tetrachloride (CCl4). In contrast, alpha subunit mRNA was detected in normal cells, but was unchanged after liver injury. Similar results were observed in lipocytes from bile duct-obstructed rats, although beta-PDGFR induction was less marked. By immunoblot, induction of beta-PDGFR protein in lipocytes isolated from CCl4-treated animals correlated with mRNA increases. In contrast to lipocytes, endothelial cells from normal liver expressed low levels of alpha- and beta-receptor subunit mRNA, which did not increase with injury. Using a beta-PDGFR antibody, receptor protein could be identified within fibrotic septa in CCl4-treated animals in regions where cells expressed proliferating cell nuclear antigen (PCNA). In cultured lipocytes activated by growth on uncoated plastic, beta-PDGFR transcripts appeared within 3 d after plating, which coincided with the onset of cellular proliferation. In contrast, quiescent cells in suspension culture had no detectable beta-PDGFR mRNA. These results indicate that beta-PDGF receptor induction by lipocytes is an early event during hepatic injury in vivo and in primary culture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachem M. G., Riess U., Gressner A. M. Liver fat storing cell proliferation is stimulated by epidermal growth factor/transforming growth factor alpha and inhibited by transforming growth factor beta. Biochem Biophys Res Commun. 1989 Jul 31;162(2):708–714. doi: 10.1016/0006-291x(89)92368-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis B. H., Rapp U. R., Davidson N. O. Retinoic acid and transforming growth factor beta differentially inhibit platelet-derived-growth-factor-induced Ito-cell activation. Biochem J. 1991 Aug 15;278(Pt 1):43–47. doi: 10.1042/bj2780043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellström B., Dimeny E., Larsson E., Klareskog L., Tufveson G., Rubin K. Importance of PDGF receptor expression in accelerated atherosclerosis-chronic rejection. Transplant Proc. 1989 Aug;21(4):3689–3691. [PubMed] [Google Scholar]
- Fellström B., Klareskog L., Heldin C. H., Larsson E., Rönnstrand L., Terracio L., Tufveson G., Wahlberg J., Rubin K. Platelet-derived growth factor receptors in the kidney--upregulated expression in inflammation. Kidney Int. 1989 Dec;36(6):1099–1102. doi: 10.1038/ki.1989.306. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Arthur M. J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Arenson D. M., Bissell D. M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989 Jun 25;264(18):10756–10762. [PubMed] [Google Scholar]
- Friedman S. L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993 Jun 24;328(25):1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Yamasaki G., Wong L. Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. Enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem. 1994 Apr 8;269(14):10551–10558. [PubMed] [Google Scholar]
- Geerts A., Lazou J. M., De Bleser P., Wisse E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology. 1991 Jun;13(6):1193–1202. [PubMed] [Google Scholar]
- Geerts A., Schellinck P., Bouwens L., Wisse E. Cell population kinetics of Kupffer cells during the onset of fibrosis in rat liver by chronic carbon tetrachloride administration. J Hepatol. 1988 Feb;6(1):50–56. doi: 10.1016/s0168-8278(88)80461-6. [DOI] [PubMed] [Google Scholar]
- Gronwald R. G., Grant F. J., Haldeman B. A., Hart C. E., O'Hara P. J., Hagen F. S., Ross R., Bowen-Pope D. F., Murray M. J. Cloning and expression of a cDNA coding for the human platelet-derived growth factor receptor: evidence for more than one receptor class. Proc Natl Acad Sci U S A. 1988 May;85(10):3435–3439. doi: 10.1073/pnas.85.10.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy P., Bishayee S., Disa S., Scher C. D. Modulation of platelet-derived growth factor receptor function in BP3T3, a chemically transformed BALB/c-3T3 cell line. Cancer Res. 1989 Jul 1;49(13):3581–3586. [PubMed] [Google Scholar]
- Heldin P., Pertoft H., Nordlinder H., Heldin C. H., Laurent T. C. Differential expression of platelet-derived growth factor alpha- and beta- receptors on fat-storing cells and endothelial cells of rat liver. Exp Cell Res. 1991 Apr;193(2):364–369. doi: 10.1016/0014-4827(91)90108-7. [DOI] [PubMed] [Google Scholar]
- Hendriks H. F., Verhoofstad W. A., Brouwer A., de Leeuw A. M., Knook D. L. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985 Sep;160(1):138–149. doi: 10.1016/0014-4827(85)90243-5. [DOI] [PubMed] [Google Scholar]
- Hines J. E., Johnson S. J., Burt A. D. In vivo responses of macrophages and perisinusoidal cells to cholestatic liver injury. Am J Pathol. 1993 Feb;142(2):511–518. [PMC free article] [PubMed] [Google Scholar]
- Horn T., Junge J., Christoffersen P. Early alcoholic liver injury: changes of the Disse space in acinar zone 3. Liver. 1985 Dec;5(6):301–310. doi: 10.1111/j.1600-0676.1985.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Iida H., Seifert R., Alpers C. E., Gronwald R. G., Phillips P. E., Pritzl P., Gordon K., Gown A. M., Ross R., Bowen-Pope D. F. Platelet-derived growth factor (PDGF) and PDGF receptor are induced in mesangial proliferative nephritis in the rat. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6560–6564. doi: 10.1073/pnas.88.15.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. J., Hines J. E., Burt A. D. Macrophage and perisinusoidal cell kinetics in acute liver injury. J Pathol. 1992 Apr;166(4):351–358. doi: 10.1002/path.1711660406. [DOI] [PubMed] [Google Scholar]
- Kountouras J., Billing B. H., Scheuer P. J. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984 Jun;65(3):305–311. [PMC free article] [PubMed] [Google Scholar]
- Kumar R. K., Bennett R. A., Brody A. R. A homologue of platelet-derived growth factor produced by rat alveolar macrophages. FASEB J. 1988 Apr;2(7):2272–2277. doi: 10.1096/fasebj.2.7.3280379. [DOI] [PubMed] [Google Scholar]
- Lee K. H., Bowen-Pope D. F., Reed R. R. Isolation and characterization of the alpha platelet-derived growth factor receptor from rat olfactory epithelium. Mol Cell Biol. 1990 May;10(5):2237–2246. doi: 10.1128/mcb.10.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka J., Grotendorst G. R. Two peptides related to platelet-derived growth factor are present in human wound fluid. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4416–4420. doi: 10.1073/pnas.86.12.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Pham N. T., Tsukamoto H. Differential effects of interleukin-1 alpha, tumor necrosis factor alpha, and transforming growth factor beta 1 on cell proliferation and collagen formation by cultured fat-storing cells. Liver. 1989 Apr;9(2):71–78. doi: 10.1111/j.1600-0676.1989.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Minato Y., Hasumura Y., Takeuchi J. The role of fat-storing cells in Disse space fibrogenesis in alcoholic liver disease. Hepatology. 1983 Jul-Aug;3(4):559–566. doi: 10.1002/hep.1840030414. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Gesualdo L., Sabbah G. M., Abboud H. E. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989 Dec;84(6):1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M., Knauss T. C., Pierce G. F., Hsieh P., Kenney W., Dubyak G. R., Abboud H. E. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol. 1991 Mar;260(3 Pt 1):C485–C491. doi: 10.1152/ajpcell.1991.260.3.C485. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Milani S., Grappone C., Weber F. L., Jr, Gentilini P., Abboud H. E. Expression of platelet-derived growth factor in a model of acute liver injury. Hepatology. 1994 Mar;19(3):701–707. doi: 10.1002/hep.1840190323. [DOI] [PubMed] [Google Scholar]
- Proctor E., Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982 Dec;83(6):1183–1190. [PubMed] [Google Scholar]
- Ramadori G., Veit T., Schwögler S., Dienes H. P., Knittel T., Rieder H., Meyer zum Büschenfelde K. H. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(6):349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- Reuterdahl C., Sundberg C., Rubin K., Funa K., Gerdin B. Tissue localization of beta receptors for platelet-derived growth factor and platelet-derived growth factor B chain during wound repair in humans. J Clin Invest. 1993 May;91(5):2065–2075. doi: 10.1172/JCI116429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. D., Dixit A., Roufa D. J. Primary structure of human ribosomal protein S14 and the gene that encodes it. Mol Cell Biol. 1986 Aug;6(8):2774–2783. doi: 10.1128/mcb.6.8.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey D. C., Boyles J. K., Gabbiani G., Friedman S. L. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [PubMed] [Google Scholar]
- Rubin K., Tingström A., Hansson G. K., Larsson E., Rönnstrand L., Klareskog L., Claesson-Welsh L., Heldin C. H., Fellström B., Terracio L. Induction of B-type receptors for platelet-derived growth factor in vascular inflammation: possible implications for development of vascular proliferative lesions. Lancet. 1988 Jun 18;1(8599):1353–1356. doi: 10.1016/s0140-6736(88)92177-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Tanaka Y., Mak K. M., Lieber C. S. Immunohistochemical detection of proliferating lipocytes in regenerating rat liver. J Pathol. 1990 Feb;160(2):129–134. doi: 10.1002/path.1711600206. [DOI] [PubMed] [Google Scholar]
- Terracio L., Rönnstrand L., Tingström A., Rubin K., Claesson-Welsh L., Funa K., Heldin C. H. Induction of platelet-derived growth factor receptor expression in smooth muscle cells and fibroblasts upon tissue culturing. J Cell Biol. 1988 Nov;107(5):1947–1957. doi: 10.1083/jcb.107.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. T., Burk R. F. Carbon tetrachloride hepatotoxicity: an example of free radical-mediated injury. Semin Liver Dis. 1990 Nov;10(4):279–284. doi: 10.1055/s-2008-1040483. [DOI] [PubMed] [Google Scholar]