Abstract
The inhibitor-of-apoptosis (IAP) proteins encoded by baculoviruses bear a striking resemblance to the cellular IAP homologs of their invertebrate hosts. By virtue of the acquired selective advantage of blocking virus-induced apoptosis, baculoviruses may have captured cellular IAP genes that subsequently evolved for virus-specific objectives. To compare viral and host IAPs, we defined antiapoptotic properties of SfIAP, the principal cellular IAP of the lepidopteran host Spodoptera frugiperda. We report here that SfIAP prevented virus-induced apoptosis as well as viral Op-IAP3 (which is encoded by the Orgyia pseudotsugata nucleopolyhedrovirus) when overexpressed from the baculovirus genome. Like Op-IAP3, SfIAP blocked apoptosis at a step prior to caspase activation. Both of the baculovirus IAP repeats (BIRs) were required for SfIAP function. Moreover, deletion of the C-terminal RING motif generated a loss-of-function SfIAP that interacted and dominantly interfered with wild-type SfIAP. Like Op-IAP3, wild-type SfIAP formed intracellular homodimers, suggesting that oligomerization is a functional requirement for both cellular and viral IAPs. SfIAP possesses a ∼100-residue N-terminal leader domain, which is absent among all viral IAPs. Remarkably, deletion of the leader yielded a fully functional SfIAP with dramatically increased protein stability. Thus, the SfIAP leader contains an instability motif that may confer regulatory options for cellular IAPs that baculovirus IAPs have evolved to bypass for maximal stability and antiapoptotic potency. Our findings that SfIAP and viral IAPs have common motifs, share multiple biochemical properties including oligomerization, and act at the same step to block apoptosis support the hypothesis that baculoviral IAPs were derived by acquisition of host insect IAPs.
Apoptosis is a prevalent host cell response to virus infection. Representing an important antivirus defense, apoptotic cell death can limit multiplication and virus dissemination in the host. Thus, the mechanisms by which a host organism detects a viral intruder and initiates the apoptotic response are critical to the outcome of the infection for both the host and virus. The cellular inhibitor-of-apoptosis (IAP) proteins are important candidates for sensing virus infection and determining cell fate by virtue of their central position in the apoptosis pathway (reviewed in references 35, 36, and 44). Affirming their importance in regulation of apoptosis, IAPs are encoded by multiple DNA viruses, including baculoviruses, entomopoxviruses, iridoviruses, and African swine fever virus (reviewed in 3). Nonetheless, the molecular mechanisms by which viral IAPs regulate virus-induced apoptosis and how they biochemically differ from cellular IAPs are poorly understood.
The IAPs were first discovered in baculoviruses because of their capacity to prevent virus-induced apoptosis and thereby facilitate virus multiplication (4, 8). The baculovirus IAPs bear a striking resemblance to the cellular IAPs carried by the host insects that they infect. Cellular IAPs are a highly conserved family of survival factors that regulate developmental and stress-induced apoptosis, as well as inflammation, the cell cycle, and other signaling processes (35, 38, 44). Importantly, misregulation or overexpression of IAPs is associated with neoplasia and tumor chemoresistance (24, 49). The IAPs are defined by the presence of one or more ∼80-residue baculovirus IAP repeat (BIR) domains. The BIRs consist of a conserved Zn2+-coordinating arrangement of Cys and His residues (CCHC) that interact with diverse proteins, including the cysteinyl aspartate-specific proteases called caspases that execute apoptosis (reviewed in 16 and 37). The antiapoptotic activity of some, but not all, IAPs is derived from their ability to bind and neutralize caspases (reviewed in 35 and 44). The BIRs also interact with proapoptotic factors that contain IAP binding motifs (IBMs). IBM-containing factors have the capacity to bind and dissociate the IAP-caspase complex, thereby liberating active caspases to execute apoptosis (16, 35, 36, 48). Many IAPs, including viral IAPs, also possess a C-terminal RING domain, which is a Zn2+-coordinating motif with E3-ubiquitin ligase activity, which can contribute to antiapoptotic activity (48).
The best-studied baculovirus IAP is Op-IAP3, which is encoded by Orgyia pseudotsugata nucleopolyhedrovirus. This small IAP (268 residues) contains two BIRs and a C-terminal RING (Fig. 1A). Both BIRs are required for Op-IAP3 antiapoptotic activity (19, 50, 53). Truncation of the Op-IAP3 RING creates a loss-of-function dominant inhibitor (19). Op-IAP3's capacity to form a complex with this RING-lacking (RINGless) dominant inhibitor and with itself suggests that oligomerization is necessary for IAP function. Upon overexpression, Op-IAP3 blocks apoptosis triggered by diverse signals in cells from certain insects and mammals, suggesting that it acts through a conserved mechanism (7, 11, 15, 33, 51, 54, 56). In the baculovirus host moth Spodoptera frugiperda (Lepidoptera: Noctuidae), Op-IAP3 prevents apoptosis by blocking the activation of effector caspases (25, 32, 40). However, in contrast to host insect IAPs, Op-IAP3 fails to inhibit active caspases (45, 51, 54). Thus, the host cell target(s) and the mechanism by which they are neutralized by this viral IAP remain unclear.
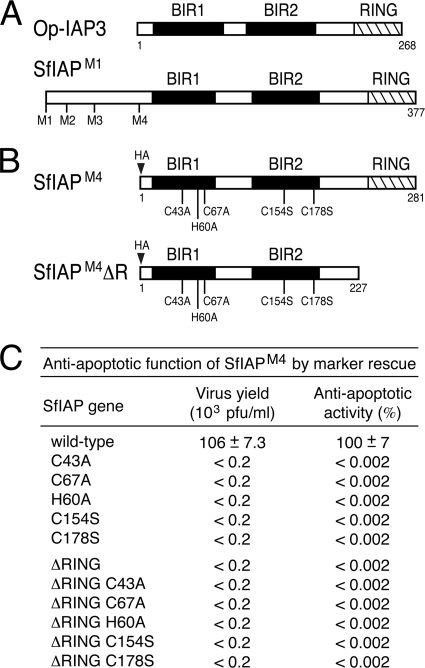
FIG. 1.
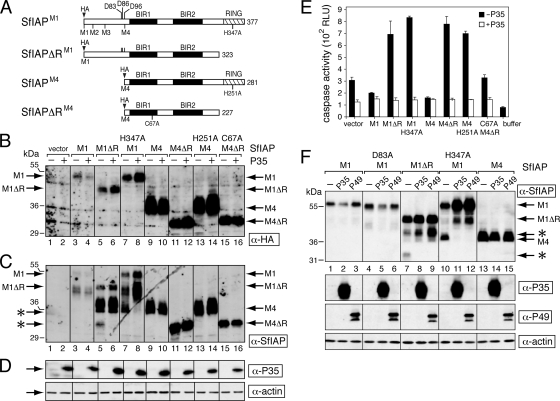
SfIAP structure and mutagenesis. (A) Viral and cellular IAPs. Viral Op-IAP3 (268 residues) and SfIAP (377 residues) each contain two BIR motifs (black boxes) and an E3 ligase RING domain (cross-hatched box). Each representing a potential start site, four methionines (M1 to M4) exist in the N-terminal leader of SfIAP. (B) SfIAPM4 mutations. SfIAPM4 (281 residues) begins with the M4 methionine. SfIAPM4ΔR (227 residues) lacks the C-terminal RING. Amino acid substitutions of Zn-coordinating residues are indicated. An epitope tag (HA) was inserted at the N terminus. (C) Marker rescue assay. The antiapoptotic activity of wild-type or mutated forms of SfIAPM4 was assayed by virus marker rescue in which replication of p35-deficient vΔp35/lacZ was restored in proportion to the antiapoptotic activity of the mutated Sfiap gene acquired by integration of the SfIAP-encoding plasmid (2). Virus yields were determined by plaque assay using apoptosis-sensitive SF21 cells. Antiapoptotic activity is reported as the ratio of nonapoptotic, lacZ-expressing plaques produced by transfection of the indicated Sfiap to those produced by wild-type Sfiap. Values shown are the averages ± standard deviations obtained from triplicate transfections.
Among the cellular IAPs, SfIAP from Spodoptera frugiperda is most closely related to viral Op-IAP3. SfIAP (Fig. 1A) is 42% identical to Op-IAP3, with a higher degree of amino acid identity localized to its two BIRs and C-terminal RING (20). As the principal IAP in Spodoptera, SfIAP suppresses a constitutive push toward apoptosis (34); ablation of SfIAP leads to immediate apoptosis of cultured Spodoptera cells. Upon overexpression, SfIAP also rescues the multiplication of apoptosis-inducing baculoviruses and can prevent apoptosis in certain mammalian cell lines (20, 26). In contrast to viral Op-IAP3, SfIAP can bind and inhibit caspases, including Spodoptera frugiperda caspase-1 (Sf-caspase-1) and human caspase-9 (20, 45). Thus, despite their structural similarities, there exist fundamental differences in the biochemical activities of these two IAPs. Importantly, SfIAP fails to prevent baculovirus-induced apoptosis when produced at endogenous levels in permissive Spodoptera cells. Thus, it is expected that SfIAP also possesses regulatory motifs that respond to cellular signals triggered upon virus infection.
SfIAP provides an unprecedented opportunity to investigate the functional and evolutionary relationships between host and viral IAPs and to test the intriguing hypothesis that viral IAPs were acquired by host gene capture (21). We have investigated the biochemical properties of SfIAP as a means to define its molecular mechanisms and to test its relatedness to viral IAPs. We report here that SfIAP shares many biochemical and functional features with viral IAPs. Like Op-IAP3, overexpressed SfIAP prevented virus-induced apoptosis at a step upstream of caspase activation by a mechanism that required BIR1, BIR2, and the RING. SfIAP formed a complex with itself and with a RINGless dominant inhibitor, suggesting that oligomerization is also required for function of cellular IAPs. Unlike viral IAPs, SfIAP possesses an N-terminal leader, which modulates intracellular SfIAP levels and may respond to apoptotic signals to regulate cell survival. Our data are consistent with a model in which baculoviruses acquired a host cell IAP and modified it for virus-specific needs, thereby increasing virus fitness by preventing virus-induced apoptosis.
MATERIALS AND METHODS
Cell lines and viruses.
Spodoptera frugiperda IPLB-SF21 cells (47) and Drosophila melanogaster DL-1 cells (39) were maintained at 27°C in TC100 (Invitrogen) and Schneider's growth medium (Invitrogen) supplemented with 10% and 15% heat-inactivated fetal bovine serum (FBS) (HyClone), respectively. Wild-type L-1 strain Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) (27) and AcMNPV recombinants vΔp35 (vΔ35K; p49− p35− iap−), vΔp35/lacZ (vΔ35K/lacZ; p49− p35− iap−), vP49 (pIE1prmp49/Δ35K/lacZ; p49+ p35− iap−), vP35 (pIE1prmp35/Δ35K/lacZ; p35+ iap−), vOpIAP (pIE1prmopiapHA/Δ35K/lacZ; Op-IAP3HA p35−), and vSfIAPHA (pIE1prmSfiapHA/Δ35K/lacZ; SfiapHA p35−) were described previously (18, 26, 56). AcMNPV recombinant rvSfIAPHA (SfiapHA/Δ35K/lacZ; SfiapHA p35−) was generated by gene insertion (32), in which a promoterless plasmid (pBS/KS+/SfiapHA) encoding SfIAPM4 (see below) was randomly integrated into the vΔp35/lacZ genome during infection; SfIAPM4 was tagged at its N terminus with the influenza virus hemagglutinin (HA) epitope (YPYDVPDYA). Recombinant viruses were identified by lacZ expression and plaque purified. Immunoblot analysis verified expression of SfiapHA.
Plasmids.
The Sfiap open reading frame (ORF) from the first methionine (M1; residue 1 for SfIAPM1) or the fourth methionine (M4; residue 97 for SfIAPM4) to residue 377 (for full-length SfIAP) or to residue 323 (for SfIAP with the RING deleted [SfIAPΔR]) was PCR amplified from an Sfiap cDNA (20) (kindly provided by J. Reed). The resulting DNA fragments were substituted for the AscI-SpeI fragment of pIE1-IAPHA/PA (32) to create expression vectors pIE1prm/hr5/M4-SfIAPHA/PA, pIE1prm/hr5/M4-SfIAPΔRHA/PA, pIE1prm/hr5/M1-SfIAPHA/PA, and pIE1prm/hr5/M1-SfIAPΔRHA/PA; each plasmid encoded N-terminal HA-tagged versions of SfIAP expressed from the highly active AcMNPV ie-1 promoter (prm). These plasmids were used to generate plasmids encoding HA-tagged SfIAPM4 and SfIAPΔRM4 containing BIR and RING substitutions C43A, C67A, H60A, C154S, C178S, C67A and C178S, and H251A, as well as SfIAPM1 with an H347A mutation (H347A-mutated SfIAPM1). pIE1prm/hr5/M4-SfIAPT7/PA, pIE1prm/hr5/M4-SfIAPΔRT7/PA, and pIE1prm/hr5/M1-SfIAPT7/PA with and without the indicated BIR or RING mutations were generated by insertion of a T7 epitope tag (MASMTGGQQMG) in place of the HA tag. pIE1prm/M4-SfIAPHA/MTprm-lacZ was generated by inserting the metallothionine promoter-driven lacZ cassette from pMT/lacZ (Invitrogen) into hr5 enhancer-less pIE1prm/M4-SfIAPHA/PA upstream of the ie-1 promoter. pIE1prm/hr5/hidHA/PA was generated by inserting the Drosophila hid ORF (kindly provided by B. Hay) with a C-terminal HA tag under the control of the ie-1 promoter in pIE1prm/hr5/PA. pIE1prm/hr5/PA encoding AcMNPV P35 or Spodoptera littoralis nucleopolyhedrovirus P49 were described previously (6, 56). All plasmids and mutations thereof were verified by nucleotide sequencing.
Transfections.
Expression plasmids (6 μg) were mixed with cationic liposomes (10 μl) consisting of DOTAP-DOPE {N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate)-l-phosphatidylethanolamine, dioeoyl (C18:1, [cis]-9)} and added to SF21 monolayers (106 cells per 60-mm plate) as described previously (25); transfection efficiencies were from 75 to 90%. For immunoprecipitations, 7 × 106 SF21 cells in 100-mm plates were transfected with 25 μg plasmid, and 40-μl DOTAP-DOPE. DL-1 monolayers (3 × 106 cells per 60-mm plate) were transfected with 2 μg plasmid and 20 μl DOTAP DOPE. All cells were harvested ∼24 h after transfection and lysed in 1% sodium dodecyl sulfate (SDS)-1% β-mercaptoethanol (βME).
Marker rescue assays.
SF21 cells were transfected with pIE1prm/hr5/PA-based plasmid vectors carrying the indicated test genes and inoculated 16 h later with AcMNPV recombinant vΔp35/lacZ as described previously (2). Extracellular virus was collected 72 h after infection and quantified by plaque assay by using SF21 cells and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) to identify rescued (nonapoptotic), lacZ-expressing virus.
Cell survival assays.
SF21 monolayers (106 cells per 60-mm plate) were inoculated with the indicated AcMNPV recombinants. Photographs were taken of four evenly distributed fields of view from each of three replicate plates by using a Zeiss Axiovert 135TV microscope as described previously (19). Intact cells were counted, and percent survival is reported as the average ratios ± standard deviations of intact, nonapoptotic cells. Values were normalized by adjusting the survival of vector-transfected cells infected with wild-type AcMNPV (p35+) to 100%.
IAP dominant inhibition assays.
For virus-based assays, triplicate plates of SF21 cells were transfected with plasmid and inoculated 24 h later with rvSfIAPHA (multiplicity of infection [MOI] = 1 to 2 PFU per cell). Cells were collected 48 h later, lysed, and assayed for β-galactosidase by using the Galacto-Light Plus β-galactosidase chemiluminescent reporter assay (Tropix), as per the manufacturer's instructions. For UV irradiation-based assays, triplicate plates of 3 × 106 DL-1 cells were transfected with the indicated lacZ reporter plasmids and irradiated 24 h later with UV-B (2 min) by using a Blak-Ray lamp (UVP, Upland, CA). Cells and associated apoptotic bodies were collected 24 h later, lysed, and assayed for β-galactosidase. All values are reported as the average activities ± standard deviations obtained with triplicate plates.
Caspase assays.
Triplicate plates of SF21 cells were transfected with the indicated pIE1prm/hr5/PA-based expression vectors, harvested 20 h later, and lysed on ice in caspase buffer (10 mM HEPES at pH 7.0, 5 mM EDTA, 0.1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, and 1 mM dithiothreitol [DTT]). After clarification by centrifugation, the caspase activity of the supernatant was measured by using the substrate Ac-DEVD-AMC (7-amino-4-methylcoumarin) as described previously (25). Values are reported as the average rates ± standard deviations of fluorescent product accumulation (in relative light units [RLU]) obtained with three independent plates.
Antisera and immunoblots.
SfIAP-specific antiserum (anti-SfIAP) was generated by immunization of New Zealand White rabbits (University of Wisconsin Medical School Polyclonal Antibody Service) with the antigen C-terminal His6-tagged residues 97 to 305 of SfIAP (SfIAP97-305; M4-SfIAPΔR) produced in Escherichia coli and purified by Ni2+ affinity chromatography as described previously (2). The serum was affinity purified using the same antigen and Hi-Trap N-hydroxysuccinimide (NHS)-activated high-performance (HP) columns (Amersham Biosciences). For immunoblotting, proteins were electrophoresed on SDS-12.5% polyacrylamide gels and transferred to nitrocellulose. The membranes were incubated with the antisera diluted as indicated in parentheses: monoclonal anti-P35 (1:5,000) (gift from Y. Lazebnik), anti-HA (1:1,000) (Covance), anti-T7 (1:10,000) (Novagen), anti-actin (1:1,000) (BD Transduction Laboratories), anti-Sf-caspase-1 (1:1,000) (25), or anti-SfIAP (1:1,000). Immunoblot detection was performed using alkaline phosphatase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G (Jackson ImmunoResearch Laboratories) and the Western-Star chemiluminescent detection system (Tropix).
Immunoprecipitations.
SF21 monolayers (7 × 106 cells per 100-mm plate) were harvested 24 h after transfection, lysed for 45 min on ice in IP buffer (10 mM sodium phosphate at pH 7.1, 150 mM NaCl, 50 mM sodium fluoride, 2 mM EDTA, 0.1% NP-40, 1× protease inhibitor [Roche]), and clarified by centrifugation (16,000 × g). The supernatant was mixed with anti-T7-conjugated agarose beads (Novagen) in IP buffer for 3 h at 4°C. The beads were collected and washed three times with IP buffer, and the proteins were eluted with sample buffer (625 mM Tris at pH 6.8, 5% βME, 2% SDS, 1.5% Ficoll) at room temperature for 20 min.
Size exclusion chromatography.
SF21 cells transfected 24 h earlier with pIE1prm/hr5/M4-SfIAPHA/PA were harvested, suspended in 250 μl buffer A (20 mM HEPES at pH 7.5, 10 mM KCl, 1 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT) per 7 × 106 cells, and lysed via three freeze-thaw cycles. After clarification (30,000 × g for 30 min), the supernatant was filtered (with 0.2-μm-pore-size polytetrafluoroethylene [Millex]) and fractionated on a Superdex 200 10/300 GL size exclusion chromatography column (GE Life Sciences) using buffer A, as described previously (13). Size calibration was conducted by using gel filtration standards (Bio-Rad).
Protein half-life measurements.
SF21 cells transfected 24 h earlier with pIE1prm-based plasmids encoding the indicated SfIAPs were overlaid with medium containing 400 μg cycloheximide per ml; this drug concentration was the minimum sufficient to block >95% of SfIAP synthesis over a 4-h period (data not shown). Serial dilutions of cell lysates prepared at the indicated times thereafter were analyzed by using anti-T7 on duplicate immunoblots. SfIAP signal intensity was quantified by densitometry (NIH ImageJ) using films exposed within the linear range. The percent protein remaining at each interval after cycloheximide treatment was calculated and plotted as a function of time to generate protein half-lives (t1/2) by best-fit analysis. Values are presented as the average values ± standard deviations of protein half-lives (in minutes) derived from triplicate experiments.
RESULTS
SfIAP blocks apoptosis by a RING-dependent mechanism.
Spodoptera frugiperda SfIAP possesses two BIRs and a C-terminal RING motif and thus closely resembles baculovirus IAPs, including best-studied Op-IAP3 (Fig. 1A). To define biochemical differences between cellular and viral homologs, we first identified the required functional motifs within SfIAP. Site-directed mutations were tested by plasmid transfection of Spodoptera frugiperda SF21 cells, which are highly sensitive to apoptotic stimuli and also permissive for baculovirus AcMNPV. The largest ORF within the Sfiap cDNA (20) is predicted to encode a 377-residue polypeptide (designated SfIAPM1) when initiated from the first available methionine (Fig. 1A). However, three other possible start codons are located N terminally to BIR1. Because it most closely resembles the size and structural organization of Op-IAP3, we first tested SfIAPM4, which initiates from the fourth methionine (Fig. 1B). When expressed from transfected plasmids using the strong AcMNPV ie-1 promoter, HA epitope-tagged SfIAPM4 was synthesized as a single 281-residue species that was abundant and fully functional in SF21 cells (see below).
To test the antiapoptotic activity of SfIAPM4, we transfected SF21 cells with SfIAP-encoding vectors and monitored cell survival after infection with the AcMNPV mutant vΔp35, which lacks caspase inhibitor P35 and causes widespread apoptosis (18). Whereas vΔp35 caused >75% apoptotic lysis of cells transfected with empty vector (Fig. 2A), overexpressed SfIAPM4 prevented apoptosis. The survival of SfIAPM4-transfected cells was more than 3-fold higher than that of control cells and comparable to that of cells infected with wild-type p35+ AcMNPV (Fig. 2B). In contrast, deletion of the C-terminal RING motif caused a loss of antiapoptotic activity (Fig. 2B); this truncation (SfIAPM4ΔR) was readily produced in transfected cells (see below), which indicated that loss of function was not due to loss of protein. SfIAPM4ΔR loss of function was confirmed by marker rescue assays in which transfection with SfIAPM4ΔR-encoding plasmid failed to restore multiplication of the p35-null mutant vΔp35/lacZ (Fig. 1C). In contrast, wild-type SfIAPM4-encoding plasmid increased vΔp35/lacZ multiplication by >500-fold. SfIAPM4, but not SfIAPM4ΔR, also protected cells from UV-induced apoptosis (see below). Thus, overexpressed SfIAP prevented apoptosis triggered by diverse signals in a RING-dependent manner, a finding consistent with earlier studies (20).
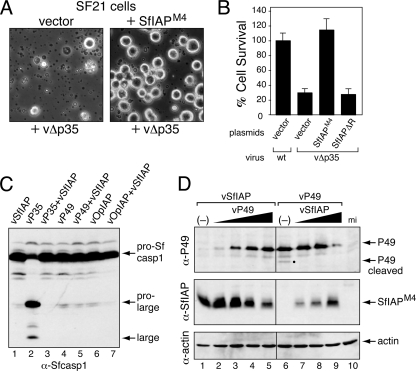
FIG. 2.
SfIAP suppression of baculovirus-induced apoptosis. (A) Morphology of infected cells. SF21 cells were transfected with empty vector (vector) or a plasmid encoding SfIAPM4, inoculated 24 h later with p35-deficient recombinant vΔp35, and photographed (magnification, ×100) after 24 h. A representative field of view is shown. (B) Cell survival. SF21 cells were transfected with empty vector or vectors encoding SfIAPM4 or SfIAPM4ΔR and inoculated 24 h later with wild-type p35+ (wt) or vΔp35 AcMNPV. Cell survival was scored 24 h later and is reported as the average values ± standard deviations of intact cells relative to those of wild-type virus-infected cells, which were normalized to 100%. (C) Activation of Sf-caspase-1. SF21 cells were inoculated with viruses encoding the indicated proteins, lysed 24 h later, and subjected to immunoblot analysis by using anti-Sf-caspase-1, which recognizes inactive uncleaved pro-Sf-caspase-1 and its active prolarge and large subunits after cleavage. (D) Effect of SfIAP on initiator caspase-mediated cleavage of P49. SF21 monolayers were mock infected (mi), inoculated (MOI = 10) with recombinant viruses vSfIAP alone (−) or vP49 alone (−), or coinoculated with an increasing ratio of the indicated viruses (total MOI ≥ 10). Cell lysates (106 cells) prepared 24 h later were subjected to immunoblot analysis by using anti-P49 (top), anti-SfIAP (middle), or anti-actin (bottom). The P49 cleavage product (dot) is indicated (lane 6).
SfIAP blocks effector caspase activation.
Apoptosis in Spodoptera involves sequential activation of initiator and effector caspases, which includes the principal effector Sf-caspase-1 (1, 25, 31). Activated first in the caspase pathway, initiator caspases proteolytically cleave and thereby activate effector caspases from their inactive proforms (16, 36, 37). Viral Op-IAP3 blocks apoptosis by preventing Sf-caspase-1 activation (25, 40, 56). To define the step at which SfIAP acts, we monitored the proteolytic processing of pro-Sf-caspase-1, which is cleaved to large and small subunits during activation (1, 25). To ensure that SfIAPM4 was overproduced in every cell triggered to undergo apoptosis, we generated an AcMNPV recombinant vSfIAP, which lacks p35 but expresses SfiapM4 from the early ie-1 promoter. Upon infection with vSfIAP, processing of pro-Sf-caspase-1 was blocked (Fig. 2C, lane 1); the abundance of SfIAPM4 (see below) indicated that infection proceeded normally. In contrast, pro-Sf-caspase-1 was processed to active subunits upon infection with p35+ recombinant vP35 (Fig. 2C, lane 2); P35 is a substrate inhibitor of effector caspases that blocks virus-induced apoptosis by inhibiting activated Sf-caspase-1 (56). We concluded that SfIAPM4 acts upstream of P35 and thus prior to effector caspase activation.
SfIAPM4 inhibition of pro-Sf-caspase-1 processing was comparable to that by P49 and Op-IAP3, produced by AcMNPV recombinants vP49 and vOpIAP, respectively (Fig. 2C, lanes 4 to 7). Bearing considerable sequence similarity to P35, baculovirus P49 is a substrate inhibitor that is cleaved by and inhibits the initiator caspase that processes Sf-caspase-1 (56). Thus, to determine if SfIAP also inhibits the P49-sensitive initiator caspase, we tested whether SfIAPM4 affects P49 cleavage. When P49 and SfIAPM4 were expressed simultaneously in cells coinfected with recombinants vP49 and vSfIAP, cleavage of P49 was blocked (Fig. 2D). P49 cleavage was detected only in the absence of vSfIAP (Fig. 2D, lane 6), indicating that SfIAPM4 inhibition was dominant to that by P49. We concluded that when overproduced, SfIAPM4 inhibits the activation or activity of the Spodoptera initiator caspase responsible for virus-induced apoptosis. Thus, SfIAP can function at a step also inhibited by viral Op-IAP3 (56).
RINGless SfIAP is a dominant inhibitor of SfIAP.
Viral Op-IAP3 RING truncations are nonfunctional and potently inhibit wild-type Op-IAP3 (19). To determine if SfIAP behaves similarly, we tested the effect of SfIAPM4 RING mutations on SfIAP function. To this end, we engineered a p35-null AcMNPV recombinant (rvSfIAP/lacZ) that produces SfIAPM4 at levels minimally sufficient to block apoptosis (see below). rvSfIAP/lacZ includes a polyhedrin promoter-directed lacZ reporter, which is expressed only when apoptosis is suppressed because the polyhedrin promoter is not activated until very late in infection (18). Thus, β-galactosidase production provided a sensitive measure of apoptotic inhibition, as used previously for studies of Op-IAP3 inhibitors (19).
SfIAPM4 produced by rvSfIAP/lacZ blocked virus-induced apoptosis (Fig. 3A); cell survival 48 h after infection was >95%. In contrast, SfIAPM4-mediated inhibition of virus-induced apoptosis was reduced by prior transfection with plasmid encoding RINGless SfIAPM4ΔR; apoptotic lysis affected >90% of the cells (Fig. 3A). SfIAPM4ΔR increased apoptosis of rvSfIAP/lacZ-infected cells in a dose-dependent manner, as indicated by decreasing β-galactosidase levels with increasing amounts of plasmid (Fig. 3B). Because transfection efficiencies were <100%, β-galactosidase activity was not reduced to zero. Immunoblots indicated that SfIAPM4ΔR was more abundant than virus-expressed SfIAPM4 in these assays (data not shown). These data suggested that SfIAPM4ΔR dominantly interferes with SfIAP.
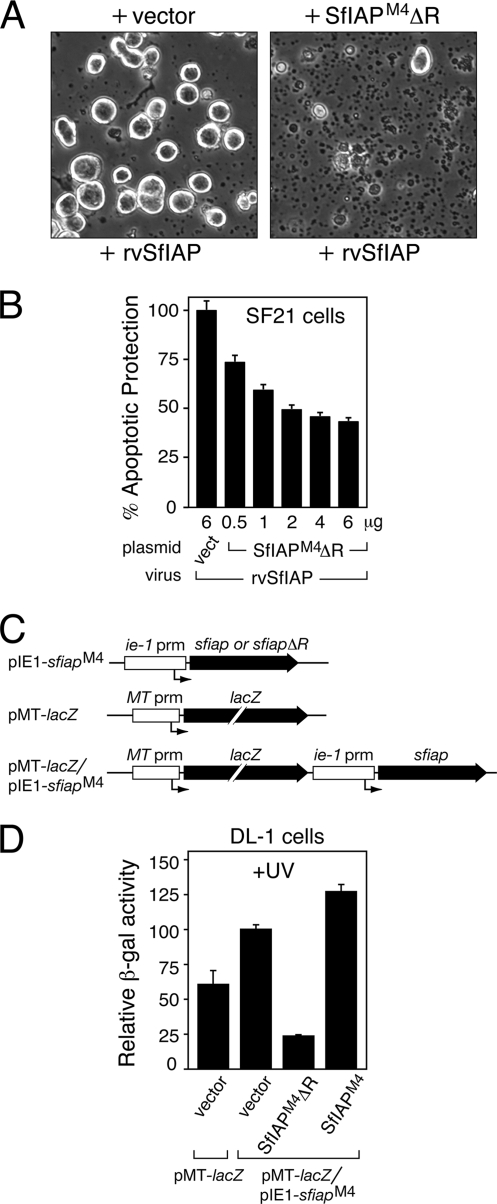
FIG. 3.
Dominant inhibition by SfIAPM4ΔR. (A) Morphology of infected cells. SF21 cells were transfected with empty vector or plasmid encoding SfIAPM4ΔR, inoculated 24 h later with SfIAPM4-encoding virus rvSfIAP/lacZ, and photographed (magnification, ×100) after 48 h. (B) Virus-based dominant inhibition assay. SF21 cells were transfected with the indicated quantities of empty vector (vect) or SfIAPM4ΔR-encoding plasmid; the total plasmid level was held constant by including empty vector. After 24 h, the cells were inoculated with rvSfIAP/lacZ, and intracellular β-galactosidase was measured 48 h later. In this assay, the yield of β-galactosidase is directly proportional to the level of apoptotic protection, which is reported as the average value ± standard deviation of activity relative to that of vector-transfected cells (normalized to 100%) obtained from triplicate infections. (C) Expression vectors. The ie-1 or metallothionine (MT) promoters (prm) were linked to SfiapM4 or lacZ, respectively, and inserted into the indicated plasmids. An arrow denotes the RNA start sites. (D) Antiapoptotic activity of SfIAPM4 in Drosophila cells. DL-1 cells were transfected with empty vector or plasmids encoding the indicated proteins, UV irradiated 24 h later, and immediately overlaid with Cu2+-containing medium to induce lacZ expression. The level of apoptotic protection was directly proportional to the yield of 24-h intracellular β-galactosidase, which is reported as the average value ± standard deviation of activity relative to that of cells cotransfected with empty vector and pMT-lacZ/pIE1-Sfiap (normalized to 100%) obtained from triplicate transfections.
To confirm this conclusion, we used heterologous Drosophila DL-1 cells that lack endogenous SfIAP and are sensitive to UV radiation-induced apoptosis. DL-1 cells were transfected with plasmids encoding either SfIAPM4 or RINGless SfIAPM4ΔR and cotransfected with plasmid pMT-lacZ/pIE1-SfiapM4 that encodes β-galactosidase under the control of the Drosophila metallothionine promoter and SfIAPM4 under the control of the ie-1 promoter (Fig. 3C). After UV irradiation, β-galactosidase production was used to monitor cell viability. Cell survival was highest when only wild-type SfIAPM4 was expressed (Fig. 3D), indicating that SfIAPM4 blocks UV-induced apoptosis. In contrast, cell survival was lowest upon coexpression of SfIAPM4ΔR and SfIAPM4; β-galactosidase was reduced by 4- to 5-fold compared to that of cells transfected with vector alone (Fig. 3D). We concluded that SfIAPM4ΔR dominantly inhibits the antiapoptotic activity of SfIAP in heterologous and homologous cells.
SfIAPΔR interacts with SfIAP.
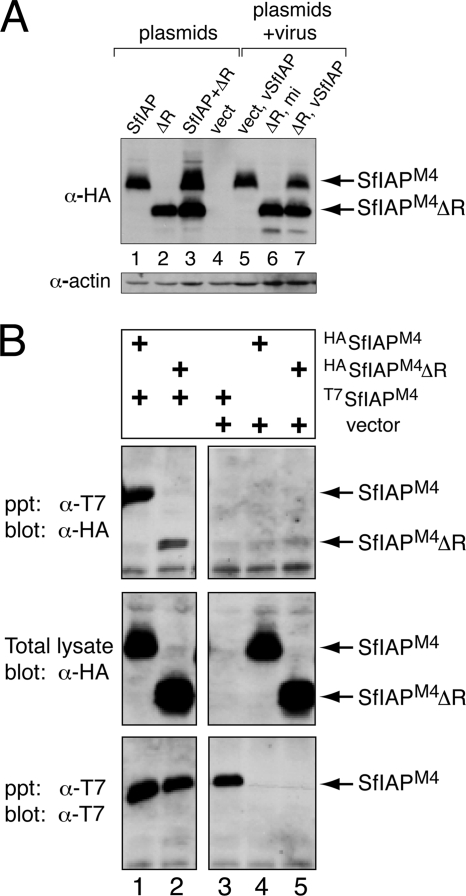
To test whether dominant inhibition involved decreased SfIAP stability, we monitored intracellular protein levels. Immunoblot analysis indicated that SfIAPM4ΔR did not affect SfIAPM4 levels either in plasmid-transfected cells (Fig. 4A, lanes 1 to 3) or in vSfIAP-infected cells (lanes 5 to 7). Consequently, we determined whether SfIAPM4ΔR interacts with SfIAPM4. In immunoprecipitation assays, HA-tagged SfIAPM4ΔR was precipitated with T7-tagged SfIAPM4 (Fig. 4B, lane 2). Little, if any, SfIAPM4ΔR precipitated nonspecifically (Fig. 4B, lane 5). These interactions were confirmed by immunoprecipitations in which the epitope tags were reversed (data not shown). Thus, SfIAPΔR forms a stable complex with SfIAP. Moreover, full-length HA-tagged SfIAPM4 coprecipitated with full-length T7-tagged SfIAPM4 (Fig. 4B, lane 1); SfIAPM4 was not precipitated in the absence of SfIAPT7 (Fig. 4B, lane 4). We concluded that SfIAP also interacts with itself and forms homo-oligomeric complexes like viral Op-IAP3 (19).
FIG. 4.
SfIAP interactions. (A) Relative stability of SfIAPM4 and SfIAPM4ΔR. SF21 cells were transfected with empty vector (vect) or plasmids encoding SfIAPM4 (SfIAP) or SfIAPM4ΔR (ΔR) (lanes 1 to 4) and lysed 24 h later. Replicate plates of 24-h transfected cells were mock infected (mi) or infected with virus vSfIAPM4 (lanes 5 to 7) and lysed 24 h later. All lysates were subjected to immunoblot analysis using anti-HA (top) and anti-actin (bottom). (B) Immunoprecipitations. SF21 cells were transfected (+) with empty vector or plasmid encoding the indicated T7- or HA-tagged proteins, and NP-40-treated extracts were prepared 24 h later. Proteins were immunoprecipitated (ppt) by using anti-T7 beads and subjected to immunoblot analysis (blot) by using anti-T7 or anti-HA. Lysates obtained prior to immunoprecipitation (total lysate) were included.
Intracellular SfIAP is dimeric.
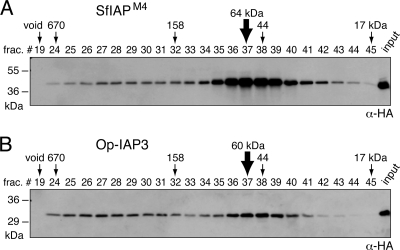
The capacity of SfIAP to interact with itself suggested that it forms oligomers. To clarify the nature of homo-oligomerization, we used size exclusion chromatography to characterize the IAP complex. The majority of SfIAPM4 obtained from transfected SF21 cells by freeze-thaw lysis eluted as a complex, with a molecular mass of 60 to 70 kDa (Fig. 5A). Since the monomeric mass of SfIAPM4 (281 residues) is 32 kDa, we concluded that the principal form of SfIAP is a ∼64-kDa dimer under these conditions. Minor amounts of SfIAP were reproducibly detected in fractions expected for larger complexes, suggesting the possibility of interaction with other cellular proteins. Collectively, our data argue that a majority of overexpressed SfIAP exists as a homodimer rather than in a complex with other apoptosis-associated molecules. When overexpressed in SF21 cells, 30-kDa Op-IAP3 (268 residues) also eluted with a size expected of a dimeric complex (Fig. 5B), consistent with its capacity to homo- oligomerize (19). Op-IAP3 had a greater tendency to form larger complexes, possibly through multimerization or interaction with cellular components.
FIG. 5.
Size exclusion chromatography of SfIAPM4 and Op-IAP3. Freeze-thaw lysates of SF21 cells transfected with plasmid encoding HA-tagged SfIAPM4 (A) or Op-IAP3 (B) were fractionated by size exclusion (Superdex 200) chromatography. Column fractions were subjected to immunoblot analysis by using anti-HA. The elution profile of protein standards (size in kDa) is indicated. Unfractionated lysates (input) and the void fraction (void) were included.
RINGless SfIAP triggers caspase activation.
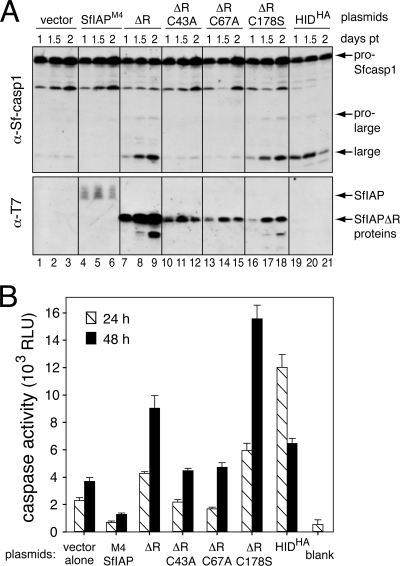
Because SF21 cells exhibit a constitutive push toward caspase activation (34), we predicted that SfIAPM4ΔR-mediated inhibition of endogenous SfIAP would trigger caspase activity. To test this possibility, we determined the effect of SfIAPM4ΔR on caspase activation in SF21 cells. In contrast to transfection of vector or SfIAPM4-encoding plasmid (Fig. 6A, lanes 1 to 3 and 4 to 6), transfection with SfIAPM4ΔR-encoding plasmid triggered the processing of Sf-caspase-1 to active subunits (lanes 7 to 9). SfIAPM4ΔR-induced processing was comparable to that induced by overexpression of Drosophila proapoptotic factor HID (Fig. 6A, lanes 19 to 21), which causes widespread apoptosis of SF21 cells (50). The intracellular level of SfIAPM4ΔR-induced caspase activity was 3-fold higher than that of vector-transfected cells and comparable to that induced by HID when measured using the effector caspase substrate DEVD-AMC (Fig. 6B). As expected, caspase activity was lowest in SfIAPM4-expressing cells (Fig. 6B). Immunoblots readily detected transfected SfIAPM4ΔR, which was more abundant than SfIAPM4 (Fig. 6A, lanes 4 to 6 and 7 to 9). We concluded that SfIAPM4ΔR triggers caspase activation and thus mimics RINGless Op-IAP3 (19).
FIG. 6.
SfIAPM4ΔR-induced caspase activation. (A) Activation of Sf-caspase-1. SF21 cells transfected with empty vector or plasmids encoding the indicated T7-tagged SfIAPM4 or HA-tagged HID were lysed 1, 1.5, or 2 days posttransfection (pt) and subjected to immunoblot analysis by using anti-Sf-caspase-1 or anti-T7. (B) Intracellular caspase activity. Extracts prepared from cells transfected 24 and 48 h previously, as described in the legend to panel A, were assayed for caspase activity by using DEVD-AMC as a substrate. Values are reported as the averages ± standard deviations in relative light units (RLU) determined from triplicate transfections. Blank, lysis buffer alone.
BIR1 of SfIAPΔR is required for dominant inhibition.
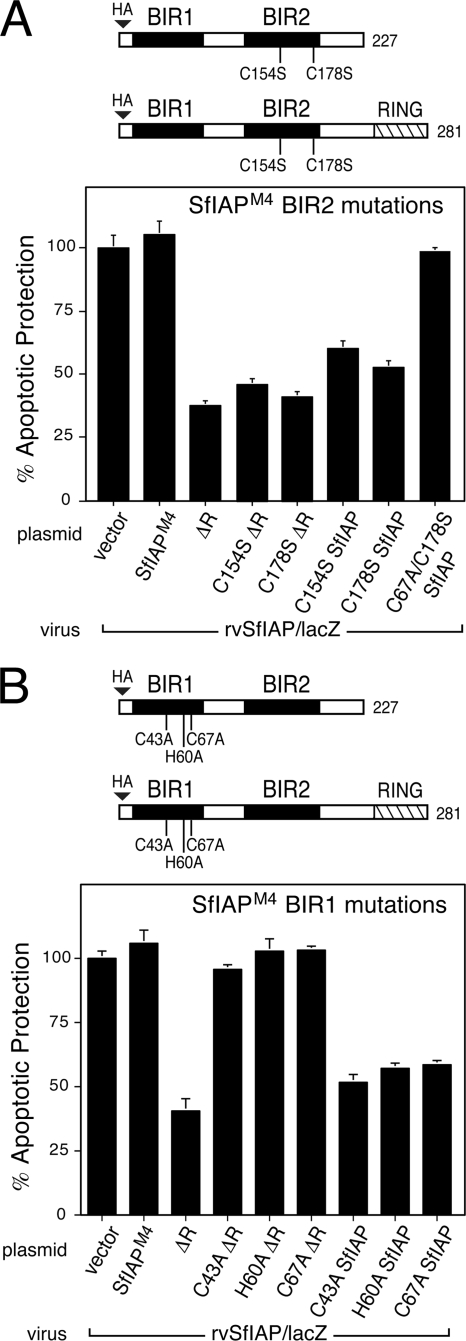
To define the domain(s) responsible for inhibition by SfIAPM4ΔR, we first examined the BIRs, which interact with known proapoptotic ligands. Substitutions of the Zn-coordinating Cys and His residues (Fig. 1B) were used to disrupt the folding of each BIR. When tested individually by marker rescue assays, BIR1 substitutions C43A, C67A, and H60A and BIR2 substitutions C154S and C178S caused loss of SfIAPM4 function (Fig. 1C), indicating that both BIRs are required for antiapoptotic activity. These BIR substitutions were then introduced into SfIAPM4ΔR for tests of dominant inhibition in our rvSfIAP/lacZ β-galactosidase assay. Upon transfection, each BIR-mutated SfIAPM4ΔR was readily detected (see below). BIR2 substitutions C154S and C178S had no effect on dominant inhibition by SfIAPM4ΔR, since both proteins reduced antiapoptotic activity of virus-encoded SfIAPM4 just as well as nonmutated SfIAPM4ΔR (Fig. 7A). Conversely, substitutions C43A, H60A, and C67A within BIR1 caused loss of dominant inhibition by SfIAPM4ΔR (Fig. 7B). C67A- and C178S-doubly mutated SfIAPM4ΔR also failed to inhibit SfIAPM4 (data not shown). Thus, only BIR1 is required for dominant inhibition.
FIG. 7.
Role of BIRs in dominant inhibition. (A) BIR2 mutations. SF21 cells transfected 24 h previously with empty vector or plasmids encoding wild-type SfIAPM4 (SfIAP), SfIAPM4ΔR (ΔR), or the indicated BIR2 substitutions of each were inoculated with rvSfIAP/lacZ. Intracellular β-galactosidase was measured 48 h later to quantify apoptotic protection. Values reported are the averages ± standard deviations of protection relative to those of empty vector-transfected cells (normalized to 100%) obtained from triplicate infections. (B) BIR1 mutations. Intracellular β-galactosidase was measured in SF21 cells transfected with the indicated plasmids, including BIR1 substitutions in SfIAPM4 or SfIAPM4ΔR, and infected with rvSfIAP/lacZ, as described in the legend to panel A.
We confirmed these findings by showing that BIR1 is also required for SfIAPM4ΔR-mediated inhibition of endogenous cellular SfIAP. Processing of Sf-caspase-1 to active subunits was blocked or reduced in the presence of BIR1 C43A- or C67A-mutated SfIAPM4ΔR (Fig. 6A, lanes 10 to 15). Conversely, processing in the presence of BIR2 C178S-mutated SfIAPM4ΔR (Fig. 6A, lanes 16 to 18) was comparable to that of dominant inhibitor SfIAPM4ΔR and HID. Intracellular caspase activity was also reduced in the presence of BIR1 C43A- or C67A-mutated SfIAPM4ΔR but was significantly increased by BIR2 C178S-mutated SfIAPM4ΔR (Fig. 6B), indicating that BIR1, not BIR2, was required for SfIAPM4ΔR-mediated inhibition.
BIR-disrupted SfIAPΔR interacts with SfIAP.
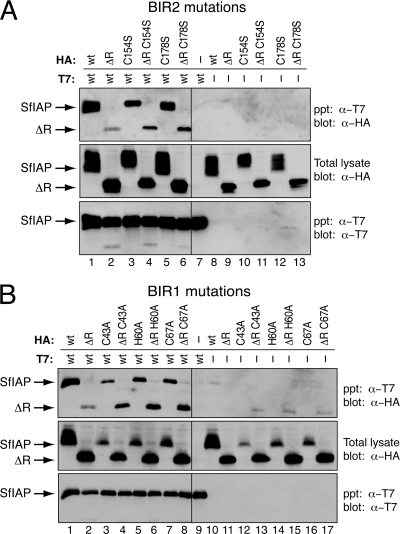
To determine whether BIR disruption interfered with SfIAPM4ΔR interaction with functional SfIAP, we used immunoprecipitation assays. Full-length T7-tagged SfIAPM4 formed a complex with BIR2 C154S- and C178S-mutated SfIAPM4ΔR (Fig. 8A, lanes 4 and 6) and with BIR1 C43A-, H60A-, and C67A-mutated SfIAPM4ΔR (Fig. 8B, lanes 4, 6, and 8). These interactions were as strong as or stronger than those with unaltered SfIAPM4ΔR (Fig. 8A and B, lane 2). Full-length T7-tagged SfIAPM4 also interacted with C67A- and C178S-mutated SfIAPM4, in which both BIRs were mutated (data not shown). None of the SfIAPM4 mutations were precipitated in the absence of full-length SfIAPT7 (Fig. 8), and all interactions were confirmed by reciprocal assays (data not shown). We concluded that the proper folding of BIR1 or BIR2 is not required for interaction between SfIAPM4ΔR and SfIAP. These findings suggested that a domain(s) outside or independent of the BIRs mediates SfIAP homophilic interactions.
FIG. 8.
Role of BIRs in SfIAP interactions. (A) BIR2 mutations. NP-40-derived extracts of SF21 cells prepared 24 h after transfection with empty vector (−) or plasmids encoding the indicated HA-tagged BIR2-mutated SfIAPM4, SfIAPM4ΔR, and T7-tagged wild-type SfIAPM4 (wt) were immunoprecipitated (ppt) by using anti-T7 beads and subjected to immunoblot analysis (blot) by using anti-T7 or anti-HA. HA-tagged proteins prior to immunoprecipitation (total lysate) were included. (B) BIR1 mutations. Immunoprecipitations of NP-40-derived extracts of SF21 cells transfected with empty vector (−) or plasmids encoding the indicated proteins were conducted, as described in the legend to panel A.
BIR disruptions within full-length SfIAP generate dominant inhibitors.
To further define the role of the BIRs in dominant inhibition, we tested the effect of BIR disruptions within full-length SfIAP. When expressed by plasmid transfection, BIR1 C43A-, H60A-, and C67A-mutated SfIAPM4 and BIR2 C154S- and C178S-mutated SfIAPM4 dominantly interfered with the antiapoptotic activity of SfIAPM4 (Fig. 7A and B); each of these mutated RING-containing proteins reduced the yield of β-galactosidase within rvSfIAP/lacZ-infected cells, indicating that SfIAP antiapoptotic activity was compromised. Dominant inhibition by these BIR-mutated proteins was comparable to that of RINGless SfIAPM4ΔR. In contrast, C67A- and C178S-mutated SfIAPM4, in which both BIRs were disrupted, failed to alter SfIAPM4 function in this assay (Fig. 7A). This doubly mutated SfIAPM4 was readily produced in transfected cells (data not shown), ruling out the possibility that protein instability caused the loss of dominant inhibition. We concluded that disruption of either BIR1 or BIR2, but not both, can generate a dominant inhibitor of functional SfIAPM4.
As demonstrated by immunoprecipitations, BIR2 C154S- and C178S-mutated SfIAPM4 formed a complex with full-length T7-tagged SfIAPM4 (Fig. 8A, lanes 3 and 5). Likewise, BIR1 C43A-, H60A-, and C67A-mutated SfIAPM4 coprecipitated with full-length SfIAPM4 (Fig. 8B, lanes 3, 5, and 7). Moreover, C67A- and C178S-doubly mutated SfIAPM4 also interacted with full-length SfIAP (data not shown). These findings confirmed our conclusion that although at least one BIR is required for dominant inhibition, properly folded BIRs are not required for SfIAP complex formation. The finding that C67A- and C178S-mutated SfIAPM4 also formed a complex with SfIAP but failed to act as a dominant inhibitor suggests that interaction with a nonfunctional IAP is necessary but not sufficient for dominant inhibition. Additional studies are required to define the mechanisms involved.
The N-terminal SfIAP leader regulates protein abundance.
A striking feature of invertebrate (lepidopteran) host IAPs is the possession of a long N-terminal leader sequence that precedes BIR1 (Fig. 1A). The absence of a comparable sequence in viral IAPs (20, 45) suggests that the leader performs a function specific for cellular IAPs. To investigate the potential role of the SfIAP leader, we generated an expression vector for SfIAPM1, which uses the first available methionine (M1) in the Sfiap ORF and thus includes the 100 residues prior to BIR1 (Fig. 9A). SfIAPM1 was engineered to include an epitope (HA) tag. Immunoblotting of transfected SF21 cells detected full-length SfIAPM1 (∼54 kDa) but at relatively low steady-state levels compared to that of SfIAPM4 (Fig. 9B and C, compare lanes 3 and 9). Smaller SfIAPM1-related products were recognized by anti-SfIAP but not by anti-HA. Because these products lacked the N-terminal HA tag, they were derived by either cleavage or internal initiation from the second or third methionine (M2 or M3) within the leader (Fig. 9A). Deletion of the RING (SfIAPΔRM1) or disruption of the E3 ubiquitin ligase activity of the RING (51) by the substitution H347A caused a modest increase in SfIAPM1 steady-state levels (Fig. 9B and C, lanes 5 and 7). Nonetheless, leaderless versions of SfIAPM4 (Fig. 9B, lanes 9, 11, 13, and 15) were routinely detected at significantly higher levels than in wild-type or RING-mutated forms of SfIAPM1.
FIG. 9.
Stability of SfIAPM1 and dominant inhibition. (A) SfIAPs. SfIAPM1 (377 residues) and SfIAPM4 (281 residues) begin from the first and fourth methionine, respectively, of the predicted Sfiap ORF. SfIAPΔRM1 and SfIAPΔRM4 each lack their C-terminal RING domain (cross-hatched box). N-terminal epitope tags (HA) were included. Only SfIAPM1 and SfIAPΔRM1 possess the caspase cleavage site at Asp83. (B to D) Protein levels. SF21 cells were transfected with empty vector or plasmids encoding the indicated SfIAPM1 (M1) or SfIAPM4 (M4) proteins with (+) or without (−) an expression vector encoding the caspase inhibitor P35. After 24 h, the cells were lysed and subjected to immunoblot analysis by using anti-HA (B), anti-SfIAP (C), or anti-P35 and anti-actin (D). Each panel represents a single, contiguous immunoblot. SfIAPM1- and SfIAPM4-related proteins are indicated. Caspase cleavage products (*) are indicated on the left. (E) Intracellular caspase activity. Extracts prepared from cells transfected with or without p35 as described in the legend to panel B were assayed for caspase activity by using substrate DEVD-AMC. Values are reported as the averages ± standard deviations in relative light units (RLU) determined from triplicate transfections. Buffer, lysis buffer alone. (F) Effect of caspase inhibitor P49. SF21 cells were transfected with plasmid encoding the indicated SfIAPM1 (M1) or SfIAPM4 (M4) proteins along with empty vector (−) or plasmids encoding P35 or P49. The effector caspase cleavage site at Asp83 was eliminated in D83A-mutated SfIAPM1. Immunoblot analysis of 24-h lysates was performed using the indicated antisera; each panel is a contiguous immunoblot. Caspase cleavage products (*) are indicated on the right.
The SfIAP leader confers protein instability.
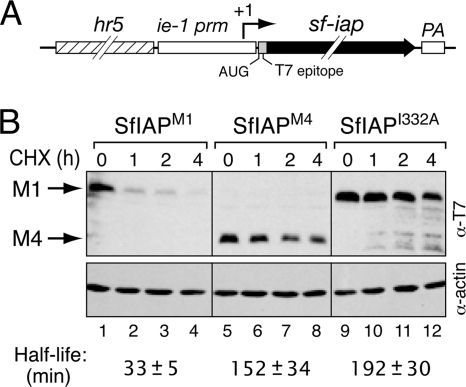
To test whether the higher accumulation of leaderless SfIAPM4 was due to increased stability, we measured the protein half-lives. T7 epitope-tagged SfIAPM1 and SfIAPM4 were overproduced in SF21 cells by using our ie-1 promoter-based expression vector (Fig. 10A). Because both proteins were translated from the same AUG start codon within the same 5′ noncoding mRNA leader, the transcriptional and translational contexts of each construct were identical. Measurements of protein stability were made after protein synthesis was blocked by cycloheximide. We discovered that leader-containing SfIAPM1 was turned over ∼5 times faster than SfIAPM4 (Fig. 10B). Whereas the 33-min half-life of SfIAPM1 was comparable to that of Drosophila IAP1 (DIAP1; t1/2 = 30 to 40 min) (52, 55), deletion of the leader increased the half-life of SfIAPM4 to 152 min. Moreover, the longer half-life of SfIAPM4 was comparable to that of I332A-mutated SfIAPM1 (Fig. 10B), which is deficient for E3 ubiquitin ligase activity of the RING (5, 17, 30). The T7 epitope tag at the N terminus had no significant effect on stability of these proteins (data not shown). We concluded that the N-terminal leader of SfIAP decreases protein stability and that both the leader and the RING contribute to SfIAP turnover under steady-state conditions.
FIG. 10.
Half-lives of SfIAPM1 and leaderless SfIAPM4. (A) SfIAP expression vector. The T7 epitope-tagged SfIAP gene was placed under the control of the ie-1 promoter linked to the AcMNPV hr5 enhancer. mRNA transcription initiates within the promoter (+1) and terminates at the polyadenylation site (PA). Translation begins at the AUG codon within the N-terminal T7 tag. Thus, the translational context of each construct is identical. (B) Protein stability assays. SF21 cells transfected 24 h earlier with plasmid encoding the indicated SfIAPs were treated with 400 μg cycloheximide (CHX) per ml. Cell lysates prepared at the indicated times thereafter were serially diluted and analyzed by immunoblotting with anti-T7 and anti-actin. A representative blot is shown; constant actin levels demonstrated comparable protein loading. Half-life values were calculated from SfIAP signal intensity on films exposed in the linear range. Values shown are the averages ± standard deviations of protein half-lives (in minutes) derived from three independent experiments.
Steady-state levels of SfIAPM1 are unaffected by caspases.
The SfIAP leader possesses three potential caspase cleavage sites at Asp83, Asp86, and Asp96, immediately N terminal to BIR1 (Fig. 9A). One of these sites, DKTD83↓N, has been implicated in SfIAP turnover and anti-caspase activity because cleavage here exposes a domain required for N-end rule ubiquitination and degradation (45). To assess the contribution of caspase-mediated cleavage to SfIAPM1 instability, we first determined the effect of caspase inhibitors on SfIAP levels. When effector caspase inhibitor P35 was coexpressed (Fig. 9D), caspase activity as measured by DEVD-AMC cleavage was reduced to background levels in cells producing dominant inhibitors of endogenous SfIAP (Fig. 9E). However, this P35 anti-caspase activity had little if any effect on the steady-state levels of SfIAPM1 (Fig. 9B and C, compare lanes 3 and 4). The level of RINGless SfIAPΔRM1 and RING-defective H347A-mutated SfIAPM1 increased modestly (Fig. 9B, compare lanes 5 and 6 to lanes 7 and 8). We attributed this small difference to P35 inhibition of caspases activated by the dominant inhibitory activity of these proteins (Fig. 9E). Consistent with caspase cleavage within the leader, SfIAPΔRM1 and H347A-mutated SfIAPM1 were accompanied by smaller fragments (Fig. 9C, asterisks) that disappeared upon P35 coexpression (Fig. 9C, lanes 5 to 8). P35 had little or no effect on SfIAPM4 (Fig. 9B, lanes 9 to 16), suggesting that leaderless SfIAP is insensitive to caspase cleavage.
To rule out the possibility that initiator caspase activity affected SfIAP stability, we also coexpressed baculovirus P49, which is a direct inhibitor of the Spodoptera initiator caspase responsible for effector caspase activation (56). When compared directly with P35, P49 had no effect on the steady-state levels of leader-containing SfIAPM1 (Fig. 9F, lanes 1 to 3 and 7 to 9). Like P35, P49 prevented the appearance of the caspase cleavage products of dominant inhibitors SfIAPΔRM1 and H347A-mutated SfIAPM1 (Fig. 9F, compare lanes 7 and 10 with lanes 9 and 12). Thus, neither initiator nor effector caspase activity affected the levels of wild-type SfIAP. Confirming this conclusion, the steady-state level of caspase cleavage-resistant D83A-mutated SfIAPM1 was comparable to that of wild-type SfIAPM1 (Fig. 9F, compare lanes 1 to 3 with lanes 4 to 6). Collectively, these data indicated that caspase activity fails to explain the reduced steady-state levels of SfIAP and suggested that the N-terminal leader regulates protein turnover by a caspase-independent mechanism.
DISCUSSION
SfIAP is the principal regulator of apoptosis in the baculovirus host Spodoptera frugiperda. We report here that on the basis of intracellular activities and biochemical properties, SfIAP closely resembles baculovirus Op-IAP3. Upon overexpression, both IAPs require the same motifs and prevent apoptosis at a step upstream of caspase activation. Nonetheless, endogenous SfIAP is a low-abundance protein that is rapidly depleted upon infection and thus fails to protect cells from virus-induced apoptosis (R. J. Cerio, K. Schultz, R. Vandergaast, and P. D. Friesen, unpublished data). As shown here, this intracellular instability of SfIAP is governed in part by its N-terminal leader sequence, which is absent within viral IAPs. These differences and similarities between cellular and viral IAPs have provided important insight into the mechanisms of IAP function in invertebrates and are consistent with the hypothesis that baculovirus IAPs were derived by capture of host cell IAP (21).
SfIAP inhibition of caspase activation and activity.
Site-directed mutagenesis demonstrated that SfIAP requires BIR1, BIR2, and its C-terminal RING motif for antiapoptotic activity when overexpressed in Spodoptera cells (Fig. 1 and 2). Given that SfIAP has high (42%) sequence similarity to baculovirus-encoded Op-IAP3 (20), the critical nature of these defining motifs was predictable. For certain cellular IAPs like DIAP1, the BIRs bind and suppress the activity of caspases (36). Indeed, SfIAP can interact with effector caspases through its BIRs (45; R. Vandergaast, D. Tran, and P. D. Friesen, unpublished data). However, when overexpressed, SfIAP functioned upstream of effector caspase activation, as SfIAP blocked the proteolytic cleavage of pro-Sf-caspase-1 in Spodoptera cells (Fig. 2D) and pro-DrICE in Drosophila cells (26). Furthermore, overexpressed SfIAP blocked the cleavage of baculovirus P49 (Fig. 2C), which is a direct substrate inhibitor of the initiator caspase that activates Sf-caspase-1 (25, 56). Thus, SfIAP can function at or upstream of the activation of the P49-sensitive initiator caspase that mediates baculovirus-induced apoptosis. As such, SfIAP probably inhibits the same step in the apoptosis pathway as baculovirus Op-IAP3 or a step adjacent to that inhibited by Op-IAP3 (56). Nonetheless, whereas SfIAP has the capacity to bind active effector caspases and block apoptosis in lepidopteran and dipteran cells, Op-IAP3 does not (26, 45, 46, 54). Thus, there exist significant differences in the core mechanisms by which SfIAP and viral IAPs block apoptosis.
Regulation of SfIAP stability.
Endogenous SfIAP is a low-abundance protein that is difficult to detect when directly compared to those produced by our plasmid expression vectors (Fig. 9B). However, by using highly purified antiserum and Sfiap-specific RNA silencing, we have determined that SfIAPM1 is the principal translation product of Sfiap in SF21 cells (R. Vandergaast, R. J. Cerio, and P. D. Friesen, unpublished data). When overproduced at equivalent levels, SfIAPM1 and SfIAPM4 have comparable antiapoptotic activity levels. Nonetheless, we demonstrated here that SfIAPM1 is significantly less stable than SfIAPM4 (Fig. 9 and 10). Full-length SfIAPM1 exhibited a half-life of ∼30 min, which is comparable to that of DIAP1, the principal IAP of Drosophila. However, removal of the 99-residue SfIAP leader increased protein stability by a factor of 5 and indicated that the N-terminal leader confers significant instability to SfIAPM1. Thus, although the N-terminal leader does not directly affect antiapoptotic function, it is a principal regulator of intracellular levels of SfIAP.
N-end rule ubiquitination and degradation has been implicated in the regulation of DIAP1 levels (10, 45). Caspase-mediated cleavage of the N-terminal DIAP1 leader at DQVD20↓N exposes an N-terminal Asn residue, thereby recruiting N-end rule ubiquitination machinery, which destabilizes DIAP1. Although caspase cleavage sites are located in the N-terminal leader of SfIAP, including DKTD83-N (Fig. 9A), our studies here suggested that caspase activity has a minimal effect on SfIAP stability. In particular, the levels of wild-type SfIAPM1 were unaffected when caspase activity was inhibited by coexpression of baculovirus caspase inhibitors P35 and P49 (Fig. 9). Confirming this conclusion, the intracellular level of caspase-resistant D83A-mutated SfIAPM1 was comparable to that of wild-type SfIAPM1 (Fig. 9F). Importantly, our study also demonstrated that leaderless SfIAPM4, which lacks all N-terminal caspase-cleavage sites, exhibited potent antiapoptotic activity in Spodoptera and Drosophila cell lines (Fig. 2 and 3) (26). These findings suggest that caspase cleavage is not required for SfIAP antiapoptotic function.
Our study also suggests that SfIAP motifs other than that of the N-terminal leader regulate protein stability. For example, disruption of BIR1 also reduced steady-state levels of leaderless SfIAPM4 (Fig. 8B). Inactivation of the C-terminal RING restored protein levels, suggesting that BIR1 and the predicted E3 ubiquitin ligase activity of the RING contribute to SfIAP stability. Loss of its RING also stabilizes DIAP1 (52). The observation that SfIAP possesses multiple determinants that affect its intracellular concentration is consistent with the critical role that this cellular IAP plays in determining cell fate, including that after virus infection.
Role of oligomerization in IAP function.
Immunoprecipitations and size exclusion chromatography (Fig. 4 and 5) indicated that SfIAPM4 exists primarily as a homodimer. The finding that RINGless SfIAPM4 forms a complex with and inhibits wild-type SfIAP (Fig. 3, 4, and 6) also suggested that oligomerization contributes to antiapoptotic function. As such, SfIAP mimics viral Op-IAP3, which also forms homodimers (Fig. 5) and is potently inhibited by RINGless mutations (19). Homo- and hetero-oligomerization have been described for human IAPs, including XIAP and c-IAP1. The domains required for IAP oligomerization vary (28, 29, 42, 43). In the case of Op-IAP3, the RING is dispensable for oligomerization (19). Likewise, the SfIAP RING and BIRs are not required for homo-oligomeric interactions (Fig. 7). Nonetheless, other experiments have indicated that the isolated SfIAP RING interacts with SfIAP (D. Tran and P. D. Friesen, unpublished data). Thus, it is likely that multiple domains contribute to SfIAP oligomerization.
The biological relevance of IAP oligomerization is unknown. Our findings here that RINGless SfIAP interacts and interferes with wild-type SfIAP suggest that two RING motifs are required for E3 ligase ubiquitination activity. Dimerized RING motifs confer optimal E3 activity from certain RING-containing proteins, including human XIAP (9, 14, 23, 43). SfIAP may require cooperative interaction between two RING motifs as well. Thus, a complex of SfIAPΔR with full-length SfIAP might have insufficient ubiquitination activity, which would increase levels of caspase normally turned over by interaction with endogenous SfIAP. Consistent with this possibility, SfIAPΔR increased caspase activation and activity in transfected cells without affecting the level of SfIAP (Fig. 4 and 6). SfIAP dimerization may also be necessary for proper binding of ligands destined for ubiquitination and turnover. Because caspases form active dimers (37), a dimeric BIR may be necessary to provide a stable interface for RING-mediated inactivation by ubiquitination. Likewise, a dimeric BIR interface may be necessary for interaction with IBM-containing prodeath factors. Our studies indicate that the oligomeric state of an IAP is an important consideration for mechanistic studies.
Dominant inhibition by loss-of-function IAPs.
Disruptions of either the RING or the BIR motifs of SfIAP functioned as dominant negative mutations (Fig. 3, 6, and 7). These findings suggest a common mode of action for cellular and viral IAPs. In contrast to RINGless Op-IAP3 (19), the dominant inhibitory, proapoptotic activity of RINGless SfIAP was selectively lost upon disruption of BIR1 (Fig. 6 and 7); BIR2 was dispensable for this activity. This finding implies that BIR1 interacts with a factor(s) involved in dominant inhibition and that the function of BIR1 is linked to that of the RING. It has been reported that BIR1-containing SfIAP truncations interact with active effector Sf-caspase-1 (45). Thus, one of several possible mechanisms for dominant inhibition by RINGless SfIAP involves the binding of caspases to BIR1 in such a way that caspase activity is not destroyed by ubiquitination. This nonproductive titration would increase intracellular caspase levels (Fig. 6) and sensitize the cell to apoptosis. Disruptions of BIR1 or BIR2 within full-length SfIAP also generated dominant inhibitors (Fig. 7). This finding is of particular interest because it indicates that a dominant inhibitor can possess a RING motif. These full-length mutations also complexed with SfIAP (Fig. 9), suggesting that their inhibitory effects involved direct interaction with SfIAP. Interestingly, BIR1- but not BIR2-disrupted SfIAP was less stable with rather than without its RING (Fig. 8). This finding suggested that BIR1-disrupted SfIAP may be more sensitive to autoubiquitination and that BIR1 regulates RING-mediated ubiquitination. Additional experiments are required to test this important possibility.
Host origin for viral IAPs?
To date, there are few unequivocal cases of direct host gene capture (transfer) by DNA viruses (12, 21, 22). On the basis of phylogenetic relatedness, it has been proposed that baculovirus-encoded IAPs were obtained through the capture of host lepidopteran IAPs on at least two independent occasions (21). Assuming that this acquisition was relatively recent, viral and cellular IAPs should share structural motifs, biochemical properties, and interactions with host components. As we have shown here, SfIAP requires the same functional motifs (BIR1, BIR2, and RING) and acts at the same step to block caspase activation and apoptosis as baculovirus Op-IAP3. Moreover, RINGless truncations of both of these IAPs act as dominant inhibitors of wild-type IAP. Both IAPs form intracellular dimers and can interact with their dominant inhibitors. Interestingly, viral Op-IAP3 can also interact with SfIAP, indicating that oligomerization domains may be shared (R. J. Cerio and P. D. Friesen, unpublished data). Collectively, these data support a common origin for both proteins.
The most striking feature that distinguishes SfIAP is its N terminus. A 30- to 100-residue leader preceding BIR1 is a prominent attribute of many cellular IAPs of lepidopteran, dipteran, and hymenopteran species. However, most, if not all, viral IAPs either lack the leader or possess just a few residues prior to BIR1. Our studies indicate that the N-terminal SfIAP leader is a principal determinant of protein turnover. We expect that the leader possesses a regulatory motif(s) that provides a critical means by which to govern intracellular concentrations of the host IAP to meet cellular demands for preventing or promoting apoptosis. In the case of virus infection, virus-induced depletion of cellular IAPs would lead to rapid apoptosis, as observed with DIAP1 in RNA virus-infected Drosophila cells (41). In contrast, viral IAPs function over a narrow window of infection to prevent apoptosis and thus would benefit from immunity to the mechanisms downregulating cellular IAP levels. Thus, a virus-encoded IAP that lacks an N-terminal regulatory leader or is deficient in host-responsive destabilizing motifs would have improved protein stability and longer-lasting antiapoptotic activity compared to those of host IAPs. Our study here suggests the simple possibility that viral IAPs evolved from cellular IAPs in a process that included the loss of the N-terminal regulatory leader. Additional studies promise to provide insight into this important aspect of host-virus evolution.
Acknowledgments
We thank John Reed for the gift of the SfIAP plasmid and Yuri Lazebnik for the anti-P35 serum. We also thank Rebecca Hozak for her preliminary work in generating and characterizing the pIE1prm-SfIAP and pIE1prm-SfIAPΔR plasmids, Melinda Brady-Osborne for anti-SfIAP preparation, Diccon Fiore for generation of recombinants vSfIAP and rvSfIAP/lacZ, and members of our lab for helpful discussions.
This work was supported in part by Public Health Service grants AI25557 and AI40482 from the National Institute of Allergy and Infectious Diseases (to P.D.F.) and NIH Predoctoral Traineeship T32 GM07215 (to R.J.C. and R.V.).
Footnotes
Published ahead of print on 25 August 2010.
REFERENCES
- 1.Ahmad, M., S. M. Srinivasula, L. Wang, G. Litwack, T. Fernandes-Alnemri, and E. S. Alnemri. 1997. Spodoptera frugiperda caspase-1, a novel insect death protease that cleaves the nuclear immunophilin FKBP46, is the target of the baculovirus antiapoptotic protein p35. J. Biol. Chem. 272:1421-1424. [DOI] [PubMed] [Google Scholar]
- 2.Bertin, J., S. M. Mendrysa, D. J. LaCount, S. Gaur, J. F. Krebs, R. C. Armstrong, K. J. Tomaselli, and P. D. Friesen. 1996. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J. Virol. 70:6251-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Best, S. M. 2008. Viral subversion of apoptotic enzymes: escape from death row. Annu. Rev. Microbiol. 62:171-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnbaum, M. J., R. J. Clem, and L. K. Miller. 1994. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J. Virol. 68:2521-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brzovic, P. S., J. R. Keeffe, H. Nishikawa, K. Miyamoto, D. Fox III, M. Fukuda, T. Ohta, and R. Klevit. 2003. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc. Natl. Acad. Sci. U. S. A. 100:5646-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartier, J. L., P. A. Hershberger, and P. D. Friesen. 1994. Suppression of apoptosis in insect cells stably transfected with baculovirus p35: dominant interference by N-terminal sequences p35(1-76). J. Virol. 68:7728-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clem, R. J., and L. K. Miller. 1994. Control of programmed cell death by the baculovirus genes p35 and iap. Mol. Cell. Biol. 14:5212-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crook, N. E., R. J. Clem, and L. K. Miller. 1993. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 67:2168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshaies, R. J., and C. A. Joazeiro. 2009. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78:399-434. [DOI] [PubMed] [Google Scholar]
- 10.Ditzel, M., R. Wilson, T. Tenev, A. Zachariou, A. Paul, E. Deas, and P. Meier. 2003. Degradation of DIAP1 by the N-end rule pathway is essential for regulating apoptosis. Nat. Cell Biol. 5:467-473. [DOI] [PubMed] [Google Scholar]
- 11.Duckett, C. S., V. E. Nava, R. W. Gedrich, R. J. Clem, J. L. Van Dongen, M. C. Gilfillan, H. Shiels, J. M. Hardwick, and C. B. Thompson. 1996. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 15:2685-2694. [PMC free article] [PubMed] [Google Scholar]
- 12.Filee, J., N. Pouget, and M. Chandler. 2008. Phylogenetic evidence for extensive lateral acquisition of cellular genes by nucleocytoplasmic large DNA viruses. BMC Evol. Biol. 8:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy, M. P., and P. D. Friesen. 2008. Reactive-site cleavage residues confer target specificity to baculovirus P49, a dimeric member of the P35 family of caspase inhibitors. J. Virol. 82:7504-7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashizume, R., M. Fukuda, I. Maeda, H. Nishikawa, D. Oyake, Y. Yabuki, H. Ogata, and T. Ohta. 2001. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 276:14537-14540. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins, C. J., P. G. Ekert, A. G. Uren, S. P. Holmgreen, and D. L. Vaux. 1998. Anti-apoptotic potential of insect cellular and viral IAPs in mammalian cells. Cell Death Differ. 5:569-576. [DOI] [PubMed] [Google Scholar]
- 16.Hay, B. A., and M. Guo. 2006. Caspase-dependent cell death in Drosophila. Annu. Rev. Cell Dev. Biol. 22:623-650. [DOI] [PubMed] [Google Scholar]
- 17.Herman-Bachinsky, Y., H. D. Ryoo, A. Ciechanover, and H. Gonen. 2007. Regulation of the Drosophila ubiquitin ligase DIAP1 is mediated via several distinct ubiquitin system pathways. Cell Death Differ. 14:861-871. [DOI] [PubMed] [Google Scholar]
- 18.Hershberger, P. A., J. A. Dickson, and P. D. Friesen. 1992. Site-specific mutagenesis of the 35-kilodalton protein gene encoded by Autographa californica nuclear polyhedrosis virus: cell line-specific effects on virus replication. J. Virol. 66:5525-5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hozak, R. R., G. A. Manji, and P. D. Friesen. 2000. The BIR motifs mediate dominant interference and oligomerization of inhibitor of apoptosis Op-IAP. Mol. Cell. Biol. 20:1877-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, Q., Q. L. Deveraux, S. Maeda, G. S. Salvesen, H. R. Stennicke, B. D. Hammock, and J. C. Reed. 2000. Evolutionary conservation of apoptosis mechanisms: lepidopteran and baculoviral inhibitor of apoptosis proteins are inhibitors of mammalian caspase-9. Proc. Natl. Acad. Sci. U. S. A. 97:1427-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes, A. L. 2002. Evolution of inhibitors of apoptosis in baculoviruses and their insect hosts. Infect. Genet. Evol. 2:3-10. [DOI] [PubMed] [Google Scholar]
- 22.Iyer, L. M., S. Balaji, E. V. Koonin, and L. Aravind. 2006. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 117:156-184. [DOI] [PubMed] [Google Scholar]
- 23.Kawai, H., V. Lopez-Pajares, M. M. Kim, D. Wiederschain, and Z. M. Yuan. 2007. RING domain-mediated interaction is a requirement for MDM2's E3 ligase activity. Cancer Res. 67:6026-6030. [DOI] [PubMed] [Google Scholar]
- 24.LaCasse, E. C., D. J. Mahoney, H. H. Cheung, S. Plenchette, S. Baird, and R. G. Korneluk. 2008. IAP-targeted therapies for cancer. Oncogene 27:6252-6275. [DOI] [PubMed] [Google Scholar]
- 25.LaCount, D. J., S. F. Hanson, C. L. Schneider, and P. D. Friesen. 2000. Caspase inhibitor P35 and inhibitor of apoptosis Op-IAP block in vivo proteolytic activation of an effector caspase at different steps. J. Biol. Chem. 275:15657-15664. [DOI] [PubMed] [Google Scholar]
- 26.Lannan, E., R. Vandergaast, and P. D. Friesen. 2007. Baculovirus caspase inhibitors P49 and P35 block virus-induced apoptosis downstream of effector caspase DrICE activation in Drosophila melanogaster cells. J. Virol. 81:9319-9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, H. H., and L. K. Miller. 1978. Isolation of genotypic variants of Autographa californica nuclear polyhedrosis virus. J. Virol. 27:754-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, S. C., Y. Huang, Y. C. Lo, M. Lu, and H. Wu. 2007. Crystal structure of the BIR1 domain of XIAP in two crystal forms. J. Mol. Biol. 372:847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu, M., S. C. Lin, Y. Huang, Y. J. Kang, R. Rich, Y. C. Lo, D. Myszka, J. Han, and H. Wu. 2007. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol. Cell 26:689-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mace, P. D., K. Linke, R. Feltham, F. R. Schumacher, C. A. Smith, D. L. Vaux, J. Silke, and C. L. Day. 2008. Structures of the cIAP2 RING domain reveal conformational changes associated with ubiquitin-conjugating enzyme (E2) recruitment. J. Biol. Chem. 283:31633-31640. [DOI] [PubMed] [Google Scholar]
- 31.Manji, G. A., and P. D. Friesen. 2001. Apoptosis in motion. An apical, P35-insensitive caspase mediates programmed cell death in insect cells. J. Biol. Chem. 276:16704-16710. [DOI] [PubMed] [Google Scholar]
- 32.Manji, G. A., R. R. Hozak, D. J. LaCount, and P. D. Friesen. 1997. Baculovirus inhibitor of apoptosis functions at or upstream of the apoptotic suppressor P35 to prevent programmed cell death. J. Virol. 71:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Means, J. C., I. Muro, and R. J. Clem. 2003. Silencing of the baculovirus Op-iap3 gene by RNA interference reveals that it is required for prevention of apoptosis during Orgyia pseudotsugata M nucleopolyhedrovirus infection of Ld652Y cells. J. Virol. 77:4481-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muro, I., B. A. Hay, and R. J. Clem. 2002. The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J. Biol. Chem. 277:49644-49650. [DOI] [PubMed] [Google Scholar]
- 35.O'Riordan, M. X., L. D. Bauler, F. L. Scott, and C. S. Duckett. 2008. Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation. Dev. Cell 15:497-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orme, M., and P. Meier. 2009. Inhibitor of apoptosis proteins in Drosophila: gatekeepers of death. Apoptosis 14:950-960. [DOI] [PubMed] [Google Scholar]
- 37.Riedl, S. J., and Y. Shi. 2004. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5:897-907. [DOI] [PubMed] [Google Scholar]
- 38.Rumble, J. M., and C. S. Duckett. 2008. Diverse functions within the IAP family. J. Cell Sci. 121:3505-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider, I. 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27:353-365. [PubMed] [Google Scholar]
- 40.Seshagiri, S., and L. K. Miller. 1997. Baculovirus inhibitors of apoptosis (IAPs) block activation of Sf-caspase-1. Proc. Natl. Acad. Sci. U. S. A. 94:13606-13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Settles, E. W., and P. D. Friesen. 2008. Flock house virus induces apoptosis by depletion of Drosophila inhibitor-of-apoptosis protein DIAP1. J. Virol. 82:1378-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silke, J., C. J. Hawkins, P. G. Ekert, J. Chew, C. L. Day, M. Pakusch, A. M. Verhagen, and D. L. Vaux. 2002. The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase 3- and caspase 9-interacting sites. J. Cell Biol. 157:115-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silke, J., T. Kratina, D. Chu, P. G. Ekert, C. L. Day, M. Pakusch, D. C. Huang, and D. L. Vaux. 2005. Determination of cell survival by RING-mediated regulation of inhibitor of apoptosis (IAP) protein abundance. Proc. Natl. Acad. Sci. U. S. A. 102:16182-16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasula, S. M., and J. D. Ashwell. 2008. IAPs: what's in a name? Mol. Cell 30:123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tenev, T., M. Ditzel, A. Zachariou, and P. Meier. 2007. The antiapoptotic activity of insect IAPs requires activation by an evolutionarily conserved mechanism. Cell Death Differ. 14:1191-1201. [DOI] [PubMed] [Google Scholar]
- 46.Tenev, T., A. Zachariou, R. Wilson, M. Ditzel, and P. Meier. 2005. IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat. Cell Biol. 7:70-77. [DOI] [PubMed] [Google Scholar]
- 47.Vaughn, J. L., R. H. Goodwin, G. J. Tompkins, and P. McCawley. 1977. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 13:213-217. [DOI] [PubMed] [Google Scholar]
- 48.Vaux, D. L., and J. Silke. 2005. IAPs, RINGs and ubiquitylation. Nat. Rev. Mol. Cell Biol. 6:287-297. [DOI] [PubMed] [Google Scholar]
- 49.Vucic, D., and W. J. Fairbrother. 2007. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin. Cancer Res. 13:5995-6000. [DOI] [PubMed] [Google Scholar]
- 50.Vucic, D., W. J. Kaiser, and L. K. Miller. 1998. A mutational analysis of the baculovirus inhibitor of apoptosis Op-IAP. J. Biol. Chem. 273:33915-33921. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson, J. C., A. S. Wilkinson, F. L. Scott, R. A. Csomos, G. S. Salvesen, and C. S. Duckett. 2004. Neutralization of Smac/Diablo by inhibitors of apoptosis (IAPs). A caspase-independent mechanism for apoptotic inhibition. J. Biol. Chem. 279:51082-51090. [DOI] [PubMed] [Google Scholar]
- 52.Wilson, R., L. Goyal, M. Ditzel, A. Zachariou, D. A. Baker, J. Agapite, H. Steller, and P. Meier. 2002. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell Biol. 4:445-450. [DOI] [PubMed] [Google Scholar]
- 53.Wright, C. W., and R. J. Clem. 2002. Sequence requirements for Hid binding and apoptosis regulation in the baculovirus inhibitor of apoptosis Op-IAP. Hid binds Op-IAP in a manner similar to Smac binding of XIAP. J. Biol. Chem. 277:2454-2462. [DOI] [PubMed] [Google Scholar]
- 54.Wright, C. W., J. C. Means, T. Penabaz, and R. J. Clem. 2005. The baculovirus anti-apoptotic protein Op-IAP does not inhibit Drosophila caspases or apoptosis in Drosophila S2 cells and instead sensitizes S2 cells to virus-induced apoptosis. Virology 335:61-71. [DOI] [PubMed] [Google Scholar]
- 55.Yoo, S. J., J. R. Huh, I. Muro, H. Yu, L. Wang, S. L. Wang, R. M. Feldman, R. J. Clem, H. A. Muller, and B. A. Hay. 2002. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 4:416-424. [DOI] [PubMed] [Google Scholar]
- 56.Zoog, S. J., J. J. Schiller, J. A. Wetter, N. Chejanovsky, and P. D. Friesen. 2002. Baculovirus apoptotic suppressor P49 is a substrate inhibitor of initiator caspases resistant to P35 in vivo. EMBO J. 21:5130-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]