Abstract
Understanding the determinants of neutralization sensitivity and resistance is important for the development of an effective human immunodeficiency virus type 1 (HIV-1) vaccine. In these studies, we have made use of the swarm of closely related envelope protein variants (quasispecies) from an extremely neutralization-resistant clinical isolate in order to identify mutations that conferred neutralization sensitivity to antibodies in sera from HIV-1-infected individuals. Here, we describe a virus with a rare mutation at position 179 in the V2 domain of gp120, where replacement of aspartic acid (D) by asparagine (N) converts a virus that is highly resistant to neutralization by multiple polyclonal and monoclonal antibodies, as well as antiviral entry inhibitors, to one that is sensitive to neutralization. Although the V2 domain sequence is highly variable, D at position 179 is highly conserved in HIV-1 and simian immunodeficiency virus (SIV) and is located within the LDI/V recognition motif of the recently described α4β7 receptor binding site. Our results suggest that the D179N mutation induces a conformational change that exposes epitopes in both the gp120 and the gp41 portions of the envelope protein, such as the CD4 binding site and the MPER, that are normally concealed by conformational masking. Our results suggest that D179 plays a central role in maintaining the conformation and infectivity of HIV-1 as well as mediating binding to α4β7.
A major goal in human immunodeficiency virus type 1 (HIV-1) vaccine research is the identification of immunogens able to elicit protective immunity from HIV-1 infection. Results from the recent RV144 clinical trial in Thailand (53) have provided evidence that immunization with vaccines containing the recombinant HIV-1 envelope glycoprotein gp120 (6, 7) can protect humans from HIV infection when incorporated in a prime/boost immunization regimen. Although the level of protection observed in the RV144 trial (31%) was modest, it represents a significant advance in HIV-1 vaccine research and has rekindled the efforts to identify improved subunit vaccine antigens that might achieve even higher levels of protection. In these studies, we have sought to understand the molecular determinants of neutralization sensitivity and resistance in HIV-1 envelope proteins for the purpose of developing improved vaccine antigens.
In previous studies (47), we have described a novel method of mutational analysis of the HIV-1 envelope protein, termed swarm analysis, for identification of mutations that confer sensitivity and/or resistance to broadly neutralizing antibodies (bNAbs). This method makes use of the natural amino acid sequence virus variation that occurs in each HIV-infected individual to establish panels of closely related envelope proteins that differ from each other by a limited number of amino acid substitutions. We have previously used this method to identify a novel amino acid substitution in gp41 that conferred sensitivity to neutralization by monoclonal and polyclonal antibodies as well as virus entry inhibitors. In this paper, we describe a mutation in the V2 domain of gp120 that similarly induces a neutralization-sensitive phenotype in an otherwise neutralization-resistant envelope sequence.
Previous studies (10, 14, 33, 40, 43, 52, 72, 74) have suggested that sequences in the V2 domain act as the “global regulator of neutralization sensitivity” and confer neutralization resistance by restricting access to epitopes located in the V3 domain, the CD4 binding site, and chemokine receptor binding sites through “conformational masking” of neutralizing epitopes. Deletion of the V2 domain markedly increases neutralization sensitivity (10, 57, 62, 74), and several envelope proteins with V2 domain deletions have been developed as candidate HIV-1 vaccines (5, 42, 61). In this paper, we show that a single substitution of asparagine (N) for aspartic acid (D) at position 179 in the C-terminal portion of the V2 domain (corresponding to position 180 in HXB2 numbering) converts a highly neutralization-resistant virus to a neutralization-sensitive virus with a phenotype similar to that described for V2 domain deletion mutants. Position 179 has recently attracted attention as a critical element of the α4β7 integrin binding site that affects virus tropism to the gut (2). Our results suggest that mutation at position 179 results in a conformational change that increases neutralization sensitivity by exposure of epitopes in both gp120 and gp41 that are normally masked in the trimeric structure of gp160 and thus are unavailable for antibody binding.
MATERIALS AND METHODS
Envelope genes and swarm analysis.
Libraries of full-length envelope genes were isolated by reverse transcription-PCR (RT-PCR) from cryopreserved plasma samples from patients who became infected with HIV-1 while participating in the VAX004 phase 3 trial of the AIDSVAX B/B vaccine (20). The specimens selected for analysis represented recent infections with a mean estimated time after infection of 109 ± 58 days (48). A panel of clade B reference isolates was obtained from the NIH AIDS Reagent Repository and included JRCSF, YU2, QHO69.42, and TRO-11 (GenBank accession numbers U63632, M93258.1, AY835439, and AY835445). The JRCSF and YU2 envelope genes were isolated from proviral clones by PCR and cloned into an expression vector. The QH0692.42 and TRO-11 envelopes were obtained as full-length Env/Rev cassettes and were subcloned directly into the standard Monogram Biosciences expression vector for pseudovirus production. The swarm analysis protocol was described previously and is an application of the clonal analysis procedure developed by Monogram Biosciences (South San Francisco, CA) (19, 21, 22, 24, 28-32, 47). Briefly, the population of viral envelope genes present in the patient plasma was amplified by RT-PCR and then cloned into expression vectors. To test individual clones derived from the envelope population, the DNA was diluted and retransformed in bacteria, and individual clones were selected and screened for infectivity using the Monogram Biosciences coreceptor tropism assay. Pseudotype viruses containing cloned envelope genes were prepared from each patient plasma sample in 293 HEK cells. Viruses from individual clones were screened for infectivity and chemokine receptor tropism in U87 cells transfected with CD4 and the CCR5 or CXCR4 chemokine receptors as described previously (69). Ten to 12 envelopes with high infectivity were selected from each individual and evaluated in virus neutralization assays (described below).
In vitro mutagenesis.
Mutations were introduced into HIV-1 envelope proteins by site-directed mutagenesis using a QuikChange Lightning kit (Agilent, Santa Clara, CA) followed by confirmatory sequencing. Chimeric envelope genes were created by transferring PCR-amplified fragments between neutralization-sensitive and -resistant mutants. To facilitate this transfer, novel restriction sites preserving the virus sequence were introduced.
Antibodies and antiviral drugs.
Four sera (Z23, Z1679, Z1684, and N16) from HIV-1 infected individuals (HIV-1-positive sera) known from previous studies (18, 59) to possess bNAbs were provided by Monogram Biosciences, Inc. (South San Francisco, CA). Six monoclonal antibodies (MAbs) with broadly neutralizing activity were obtained from the NIH AIDS Reagent Repository and/or Polymun AG (Vienna, Austria). These included 2G12, b12, 17b, 2F5, 4E10, and 447D-52 (4, 9, 15, 26, 46, 64, 66, 67, 77). MAbs to the α4β7 integrins were obtained from two sources. The Act-1 MAb (38) was obtained from the NIH AIDS Reagent Repository, and the α4/VLA-4/CD49d MAb was purchased from R&D Bio-systems (Minneapolis, MN). A cyclized synthetic peptide (CWLDVC) reported to be a ligand for α4β7 (2) was obtained from GenScript (Piscataway, NJ). The antiviral compound CD4-IgG was described previously (3, 11) and provided by GSID (South San Francisco, CA). The peptide-based antiviral drug enfuvirtide (Fuzeon) was commercially available and produced by Roche, Inc. (Basel, Switzerland).
Virus neutralization assay.
The study utilized a high-throughput virus neutralization assay to measure the ability of monoclonal antibodies and antibodies in HIV-1-positive plasma to inhibit infection of pseudotype viruses (17, 49, 55, 59). Briefly, pseudotype viruses were prepared by cotransfecting 293 cells with an envelope expression vector and an envelope-deficient HIV-1 genomic vector carrying a luciferase reporter gene. The virus-antibody mixture was incubated for 1 h prior to inoculation of U87 cells expressing CD4, CCR5, and CXCR4. Cells were then incubated for 3 days, and then viral infectivity was measured by luciferase expression. Neutralization data were reported as the 50% inhibitory concentration (IC50) calculated from serum dilution curves. The positive controls included pseudoviruses prepared from the neutralization-sensitive HIV-1 isolate NL43 and the less neutralization-sensitive primary isolate JRCSF. The negative virus control consisted of pseudotype viruses prepared from the envelope of the amphotropic murine leukemia virus (aMLV). HIV-1 neutralization titers were considered significant only if they were greater than three times the aMLV titers.
Sequence analysis.
The Los Alamos HIV database (http://hiv.lanl.gov/), the GSID HIV Data Browser (http://www.gsid.org/gsid_hiv_data_browser.html), and the NCBI nucleotide database (http://www.ncbi.nlm.nih.gov/nuccore) were interrogated to determine the degree of amino acid conservation at position 179 (180 according to HXB2 numbering). Alignments were performed using the EMBOSS suite (54). Because of insertions and deletions, it was not practical to identify each amino acid by use of the standard HXB2 numbering. Amino acid positions are provided with reference to the sequences from the envelope genes from clones 108051-005 and 108051-006 (GenBank accession numbers HM769943 and HM769944, respectively). Wherever possible, corresponding HXB2 numbering is provided in the text along with the 108051 numbering. An amino acid sequence alignment of the envelope proteins from clones 005 and 006 of the 108051 virus as well as the HXB2 envelope reference sequence is provided in Fig. S1 in the supplemental material.
RESULTS
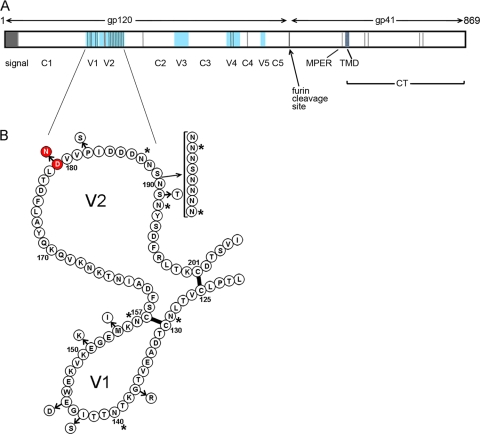
In previous studies (47), we described the analysis of clade B envelope genes obtained from a cohort of 28 individuals infected with HIV-1 during the course of the VAX004 HIV vaccine trial that ran from 1998 to 2003 (20). In these studies, we identified seven cases where neutralization-sensitive and neutralization-resistant clones were both observed in the same individual. The first pair of envelopes analyzed was obtained from subject 108060 and allowed us to identify a mutation in a previously unexplored hydrogen-bonded ring structure that conferred sensitivity and resistance to bNAbs. In this paper, we report the analysis of viruses obtained from another individual (108051) in this cohort. The envelope genes from subject 108051 were amplified by RT-PCR from cryopreserved plasma collected at the first postdiagnosis blood draw. Envelope genes were analyzed for infectivity and chemokine receptor usage, and 10 envelopes with robust infectivity were isolated and evaluated in virus neutralization assays against a panel of HIV-1-positive sera, Z23, Z1679, Z1684, and N16, known to possess bNAbs (59). As can be seen in Table 1, most of the clones from patient 108051 were highly resistant to neutralization by all four sera, with only 2 of 10 (clones 006 and 015) being sensitive to neutralization. Based on the magnitude of the difference in neutralization titers, we selected clones 005 and 006 for further studies. Clone 015 gave a somewhat different pattern of neutralization sensitivity and was set aside for future studies. When we sequenced and aligned the translated gene products, we found a total of 25 individual amino acid differences between the sensitive and resistant clones (Fig. 1 A). Some differences were due to isolated amino acid substitutions, and others represented clusters of differences resulting from deletions and insertions. Further examination revealed that 16 of the 25 amino acid differences were located in the V1 and V2 domains (Fig. 1B). To localize the amino acids responsible for the difference in neutralization sensitivity, we systematically transferred sequences individually and in clusters from the sensitive clone 006 envelope protein into the resistant clone 005 protein.
TABLE 1.
Neutralization of pseudoviruses containing HIV-1 envelope genes from subject 108051
| Clonea | Neutralizing antibody titer (IC50) for indicated human HIV-positive serum sampleb |
|||
|---|---|---|---|---|
| Z23 | N16 | Z1684 | Z1679 | |
| 005 | <40 | <40 | <40 | <40 |
| 006 | 1,114 | 354 | 490 | 824 |
| 009 | <40 | <40 | <40 | <40 |
| 011 | <40 | <40 | <40 | <40 |
| 013 | <40 | <40 | <40 | <40 |
| 015 | 96 | 164 | 87 | 172 |
| 016 | <40 | <40 | <40 | <40 |
| 018 | 42 | 73 | <40 | 56 |
| 021 | <40 | <40 | <40 | <40 |
| 022 | 45 | 50 | 43 | 72 |
“Clone” indicates the pseudotype virus prepared using the specified gp160 envelope genes. All clones tested were CCR5 tropic.
HIV-positive sera Z23, N16, Z1684, and Z1679, known to possess broadly neutralizing antibodies (bNAbs). The neutralizing antibody titer (IC50) is defined as the reciprocal of the plasma dilution that produces a 50% inhibition in target cell infection. Values in bold represent significant neutralization titers that are at least 3 times greater than those observed for the negative-control virus (aMLV).
FIG. 1.
Location of amino acid differences between neutralization-sensitive and -resistant clones isolated from subject 108051. HIV-1 envelope genes were isolated from the swarm of variants in plasma from subject 108051 and tested for sensitivity and resistance to neutralization. (A) The sequences of the neutralization-resistant clone 005 and the neutralization-sensitive clone 006 were aligned, and amino acid differences (vertical lines) were located on the linear sequence. Conserved (C) and variable (V) domains (blue) of gp120 are indicated, as well as the locations of the signal sequence (signal), membrane-proximal external domain (MPER), transmembrane domain (TMD), and cytoplasmic tail (CT). (B) Amino acid sequence differences in the V1 and V2 domains between the neutralization-resistant clone 005 (contiguous sequence) and neutralization-sensitive clone 006 (circles with arrows). Red circles indicate the locations of the D179N mutation. Open circles indicate the positions of other amino acid substitutions. Asterisks indicate the positions of N-linked glycosylation sites.
Identification of a mutation in gp160 from subject 108051 that confers sensitivity to neutralization by HIV-1-positive sera.
When the panel of mutants was examined (Table 2), we found that single-amino-acid substitutions at positions 272, 462, and 644 had no effect on neutralization sensitivity or resistance. Similarly, a cluster of mutations in the cytoplasmic tail at positions 746, 748, 846, and 847 had no effect on sensitivity or resistance. Likewise, a cluster of amino acid substitutions at the C-terminal portion of gp120, including substitutions at positions 412, 413, and 462, had no effect on sensitivity. However, we found that the chimeric envelope protein containing the V1/V2 domain from clone 006 inserted into the backbone of the 005 envelope gene markedly increased sensitivity and exhibited neutralization titers comparable to those seen with the neutralization-sensitive clone 006.
TABLE 2.
Neutralization of pseudotype viruses with wild-type and mutated envelope genes from subject 108051 by HIV-positive sera possessing broadly neutralizing antibodies
| Clonea | Mutation(s) | Neutralization antibody titer (IC50) for indicated serum sampleb |
|||
|---|---|---|---|---|---|
| Z23 | N16 | Z1684 | Z1679 | ||
| 005 | wtR | <100 | <20 | <20 | <20 |
| 006 | wtS | 1,805 | 609 | 1,023 | 2,194 |
| 005 | T746I, K748E, V846R, R847Q | <100 | <20 | <20 | <20 |
| 005 | Y412Δ, T413S,G462D | <100 | <20 | <20 | <20 |
| 005 | E272K | <100 | 27 | <20 | <20 |
| 005 | G462D | <100 | <20 | <20 | <20 |
| 005 | Q644R | <100 | <20 | <20 | 26 |
| 005 | V1/V2_006 | 2,123 | 749 | 1,328 | 2,177 |
| 005 | V1_006 | <100 | <20 | <20 | <20 |
| 005 | V2_006 | 1,457 | 390 | 827 | 1,412 |
| 006 | V1_005 | 1,930 | 827 | 763 | 2,786 |
| 006 | V2_005 | <100 | <20 | <20 | <20 |
| 005 | 189 insert NNNSNNN, S191T | <100 | <20 | <20 | <20 |
| 005 | P182S | <100 | <20 | <20 | <20 |
| 005 | D179N | 1,875 | 272 | 1,024 | 3,094 |
| 005 | D179N, P182S | 1,391 | 773 | 584 | 2,731 |
| 006 | N179D | <100 | <20 | <20 | <20 |
| 006 | N179D, S182P | <100 | <20 | <20 | <20 |
Wild-type resistant (wtR) and wild-type sensitive (wtS) clones from subject 108051 are indicated. The V1_006 and V2_006 designations indicate chimeric envelope proteins in which the V1 and/or V2 domain of clone 006 replaces that of clone 005. V1_005 and V2_005 designations indicate chimeric envelope proteins in which the V1 and/or V2 domain of clone 005 replaces that of clone 006. “Δ” indicates deletion.
The neutralizing antibody titer (IC50) is defined as the reciprocal of the plasma dilution that produces a 50% inhibition in target cell infection. Values in bold represent neutralization titers that are at least 3 times greater than those observed for the negative control (aMLV). All clones tested were CCR5 tropic.
These studies located the sequences responsible for increased neutralization sensitivity to either the V1 domain or the V2 domain. We then carried out further experiments to determine which domain was responsible (Table 2). We found that replacement of the clone 005 V1 domain with the V1 domain from clone 006 (V1_006) did not confer increased neutralization sensitivity. However, replacement of the V2 domain of clone 005 with that from clone 006 resulted in increased sensitivity, similar to that of the neutralization-sensitive clone 006 (Table 2). In the converse experiment, we transferred the V1 and V2 sequences of the resistant clone 005 envelope protein into the sensitive clone 006 envelope protein. Transferring the V1 domain preserved the neutralization-sensitive phenotype, whereas transferring the V2 domain resulted in loss of the neutralization-sensitive phenotype. Together, these studies clearly indicated that the difference in neutralization sensitivity between clones 005 and 006 could be attributed to the differences in the V2 domain.
Further studies were carried out to determine whether the increase in neutralization sensitivity could be localized to specific amino acid substitutions in the V2 domain. As described above, there were 11 amino acid differences in the V2 domain between the sensitive and resistant clones of the 108051 virus. Further mutagenesis enabled us to rule out an eight-amino-acid insertion between positions 189 and 190 as well as single-amino-acid changes at positions 182 and 191 (Table 2). However, the single-amino-acid substitution of asparagine (N) for aspartic acid (D) at position 179 (corresponding to HXB2 position 180) markedly increased neutralization sensitivity and clearly accounted for the difference in neutralization between the neutralization-resistant clone 005 and the neutralization-sensitive clone 006.
In these studies, it can be seen that the largest increases in neutralization sensitivity occurred with the Z1679 and Z1684 sera, where neutralization sensitivity increased by 150-fold and 50-fold, respectively, compared to the level for the neutralization-resistant clone 005. The effect on sensitivity to N16 and Z23 was more moderate, with 13- and 18-fold increases, respectively, possibly indicating some differences in the magnitude and/or specificity of particular neutralizing antibody populations in each of the sera. The reverse mutation of N to D at position 179 conferred neutralization resistance to the neutralization-sensitive 006 clone, unambiguously confirming the importance of D at position 179 in conferring the sensitive phenotype. The fact that multiple single-amino-acid substitutions or clusters of substitutions at other locations within gp160 had no effect on the neutralization phenotype showed that increased neutralization sensitivity is not a trivial artifact. Rather, only specific amino acid substitutions at specific sites are able to convert a neutralization-resistant virus to a neutralization-sensitive virus (Tables 2 and 3 ).
TABLE 3.
Sensitivity of 10851 mutants to neutralizing monoclonal antibodies and entry inhibitors
| Clonea | Mutation(s) | IC50 (μg/ml) of indicated MAb or fusion inhibitorb |
|||||
|---|---|---|---|---|---|---|---|
| CD4-IgG | 2F5 | 4E10 | 447-D52 | 17b | Enfuvirtide | ||
| 005 | wtR | >20 | >20 | >20 | >20 | >20 | 0.308 |
| 006 | wtS | 0.14 | 1.29 | 0.21 | >20 | 13.34 | 0.020 |
| 005 | D179N | 0.10 | 0.96 | 0.15 | >20 | 4.25 | 0.023 |
| 006 | N179D | >20 | 16.29 | 8.02 | >20 | >20 | 0.178 |
| 005 | D179E | 0.18 | 1.74 | 0.59 | >20 | 12.11 | 0.091 |
| 005 | D179Q | 0.17 | 0.43 | 0.14 | >20 | 2.64 | 0.036 |
| 005 | D179H | 0.11 | 0.55 | 0.11 | >20 | 3.29 | 0.032 |
| 005 | D179S | 0.15 | 0.53 | 0.15 | >20 | 5.60 | 0.037 |
| 005 | D179A | 0.09 | 0.66 | 0.10 | >20 | 4.35 | 0.037 |
| 005 | P182S | >20 | >20 | >20 | ND | ND | 0.182 |
| 005 | D179N, P182S | 0.221 | 1.314 | 1.585 | ND | ND | 0.018 |
| 005 | 189 insert NNNSNNN, S191T | >20 | >20 | >20 | ND | ND | 0.096 |
| 005 | V1/V2_006 sensitive | 0.097 | 0.935 | 1.117 | ND | ND | 0.018 |
| 005 | V1_006 sensitive | >20 | 19.04 | >20 | ND | ND | 0.173 |
| 005 | V2_006 sensitive | 0.357 | 1.670 | 2.135 | ND | ND | 0.017 |
| 006 | V1_005 resistant | 0.146 | 0.641 | 0.965 | ND | ND | 0.010 |
| 006 | V2_005 resistant | >20 | >20 | >20 | ND | ND | 0.096 |
wtR, wild-type resistant clone; wtS, wild-type sensitive clone; ND, not done. The V1_006 and V2_006 designations indicate chimeric envelope proteins in which the V1 and/or V2 domain of clone 006 replaces that of clone 005. The V1_005 and V2_005 designations indicate chimeric envelope proteins in which the V1 and/or V2 domain of clone 005 replaces that of clone 006. All clones tested were CCR5 tropic. The IC50s for b12 and 2G12 for all clones and mutations tested were >20 μg/ml.
The neutralizing antibody titer (IC50) is defined as the concentration of monoclonal antibodies or antiviral entry inhibitor that produces a 50% inhibition in target cell infection. IC50 values in bold print are at least 3 times greater than the IC50 values measured for the specificity control virus (aMLV) and are therefore considered positive for neutralization in this assay.
Sensitivity to neutralization by MAbs and virus entry inhibitors.
In order to investigate the mechanism by which the replacement of D with N at position 179 in the V2 loop alters neutralization sensitivity, we investigated the effects of monoclonal antibodies (MAbs) and virus entry inhibitors that target defined regions of the envelope protein (Table 3). These included the b12, 17b, 2G12, and 447D-52 MAbs, known to neutralize HIV-1 and bind to epitopes in gp120 (4, 9, 15, 26, 66, 67), and the 2F5 and 4E10 MAbs, known to bind to epitopes in gp41 (46, 64, 77). In addition to these MAbs, we also made use of the antiviral entry inhibitors CD4-IgG and enfuvirtide to further define the mechanism of neutralization sensitivity. The antiviral entry inhibitor CD4-IgG binds to the CD4 binding site in gp120 and is able to neutralize laboratory-adapted CXCR4-dependent isolates at low concentration (0.01 to 0.1 μg/ml) and primary CCR5-dependent primary isolates of HIV-1 (16) at a high concentration (10 to 100 μg/ml). Enfuvirtide is a peptide-based virus entry inhibitor (39, 70) that is thought to interfere with the formation of the six-helix bundle that is required for virus fusion. It is thought to bind to the prehairpin intermediate structure of gp41 that is transiently formed in gp41 (45) after binding of CD4 to the gp160 trimer (12, 23, 36).
The results obtained with this panel of inhibitors are shown in Table 3. We found that the wild-type neutralization-resistant clone 005 was resistant to all of the MAbs in the panel as well as to CD4-IgG. Because of its resistance to four HIV-1-positive sera possessing bNAbs, as well as its resistance to the broadly neutralizing MAbs 2F5, 4E10, 2G12, b12, and 447-D and to CD4-IgG, clone 005 appears to be a tier 3 (44) neutralization-resistant virus (D. Montefiori, personal communication). When we examined the properties of clone 006, we found that it was also completely resistant to neutralization by the b12, 2G12, and 447D-52 antibodies. Examination of the amino acid sequences of clones 005 and 006 showed that both envelopes contained polymorphisms in the epitopes recognized by these three MAbs. The 108051 envelope proteins all possess a GPGG sequence at the tip of the V3 loop rather than the clade B consensus GPGR motif required for 447D-52 binding (15, 26, 63). Similarly, the 108051 residues T286, T375, and M376 are known to be common in b12-resistant viruses and differed from the A281, V372, and T373 (HXB2 numbering) residues, common in b12-sensitive viruses (71, 75). Finally, the lack of inhibition by the 2G12 antibody could be attributed to the fact that the 108051 envelope protein lacks two of four glycosylation sites at positions 300 and 395 (corresponding to HXB2 positions 295 and 392) essential for binding by this antibody (58). Thus, the resistance of clones 005 and 006 to the b12, 2G12, and 447D-52 antibodies could be attributed to polymorphisms at neutralizing epitopes. However, since antibodies with specificities similar to those of b12 and 2G12 are rare in HIV-1-positive sera (8, 56), another explanation was required to account for the neutralization resistance of clone 005 to polyclonal sera (Table 1) and the remaining monoclonal antibodies in this panel (Table 3).
First, we examined the sensitivity of the neutralization-sensitive and -resistant variants to CD4-IgG (Table 3). Previous studies (36, 37, 41) have shown that the CD4 binding site is located entirely within the gp120 portion of the HIV-1 envelope protein, recessed deeply below the apex in the native trimer. We found that a high CD4-IgG concentration (>20 μg/ml) was required for neutralization of 108051 clone 005, which was consistent with the concentration required to neutralize other primary isolates of CCR5-dependent viruses (16). Replacement of D with N at position 179 in clone 005 increased sensitivity to CD4-IgG approximately 200 times compared to the level for the wild-type neutralization-resistant clone 005 envelope. Conversely, we found that the neutralization-sensitive clone 006 could be converted to the CD4-IgG-resistant phenotype by replacement of N with D at position 179.
We next examined the effect of the D179N mutation on 17b, a neutralizing MAb known to target a conserved CD4-induced (CD4i) epitope on gp120 overlapping the coreceptor binding region (36, 66, 67). Clone 005 was resistant to neutralization by 17b at 20 μg/ml, and the neutralization-sensitive clone 006 was marginally more sensitive, with an IC50 of 13.3 μg/ml (Table 3). However, the clone 005 envelope with the D179N mutation was approximately 5-fold more sensitive to neutralization by this antibody. This result suggests that the D179N mutation enhances neutralization by the 17b MAb but suggests that other sequence differences between clone 005 and clone 006 also affect the binding of this antibody.
We next considered the effect of the D179N mutation on sensitivity to MAbs and entry inhibitors that target sites in the gp41 protein (Table 3). Interestingly, replacement of D with N at position 179 in the V2 domain had a significant effect on sensitivity to neutralization by the two broadly neutralizing MAbs 4E10 and 2F5, directed to the membrane-proximal external region (MPER) of gp41. The epitopes recognized by these antibodies are well defined, with the 2F5 MAb recognizing the ELDWA sequence and the 4E10 MAb recognizing the adjacent NWF(D/N)IT sequence (46, 76, 77). Recent studies suggest that the peptide in which these sequences occur is partially embedded in the lipid bilayer (60). We found that the D179N mutation increased neutralization sensitivity approximately 20-fold in the case of 2F5 and between 100- and 200-fold in the case of 4E10. This result showed that a single-amino-acid substitution in gp120 could have a dramatic effect on the neutralizing activity of antibodies directed to the gp41 domain. Similar results were obtained with the antiviral entry inhibitor enfuvirtide. This drug consists of a peptide derived from gp41 sequences that overlap the MPER domain and the C34 helix (70). The binding of enfuvirtide to gp41 is thought to depend on CD4 binding which induces a conformational change that exposes a binding site involving the HR1 domain of gp41 (25). The observation that sensitivity to enfuvirtide was increased 13-fold (Table 3) in the D179N mutant provides additional evidence that a mutation in the V2 domain of gp120 can modulate the potency of antiviral compounds targeting the gp41-mediated virus fusion mechanism.
Conservation of aspartic acid at position 179.
Comparative sequence analysis showed that position 179 (corresponding to HXB2 position 180) is highly conserved across all clades of HIV-1. We analyzed 5,918 sequences from 2,414 individuals in three datasets, including 1,963 curated and aligned sequences from the Los Alamos HIV Sequence database (HIV-1/SIVcpz; 2008) that listed one sequence per individual, a set of 2,908 sequences from 102 individuals with acute infections (1, 34), and 1,047 sequences from 349 individuals with recent infections from the VAX004 HIV vaccine trial (GSID HIV Data Browser [http://www.gsid.org/gsid_hiv_data_browser.html]). We found only a single naturally occurring HIV-1 sequence, other than the 108051 sequence from the GSID HIV Sequence database, where N replaced D at position 179 (GenBank accession number AF321080). Interestingly, we also found that the D in the LDI/LDV motif was conserved in simian immunodeficiency virus (SIV) and HIV-2, where it corresponded to position 201 in the SIV reference sequence (GenBank accession number M33262). We found that this residue was conserved in all 69 different HIV-2 and SIV sequences in the HIV-2/SIV/MN 2008 Los Alamos HIV Sequence database. Given the high degree of sequence variation among these primate lentiviruses, D179 would be preserved over time and across species only if it played an important role in the survival of these viruses.
Further studies were carried out to try to understand the mechanism by which aspartic acid at position 179 modulates neutralization sensitivity in 108051. In these studies, we constructed a series of mutants where D at position 179 was replaced by other amino acids (Tables 3 and 4). We found that replacement of D at 179 with the hydrophilic, basic amino acids arginine (R) and lysine (K) or the hydrophobic branched-chain isoleucine (I) residue failed to yield infectious virus. This result suggests that D179 must interact with other parts of the envelope protein and that these interactions can alter virus infectivity. In contrast, it was possible to replace D179 with other amino acids that preserved virus infectivity. For example, replacement of D with amino acids with short side chains, such as alanine (A), serine (S), and glycine (G), resulted in infectious viruses. Replacement of D179 with bulky side chains, such as histidine (H), glutamine (Q), or the negatively charged glutamic acid (E), also resulted in infectious viruses. However, all of these replacements increased sensitivity to neutralization by the polyclonal HIV-positive sera (Table 4) and the 2F5, 4E10, and 17b MAbs as well as CD4-IgG and enfuvirtide (Table 3). Replacement of D with glutamic acid (E), whose acidic side chain is only 1 carbon longer than D, preserved CCR5 tropism but similarly increased neutralization sensitivity. This result indicates that there must be an extremely restrictive structural constraint required to preserve neutralization resistance. Thus, the only amino acid that we have found that can maintain the neutralization-resistant phenotype is D at position 179.
TABLE 4.
Envelope protein mutagenesis for investigation of the significance of aspartic acid at position 179 in clones of the 108051 and 108048 envelope proteins
| Clonea | Mutation(s) | Neutralization antibody titer (IC50) for indicated serum sampleb |
|||
|---|---|---|---|---|---|
| Z23 | N16 | Z1684 | Z1679 | ||
| 005 | wtR | <100 | <40 | <40 | <40 |
| 006 | wtS | 1,524 | 276 | 832 | 705 |
| 005 | D179N | 3,963 | 662 | 873 | 602 |
| 006 | N179D | <100 | <40 | <40 | <40 |
| 005 | D179E | 1,792 | 167 | 611 | 261 |
| 005 | D179Q | 4,997 | 524 | 1,661 | 1,020 |
| 005 | D179H | 5,121 | 623 | 1,078 | 1,410 |
| 005 | D179S | 3,626 | 379 | 859 | 816 |
| 005 | D179A | 3,804 | 503 | 1,056 | 1,137 |
| 005 | L178R, D179G, V180D | 3,001 | 289 | 563 | 921 |
| 108048_002 | wtR | 119 | 55 | <40 | <40 |
| 108048_002 | D179N | NI | NI | NI | NI |
| 108048_002 | V1/V2_006 | 12,541 | 1,729 | 2,729 | 1,528 |
Neutralization-resistant and -sensitive clones were obtained from subject 108051 (clones 005 and 006) or from subject 108048. V1/V2_006 represents a chimeric envelope where the V1 and V2 domains of 108051_006 replaced the V1 and V2 domains of the 108048_002 envelope. “NI” indicates no infectivity.
The neutralizing antibody titer (IC50) is defined as the reciprocal of the plasma dilution that produces a 50% inhibition in target cell infection. Values in bold represent neutralization titers that are at least 3 times greater than those observed for the negative control (aMLV). All clones tested were CCR5 tropic.
In theory, the high level of conservation of D179 might be critical for maintaining the conformation of the envelope protein or might be involved with receptor binding. Indeed, D179 has recently been highlighted as part of the LDV/I recognition motif that forms the newly described α4β7 receptor binding site on gp120 (2). Based on this observation, we examined the effect of α4β7 binding inhibitors on virus neutralization in order to determine if disruption of α4β7 binding could account for the observed increase in neutralization sensitivity associated with the D179N mutation. We found (see Table S1 in the supplemental material) that neither the Act-1 MAb to α4β7 nor the cyclic peptide inhibitor CWLDVC (2) was able to inhibit the infectivity of JRCSF, NL43, or the wild-type neutralization-sensitive and -resistant clones of 108051 in the U87 cell pseudotype neutralization assay. However, both inhibitors (Act-1 and cyclic CWLDVC) were able to prevent the binding of recombinant gp120 to a cell line (65) expressing α4β7 in a flow cytometry assay (D. Fonseca and P. Berman, unpublished results). These results suggest that the U87 target cells used in our assay lack the α4β7 receptor and demonstrate that the increased neutralization sensitivity of the D179N mutant cannot be attributed to disruption of interactions mediated by α4β7 in our assay system. However, this mutation might be expected to interfere with infectivity in systems where the α4β7 receptor is expressed on target cells.
Transfer of the D179N mutation to other viruses.
To investigate possible strain-specific differences of the D179N mutation on increased neutralization sensitivity, we attempted to transfer this mutation to five other, unrelated viruses. For this purpose, we selected four commonly used tier 2 viruses from standard neutralization panels exhibiting a range of neutralization sensitivities, specifically JRCSF, YU2, QH0692, and TRO-11. In addition, we also examined neutralization sensitivity in another virus, 108048, from the VAX004 trial. All five viruses possessed D at the position corresponding to position 179 of the 108051 virus. Whereas all five wild-type viruses were infectious in the U87 pseudotype assay, we found that replacement of D with N at positions corresponding to 108051 position 179 resulted in viruses with little or no infectivity. This result suggested that D179N is essential for infectivity and suggests that compensatory mutations may be necessary to preserve infectivity when D is replaced by N at this position. To further explore this possibility, we replaced the entire V1 and V2 domains of the neutralization-resistant 108048 virus with that of the 108051 virus containing the D179N mutation. As can be seen in Table 4, replacement of the entire V1 and V2 domains from 108051 markedly increased sensitivity to neutralization by the 4 HIV-1-positive sera, suggesting that the compensatory mutations required to increase neutralization sensitivity while preserving infectivity are located within the V1 or V2 domains.
DISCUSSION
The results presented in this study show that a single-amino-acid mutation, D179N, in the V2 domain of gp120 can convert a highly neutralization-resistant virus to a neutralization-sensitive virus. The fact that the D179N mutation increased sensitivity to neutralization by MAbs and antiviral drugs, targeting both gp120 and gp41, suggests that the D179N mutation induces a conformation change that affects accessibility of multiple neutralizing epitopes, rather than affecting the contact residues of a single neutralizing antibody binding site. These results suggest a far greater level of interaction between these two subunits, with respect to neutralization sensitivity, than was previously appreciated. The fact that D179 is conserved in HIV-1, SIV, and HIV-2 suggests that D at position 179 may have been preserved throughout evolution in order to preserve resistance to neutralization by antibodies targeting epitopes in both gp120 and gp41.
Our results are consistent with previous studies that have identified the V2 domain of gp120 as the “global regulator of neutralization sensitivity” (51, 52). Because the V2 domain can be deleted entirely in some viruses while preserving virus viability (10, 57, 62), it seems unlikely that the V2 domain provides a contact surface required for infectivity or virus fusion. Rather, it appears to provide an epitope-“masking” function that is thought to conceal important neutralizing epitopes from neutralizing antibodies until the envelope protein undergoes a conformational change triggered by CD4 binding (35, 36, 41). This hypothesis is supported by studies showing increased binding of antibodies to neutralizing epitopes in the V3 and C4 domains by envelope proteins lacking the V2 domain (10, 50, 52, 62, 72). In this regard, the single-amino-acid substitution of N for D at position 179 appears to confer the same phenotype as that observed when the entire V2 domain is deleted from the SF162 virus (5, 10, 27, 61, 62, 72-74). Further data supporting the role of the V2 domain in regulating neutralization sensitivity is provided by studies showing that sensitivity and resistance to neutralization can be transferred by moving the V2 domain from a neutralization-sensitive virus (e.g., SF162) onto a neutralization-resistant virus (e.g., JR-FL) backbone. Conversely, the neutralization-sensitive SF162 virus can be converted to a neutralization-resistant virus by exchange of the V2 domain with that of JR-FL (52).
Although conformational masking by the V2 domain appears to explain most of the data relating to the ability of the V2 domain to modulate neutralization sensitivity and resistance (10, 43, 50, 52, 62, 72, 74), the molecular interactions determining how the mask is “raised and lowered” have not been characterized. Our results suggest that D179 mediates a key interaction required for maintenance of the neutralization-resistant, “masked” state. Replacement of D with N at position 179 seems to open up the structure of the gp160 trimer and makes the virus more sensitive to neutralization by exposing epitopes in both gp120 and gp41. Aspartic acid at position 179 appears to be unique, since it appears in all but two of more than 5,918 virus sequences in the 3 datasets examined and since all of the other mutations created in vitro at this position resulted in either noninfectious viruses or viruses with increased neutralization sensitivity. The lack of representation of viruses with mutations at position 179 in other data sets might reflect the fact that all other variants are noninfectious or are so sensitive to neutralization that they are rapidly eliminated from circulation once envelope-specific antibody responses have developed. The fact that transfer of the D179N mutation to five unrelated viruses (YU2, JRCSF, QH0692.42, TRO-11, and 108048) all resulted in noninfectious viruses is consistent with the importance of D179 in preserving the functional structure of the envelope protein and suggests that compensatory mutations are required in other parts of the molecule to preserve infectivity when D179 is replaced with N. In this regard, the need for compensatory mutations may be similar to that observed with V2 domain deletions where deletion of the V2 domain in the SF162 strain results in infectious viruses, whereas deletion of the V2 domain in other strains (e.g., HXB2) requires compensatory mutations to maintain virus infectivity (57). This possibility is supported by the V1/V2 domain replacement experiment (Table 4), where it was found that replacement of the entire V1/V2 domain could increase sensitivity to neutralization by HIV-1-positive sera, while preserving infectivity. With respect to mutations at position 179, the amino acid substitutions that destroyed infectivity may have stabilized the masking function to such an extent as to prevent the conformational changes required for infectivity following receptor binding.
Our data are also consistent with the hypothesis that the V2 masking function is dependent on quaternary interactions between the gp160 subunits that associate to form the trimeric envelope structure that mediates virus infectivity and fusion (13, 35, 36). Based on structural studies involving cryoelectron tomography and X-ray data fitting, the V1 and V2 domains appear to be located at the apex of an intermolecular contact region within the envelope glycoprotein trimer (41). According to this model, the native trimer is held together by strong contacts at the gp41 base and the V1/V2 regions, with little or no contact elsewhere. Upon CD4 binding, the monomers rotate with respect to the core of the trimer to “open” the center of the trimer, exposing CCR5 binding sites, shifting gp41 up toward the cell membrane to form the six-helix bundle, and exposing the fusion peptide at the target cell membrane (see Fig. S2 in the supplemental material). When viewed in the context of these observations, our data are consistent with the possibility that D179 provides interactions required to maintain the unligated trimeric structure. Accordingly, mutations at position 179 may weaken the quaternary, intersubunit interactions, thereby providing increased access of antibodies to parts of the molecule, such as the V3 domain, the CD4 binding site, and the MPER, that are normally located in the interior of the molecule and exposed only after CD4 binding. Further investigations using conformation-dependent antibodies to the V2 domain, such as the newly described PG9 and PG16 antibodies (68), might provide additional support for this model; studies using these antibodies as well as cryoelectron tomography are planned to further investigate this mutation.
The results reported herein confirm and extend our previous studies, in which swarm analysis has proved useful in identifying single-amino-acid substitutions that appear to trigger conformational changes that expose or conceal epitopes recognized by bNAbs. Envelopes with exposed neutralizing epitopes may represent a source of immunogens potentially more effective in eliciting bNAbs than those previously tested. Envelope proteins with deleted V2 domains have been tested as candidate HIV-1 vaccine antigens and were shown to elicit higher titers of neutralizing antibodies than wild-type proteins (5, 42, 61, 73). Studies are in progress to determine whether immunization with the D179N mutant described in these studies exhibits broader neutralizing activity, as seen with the V2-deleted envelope antigens.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Bill & Melinda Gates Foundation to Global Solutions for Infectious Diseases (South San Francisco, CA) and by funding provided by the University of California, Santa Cruz.
We thank Julie Goss (Monogram Biosciences) for her role in project management and Ann Durbin (UCSC) for expert technical assistance in the preparation of the manuscript. We thank D. Burton, J. Robinson, S. Zolla-Pazner, H. Kattinger, and A. A. Ansari for providing monoclonal antibodies through the NIH AIDS Research and Reference Reagent Program. We also thank I. S. Chen and Y. Yoyanagi for the pYKJRCSF clone, B. Hahn and G. Shaw for the pYU2 clone, and M. Li, F. Gao, and D. Montefiori for the QH0692.42 and TRO-11 envelope genes, also provided by the NIH AIDS Research and Reference Reagent Program.
Footnotes
Published ahead of print on 11 August 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abrahams, M. R., J. A. Anderson, E. E. Giorgi, C. Seoighe, K. Mlisana, L. H. Ping, G. S. Athreya, F. K. Treurnicht, B. F. Keele, N. Wood, J. F. Salazar-Gonzalez, T. Bhattacharya, H. Chu, I. Hoffman, S. Galvin, C. Mapanje, P. Kazembe, R. Thebus, S. Fiscus, W. Hide, M. S. Cohen, S. A. Karim, B. F. Haynes, G. M. Shaw, B. H. Hahn, B. T. Korber, R. Swanstrom, and C. Williamson. 2009. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J. Virol. 83:3556-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9:301-309. [DOI] [PubMed] [Google Scholar]
- 3.Ashkenazi, A., D. H. Smith, S. A. Marsters, L. Riddle, T. J. Gregory, D. D. Ho, and D. J. Capon. 1991. Resistance of primary isolates of human immunodeficiency virus type 1 to soluble CD4 is independent of CD4-rgp120 binding affinity. Proc. Natl. Acad. Sci. U. S. A. 88:7056-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbas, C. F., III, D. Hu, N. Dunlop, L. Sawyer, D. Cababa, R. M. Hendry, P. L. Nara, and D. R. Burton. 1994. In vitro evolution of a neutralizing human antibody to human immunodeficiency virus type 1 to enhance affinity and broaden strain cross-reactivity. Proc. Natl. Acad. Sci. U. S. A. 91:3809-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman, P. W. 1998. Development of bivalent rgp120 vaccines to prevent HIV type 1 infection. AIDS Res. Hum. Retroviruses 14(Suppl. 3):S277-S289. [PubMed] [Google Scholar]
- 7.Berman, P. W., W. Huang, L. Riddle, A. M. Gray, T. Wrin, J. Vennari, A. Johnson, M. Klaussen, H. Prashad, C. Kohne, C. deWit, and T. J. Gregory. 1999. Development of bivalent (B/E) vaccines able to neutralize CCR5-dependent viruses from the United States and Thailand. Virology 265:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, D. R., and C. F. Barbas III. 1994. Human antibodies from combinatorial libraries. Adv. Immunol. 57:191-280. [DOI] [PubMed] [Google Scholar]
- 10.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capon, D. J., S. M. Chamow, J. Mordenti, S. A. Marsters, T. Gregory, H. Mitsuya, R. A. Byrn, C. Lucas, F. M. Wurm, J. E. Groopman, S. Broder, and D. H. Smith. 1989. Designing CD4 immunoadhesins for AIDS therapy. Nature 337:525-531. [DOI] [PubMed] [Google Scholar]
- 12.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 13.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 14.Cheng-Mayer, C., A. Brown, J. Harouse, P. A. Luciw, and A. J. Mayer. 1999. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J. Virol. 73:5294-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conley, A. J., M. K. Gorny, J. A. Kessler II, L. J. Boots, M. Ossorio-Castro, S. Koenig, D. W. Lineberger, E. A. Emini, C. Williams, and S. Zolla-Pazner. 1994. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J. Virol. 68:6994-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daar, E. S., X. L. Li, T. Moudgil, and D. D. Ho. 1990. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc. Natl. Acad. Sci. U. S. A. 87:6574-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeks, S. G., B. Schweighardt, T. Wrin, J. Galovich, R. Hoh, E. Sinclair, P. Hunt, J. M. McCune, J. N. Martin, C. J. Petropoulos, and F. M. Hecht. 2006. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J. Virol. 80:6155-6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhillon, A. K., H. Donners, R. Pantophlet, W. E. Johnson, J. M. Decker, G. M. Shaw, F. H. Lee, D. D. Richman, R. W. Doms, G. Vanham, and D. R. Burton. 2007. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J. Virol. 81:6548-6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eshleman, S. H., D. Jones, J. Galovich, E. E. Paxinos, C. J. Petropoulos, J. B. Jackson, and N. Parkin. 2006. Phenotypic drug resistance patterns in subtype A HIV-1 clones with nonnucleoside reverse transcriptase resistance mutations. AIDS Res. Hum. Retroviruses 22:289-293. [DOI] [PubMed] [Google Scholar]
- 20.Flynn, N. M., D. N. Forthal, C. D. Harro, F. N. Judson, K. H. Mayer, and M. F. Para. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654-665. [DOI] [PubMed] [Google Scholar]
- 21.Fransen, S., G. Bridger, J. M. Whitcomb, J. Toma, E. Stawiski, N. Parkin, C. J. Petropoulos, and W. Huang. 2008. Suppression of dualtropic human immunodeficiency virus type 1 by the CXCR4 antagonist AMD3100 is associated with efficiency of CXCR4 use and baseline virus composition. Antimicrob. Agents Chemother. 52:2608-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fransen, S., M. Karmochkine, W. Huang, L. Weiss, C. J. Petropoulos, and C. Charpentier. 2009. Longitudinal analysis of raltegravir susceptibility and integrase replication capacity of human immunodeficiency virus type 1 during virologic failure. Antimicrob. Agents Chemother. 53:4522-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frey, G., H. Peng, S. Rits-Volloch, M. Morelli, Y. Cheng, and B. Chen. 2008. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. U. S. A. 105:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost, S. D., Y. Liu, S. L. Pond, C. Chappey, T. Wrin, C. J. Petropoulos, S. J. Little, and D. D. Richman. 2005. Characterization of human immunodeficiency virus type 1 (HIV-1) envelope variation and neutralizing antibody responses during transmission of HIV-1 subtype B. J. Virol. 79:6523-6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 26.Gorny, M. K., J. Y. Xu, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J. Immunol. 150:635-643. [PubMed] [Google Scholar]
- 27.Gzyl, J., E. Bolesta, A. Wierzbicki, D. Kmieciak, T. Naito, M. Honda, K. Komuro, Y. Kaneko, and D. Kozbor. 2004. Effect of partial and complete variable loop deletions of the human immunodeficiency virus type 1 envelope glycoprotein on the breadth of gp160-specific immune responses. Virology 318:493-506. [DOI] [PubMed] [Google Scholar]
- 28.Huang, W., S. H. Eshleman, J. Toma, S. Fransen, E. Stawiski, E. E. Paxinos, J. M. Whitcomb, A. M. Young, D. Donnell, F. Mmiro, P. Musoke, L. A. Guay, J. B. Jackson, N. T. Parkin, and C. J. Petropoulos. 2007. Coreceptor tropism in human immunodeficiency virus type 1 subtype D: high prevalence of CXCR4 tropism and heterogeneous composition of viral populations. J. Virol. 81:7885-7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang, W., S. H. Eshleman, J. Toma, E. Stawiski, J. M. Whitcomb, J. B. Jackson, L. Guay, P. Musoke, N. Parkin, and C. J. Petropoulos. 2009. Vertical transmission of X4-tropic and dual-tropic HIV-1 in five Ugandan mother-infant pairs. AIDS 23:1903-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, W., A. Gamarnik, K. Limoli, C. J. Petropoulos, and J. M. Whitcomb. 2003. Amino acid substitutions at position 190 of human immunodeficiency virus type 1 reverse transcriptase increase susceptibility to delavirdine and impair virus replication. J. Virol. 77:1512-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, W., J. Toma, S. Fransen, E. Stawiski, J. D. Reeves, J. M. Whitcomb, N. Parkin, and C. J. Petropoulos. 2008. Coreceptor tropism can be influenced by amino acid substitutions in the gp41 transmembrane subunit of human immunodeficiency virus type 1 envelope protein. J. Virol. 82:5584-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang, W., J. Toma, E. Stawiski, S. Fransen, T. Wrin, N. Parkin, J. M. Whitcomb, E. Coakley, F. M. Hecht, S. G. Deeks, R. T. Gandhi, S. H. Eshleman, and C. J. Petropoulos. 2009. Characterization of human immunodeficiency virus type 1 populations containing CXCR4-using variants from recently infected individuals. AIDS Res. Hum. Retroviruses 25:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, W. E., H. Sanford, L. Schwall, D. R. Burton, P. W. Parren, J. E. Robinson, and R. C. Desrosiers. 2003. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J. Virol. 77:9993-10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keele, B. F., E. E. Giorgi, J. F. Salazar-Gonzalez, J. M. Decker, K. T. Pham, M. G. Salazar, C. Sun, T. Grayson, S. Wang, H. Li, X. Wei, C. Jiang, J. L. Kirchherr, F. Gao, J. A. Anderson, L. H. Ping, R. Swanstrom, G. D. Tomaras, W. A. Blattner, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, M. S. Cohen, D. C. Montefiori, B. F. Haynes, B. Gaschen, G. S. Athreya, H. Y. Lee, N. Wood, C. Seoighe, A. S. Perelson, T. Bhattacharya, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 36.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lasky, L. A., G. Nakamura, D. H. Smith, C. Fennie, C. Shimasaki, E. Patzer, P. Berman, T. Gregory, and D. J. Capon. 1987. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell 50:975-985. [DOI] [PubMed] [Google Scholar]
- 38.Lazarovits, A. I., R. A. Moscicki, J. T. Kurnick, D. Camerini, A. K. Bhan, L. G. Baird, M. Erikson, and R. B. Colvin. 1984. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J. Immunol. 133:1857-1862. [PubMed] [Google Scholar]
- 39.Lazzarin, A., B. Clotet, D. Cooper, J. Reynes, K. Arasteh, M. Nelson, C. Katlama, H. J. Stellbrink, J. F. Delfraissy, J. Lange, L. Huson, R. DeMasi, C. Wat, J. Delehanty, C. Drobnes, and M. Salgo. 2003. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N. Engl. J. Med. 348:2186-2195. [DOI] [PubMed] [Google Scholar]
- 40.Li, Y., B. Cleveland, I. Klots, B. Travis, B. A. Richardson, D. Anderson, D. Montefiori, P. Polacino, and S. L. Hu. 2008. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J. Virol. 82:638-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, J., A. Bartesaghi, M. J. Borgnia, G. Sapiro, and S. Subramaniam. 2008. Molecular architecture of native HIV-1 gp120 trimers. Nature 455:109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu, S., R. Wyatt, J. F. Richmond, F. Mustafa, S. Wang, J. Weng, D. C. Montefiori, J. Sodroski, and H. L. Robinson. 1998. Immunogenicity of DNA vaccines expressing human immunodeficiency virus type 1 envelope glycoprotein with and without deletions in the V1/2 and V3 regions. AIDS Res. Hum. Retroviruses 14:151-155. [DOI] [PubMed] [Google Scholar]
- 43.Ly, A., and L. Stamatatos. 2000. V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies. J. Virol. 74:6769-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mascola, J. R., P. D'Souza, P. Gilbert, B. H. Hahn, N. L. Haigwood, L. Morris, C. J. Petropoulos, V. R. Polonis, M. Sarzotti, and D. C. Montefiori. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 79:10103-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muñoz-Barroso, I., S. Durell, K. Sakaguchi, E. Appella, and R. Blumenthal. 1998. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 140:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Rourke, S. M., B. Schweighardt, W. G. Scott, T. Wrin, D. P. Fonseca, F. Sinangil, and P. W. Berman. 2009. Novel ring structure in the gp41 trimer of human immunodeficiency virus type 1 that modulates sensitivity and resistance to broadly neutralizing antibodies. J. Virol. 83:7728-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Losada, M., D. V. Jobes, F. Sinangil, K. A. Crandall, D. Posada, and P. W. Berman. 2010. Phylodynamics of HIV-1 from a phase-III AIDS vaccine trial in North America. Mol. Biol. Evol. 27:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinter, A. 2007. Roles of HIV-1 Env variable regions in viral neutralization and vaccine development. Curr. HIV Res. 5:542-553. [DOI] [PubMed] [Google Scholar]
- 51.Pinter, A., W. J. Honnen, P. D'Agostino, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2005. The C108g epitope in the V2 domain of gp120 functions as a potent neutralization target when introduced into envelope proteins derived from human immunodeficiency virus type 1 primary isolates. J. Virol. 79:6909-6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinter, A., W. J. Honnen, Y. He, M. K. Gorny, S. Zolla-Pazner, and S. C. Kayman. 2004. The V1/V2 domain of gp120 is a global regulator of the sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J. Virol. 78:5205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rerks-Ngarm, S., P. Pitisuttithum, S. Nitayaphan, J. Kaewkungwal, J. Chiu, R. Paris, N. Premsri, C. Namwat, M. de Souza, E. Adams, M. Benenson, S. Gurunathan, J. Tartaglia, J. G. McNeil, D. P. Francis, D. Stablein, D. L. Birx, S. Chunsuttiwat, C. Khamboonruang, P. Thongcharoen, M. L. Robb, N. L. Michael, P. Kunasol, and J. H. Kim. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209-2220. [DOI] [PubMed] [Google Scholar]
- 54.Rice, P., I. Longden, and A. Bleasby. 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16:276-277. [DOI] [PubMed] [Google Scholar]
- 55.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sather, D. N., J. Armann, L. K. Ching, A. Mavrantoni, G. Sellhorn, Z. Caldwell, X. Yu, B. Wood, S. Self, S. Kalams, and L. Stamatatos. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saunders, C. J., R. A. McCaffrey, I. Zharkikh, Z. Kraft, S. E. Malenbaum, B. Burke, C. Cheng-Mayer, and L. Stamatatos. 2005. The V1, V2, and V3 regions of the human immunodeficiency virus type 1 envelope differentially affect the viral phenotype in an isolate-dependent manner. J. Virol. 79:9069-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schweighardt, B., Y. Liu, W. Huang, C. Chappey, Y. S. Lie, C. J. Petropoulos, and T. Wrin. 2007. Development of an HIV-1 reference panel of subtype B envelope clones isolated from the plasma of recently infected individuals. J. Acquir. Immune Defic. Syndr. 46:1-11. [DOI] [PubMed] [Google Scholar]
- 60.Song, L., Z. Y. Sun, K. E. Coleman, M. B. Zwick, J. S. Gach, J. H. Wang, E. L. Reinherz, G. Wagner, and M. Kim. 2009. Broadly neutralizing anti-HIV-1 antibodies disrupt a hinge-related function of gp41 at the membrane interface. Proc. Natl. Acad. Sci. U. S. A. 106:9057-9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srivastava, I. K., K. VanDorsten, L. Vojtech, S. W. Barnett, and L. Stamatatos. 2003. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J. Virol. 77:2310-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stamatatos, L., and C. Cheng-Mayer. 1998. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J. Virol. 72:7840-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanfield, R. L., M. K. Gorny, C. Williams, S. Zolla-Pazner, and I. A. Wilson. 2004. Structural rationale for the broad neutralization of HIV-1 by human monoclonal antibody 447-52D. Structure 12:193-204. [DOI] [PubMed] [Google Scholar]
- 64.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 17:1757-1765. [DOI] [PubMed] [Google Scholar]
- 65.Stupack, D. G., S. Stewart, W. G. Carter, E. A. Wayner, and J. A. Wilkins. 1991. B lymphocyte fibronectin receptors: expression and utilization. Scand. J. Immunol. 34:761-769. [DOI] [PubMed] [Google Scholar]
- 66.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 68.Walker, L. M., S. K. Phogat, P. Y. Chan-Hui, D. Wagner, P. Phung, J. L. Goss, T. Wrin, M. D. Simek, S. Fling, J. L. Mitcham, J. K. Lehrman, F. H. Priddy, O. A. Olsen, S. M. Frey, P. W. Hammond, S. Kaminsky, T. Zamb, M. Moyle, W. C. Koff, P. Poignard, and D. R. Burton. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whitcomb, J. M., W. Huang, S. Fransen, K. Limoli, J. Toma, T. Wrin, C. Chappey, L. D. Kiss, E. E. Paxinos, and C. J. Petropoulos. 2007. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob. Agents Chemother. 51:566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wild, C., T. Oas, C. McDanal, D. Bolognesi, and T. Matthews. 1992. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. U. S. A. 89:10537-10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu, X., T. Zhou, S. O'Dell, R. T. Wyatt, P. D. Kwong, and J. R. Mascola. 2009. Mechanism of human immunodeficiency virus type 1 resistance to monoclonal antibody B12 that effectively targets the site of CD4 attachment. J. Virol. 83:10892-10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyatt, R., J. Moore, M. Accola, E. Desjardin, J. Robinson, and J. Sodroski. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang, Z. Y., B. K. Chakrabarti, L. Xu, B. Welcher, W. P. Kong, K. Leung, A. Panet, J. R. Mascola, and G. J. Nabel. 2004. Selective modification of variable loops alters tropism and enhances immunogenicity of human immunodeficiency virus type 1 envelope. J. Virol. 78:4029-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye, Y., Z. H. Si, J. P. Moore, and J. Sodroski. 2000. Association of structural changes in the V2 and V3 loops of the gp120 envelope glycoprotein with acquisition of neutralization resistance in a simian-human immunodeficiency virus passaged in vivo. J. Virol. 74:11955-11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou, T., L. Xu, B. Dey, A. J. Hessell, D. Van Ryk, S. H. Xiang, X. Yang, M. Y. Zhang, M. B. Zwick, J. Arthos, D. R. Burton, D. S. Dimitrov, J. Sodroski, R. Wyatt, G. J. Nabel, and P. D. Kwong. 2007. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445:732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zwick, M. B., L. L. Bonnycastle, A. Menendez, M. B. Irving, C. F. Barbas III, P. W. Parren, D. R. Burton, and J. K. Scott. 2001. Identification and characterization of a peptide that specifically binds the human, broadly neutralizing anti-human immunodeficiency virus type 1 antibody b12. J. Virol. 75:6692-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zwick, M. B., R. Jensen, S. Church, M. Wang, G. Stiegler, R. Kunert, H. Katinger, and D. R. Burton. 2005. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J. Virol. 79:1252-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.