Abstract
Hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS) are severe diseases associated with hantavirus infection. High levels of virus replication occur in microvascular endothelial cells but without a virus-induced cytopathic effect. However, virus infection results in microvascular leakage, which is the hallmark of these diseases. VE-cadherin is a major component of adherens junctions, and its interaction with the vascular endothelial growth factor (VEGF) receptor, VEGF-R2, is important for maintaining the integrity of the endothelial barrier. Here we report that increased secreted VEGF and concomitant decreased VE-cadherin are seen at early times postinfection of human primary lung endothelial cells with an HPS-associated hantavirus, Andes virus. Furthermore, active virus replication results in increased permeability and loss of the integrity of the endothelial cell barrier. VEGF binding to VEGF-R2 is known to result in dissociation of VEGF-R2 from VE-cadherin and in VE-cadherin activation, internalization, and degradation. Consistent with this, we showed that an antibody which blocks VEGF-R2 activation resulted in inhibition of the Andes virus-induced VE-cadherin reduction. These data implicate virus induction of VEGF and reduction in VE-cadherin in the endothelial cell permeability seen in HPS and suggest potential immunotherapeutic targets for the treatment of the disease.
Hantaviruses, of the family Bunyaviridae, are rodent-borne RNA viruses. Members of the Hantavirus genus have been identified as etiologic agents of two severe human diseases: hemorrhagic fever with renal syndrome (HFRS), which is caused by the Old World hantaviruses, and hantavirus pulmonary syndrome (HPS), which is caused by the New World hantaviruses (38, 39). Sin Nombre virus (SNV) and Andes virus (ANDV) are the main causes of HPS in the Americas. The major hantavirus target in humans is the microvascular endothelium, and the basis of HPS and HFRS is attributed to microvascular leakage (9, 34, 57). Common clinical features of HPS are interstitial pneumonitis with variable amounts of mononuclear cell infiltration, congestion, and both interstitial and alveolar edema (4, 34, 57). Despite the prominent accumulation of viral antigen in the infected vascular endothelium, no evidence of cellular destruction has been observed (57). Absence of a cytopathic effect has also been reported in in vitro studies of hantavirus infection of human primary endothelial cells (35, 46). In general, it is believed that induction of an uncontrolled immune response to the hantavirus infection, rather than the viral infection per se, is the cause of the microvascular leakage and ultimately HPS and HFRS (3, 48, 57). So far, a limited number of in vitro permeability studies have reported either no significant changes in the vascular permeability upon hantavirus infection or a significant increase only when mediators of increased permeability are exogenously added to the hantavirus-infected cells (12, 22, 46).
Endothelial cell permeability is a highly regulated process and is maintained by both tight and adherens junctions (47). The disruption of adherens junctions is sufficient to disturb the endothelium barrier function and cause an increase in permeability and formation of edema (25, 47). Adherens junctions are largely composed of vascular endothelial (VE) cadherin (VE-cadherin), an endothelial cell-specific member of the cadherin family of adhesion protein (51, 52). Adherens junctions and in particular VE-cadherin are targets of the signaling pathway of agents that increase vascular permeability (7, 8, 10). Vascular endothelial growth factor (VEGF), one of the most potent vascular permeability agents, exerts its effects after binding to its homologous membrane tyrosine kinase receptor, VEGF-R2, whose expression is restricted to endothelial cells. It is known that VEGF-R2 interacts with VE-cadherin, and together they maintain the endothelial cell barrier (26). When VEGF is present, it binds to VEGF-R2, and that initiates the internalization and degradation of VE-cadherin and disruption of the adherens junctions (10, 54).
In general, increase of vascular permeability is an important component of severe disease progression in hemorrhagic fevers (36). A number of studies have investigated the cause of increased vascular permeability in viral hemorrhagic fevers induced by viruses such as Dengue virus or Ebola virus (41, 42, 50, 53, 56). Studies of vascular permeability during hantavirus infection in vitro have mainly been performed in the presence of various inflammatory agents and growth factors (12, 15, 19, 22, 46). A recent study demonstrated that pathogenic hantaviruses sensitize the endothelium and cause hyperpermeability in response to high levels of exogenously added VEGF (12). We show here that VE-cadherin downregulation can be observed in ANDV-infected cells in the absence of exogenous VEGF. The downregulation of VE-cadherin in the absence of exogenous VEGF led us to the discovery that endothelial cells infected with ANDV induce the production of VEGF at early times postinfection. The early increased secretion of VEGF coincides with the initiation of downregulation of the adherent junction protein VE-cadherin and an increase in permeability of endothelial cells. The involvement of VEGF-R2 in VE-cadherin downregulation was demonstrated by antibody blockage of VEGF-R2 that resulted in significant recovery of VE-cadherin levels. These data indicate that the increased vascular permeability seen in HPS could be a direct result of hantavirus infection of the endothelium and may occur through a pathway involving VEGF-induced downregulation of VE-cadherin at early times postinfection.
MATERIALS AND METHODS
Cell culture and virus infection.
Primary cultures of human pulmonary microvascular endothelial cells (HMVEC-L) isolated from individual donors were obtained at passage 3 from Clonetics Corporation (Walkersville, MD). The cells were seeded into tissue culture flasks precoated with 0.2% gelatin (Sigma, St. Louis, MO). Cells were grown in EGM-2MV growth medium (Clonetics) consisting of modified endothelial cell baseal growth medium (EGM) supplemented with human recombinant epidermal growth factor (10 ng/ml), hydrocortisone (1 μg/ml), gentamicin (50 μg/ml), amphotericin B (50 μg/ml), bovine brain extract (3 mg/ml), and fetal calf serum (5% [vol/vol]). All experiments were performed at cell passages 5 to 7. African green monkey kidney cells (Vero-E6) were maintained in Dulbecco's modified Eagle medium (Gibco BRL, Grand Island, NY) containing 10% fetal calf serum (HyClone, Logan, UT). Stocks of ANDV (strain Chile 9717869; Vero-E6+7) and SNV (strain MMR11; Vero-E6+13) were prepared in Vero-E6 cells, and virus titers were determined by limited dilution as described previously (16). For control experiments, noninfectious virus was prepared by gamma radiation inactivation of virus by using a high-energy 60Co source. The work with ANDV was done under biosafety level 3 conditions.
Antibodies.
The following antibodies were used in this study: rabbit antisera against ANDV; a Puumala anti-N monoclonal antibody (GB04-BF07) which is cross-reactive with all hantaviruses; mouse monoclonal antibody against VE-cadherin (BD Biosciences); mouse monoclonal anti-actin antibody (Sigma). Monoclonal antibody to human VEGF-R2 and IgG were purchased from R&D Systems and Santa Cruz Biotechnology, respectively.
Western blotting and enzyme-linked immunosorbent assay (ELISA).
Cells were lysed by addition of lysis buffer containing 10 mM sodium orthovanadate and 1% sodium dodecyl sulfate (SDS), heated to 90°C. Proteins were electrophoretically separated on 4-to-12% Nupage (Invitrogen) gels and transferred to nitrocellulose. The membranes were blocked with buffer containing Tris-buffered saline and 0.1% Tween 20 with 5% nonfat dry milk for 1 h and then probed overnight at 4°C with specific antibody. Membranes were developed using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence. After detection of VE-cadherin, the membrane was stripped twice, first to reprobe with antibody reactive with hantaviral nucleocapsid protein and then with actin antibody for detection of total protein. To study the effects of blocking VEGF-R2, cells were incubated with anti-VEGF-R2 (500 ng/ml) or mouse IgG antibody (500 ng/ml) after 1 h of virus absorption and then incubated at 37°C for different periods.
Assay of free VEGF.
For the VEGF determination, a quantitative sandwich ELISA technique was used (Biosource-Invitrogen). The system used a solid-phase monoclonal antibody (mouse anti-human VEGF165 IgG) and a biotin-conjugated rabbit polyclonal antibody against recombinant human VEGF165. For the assay, 50 μl of cell culture supernatant was used. The optical densities were determined at 450 nm. The concentration of VEGF in the tested samples was estimated from the standard curve as determined with serially diluted standards. Concentrations are reported in pg/ml.
Transwell endothelial permeability assay.
An in vitro cell permeability assay was carried out according to the protocol of the Chemicon in vitro vascular permeability assay kit (catalog number ECM640; Millipore). Briefly, HMVEC-L cells plated onto collagen-coated transwell inserts (1.0-μm pore size; Chemicon-Millipore) in permeability chambers were left for 2 days to form mature monolayers. Confluent endothelial monolayers were infected in quadruplicate either with ANDV, treated with ANDV inactivated by gamma radiation, or left untreated (mock). After 12, 16, 24, or 72 h postinfection, fluorescein isothiocyanate (FITC)-dextran (1:20 dilution) was added to the upper chamber of monolayers and incubated for 5, 15, or 30 min at room temperature. Samples (100 μl) from the lower chamber were transferred to a 96-well plate and were read using a Biotek Synergy 4 microplate reader (excitation/emission wavelengths, 490/530 nm). Fluorescence intensity measurements were expressed as the increase over the basal permeability of the cell monolayer.
Immunofluorescence.
HMVEC-L cells grown on coverslips were infected with ANDV. At certain time points postinfection, the cells were washed twice with phosphate-buffered saline (PBS) and fixed with 2% formaldehyde at room temperature for 15 min. After formaldehyde fixation, the cells were washed three times with PBS and permeabilized with 0.1% Triton X-100 for intracellular staining. The primary antibodies were added at a 1:200 dilution in 1% bovine serum albumin in PBS for 1 h. The cells were then washed three times with PBS and incubated for 30 min with the secondary antibodies diluted 1:1,000 in 1% bovine serum albumin in PBS. Multiple final washes were done, and the cells were mounted on microscope slides and viewed using a Zeiss microscope.
RESULTS
Reduction of VE-cadherin protein levels in ANDV-infected endothelial cells in the absence of added VEGF.
Previously it had been shown that ANDV-infected endothelial cells treated with high levels of exogenous VEGF displayed a dramatic increase in permeability in comparison to either uninfected cells treated with VEGF or cells infected with ANDV alone (12, 28). Since endothelial cell permeability is regulated by adherens junctions, it may be that the increased permeability observed was linked to a VEGF interaction with VEGF-R2, resulting in internalization and degradation of VE-cadherin and vascular leakage. The earlier in vitro study had shown that exogenously VEGF added at the level of 100 ng/ml induced increased permeability in hantavirus-infected endothelial cells. However, this is in considerable excess over physiological concentrations of VEGF present in human plasma (approximately 20 to 30 pg/ml) and sera (40 to 200 pg/ml) (23, 43). The question remains as to what extent one can extrapolate from such results, based on nonphysiological concentrations, the effects of VEGF on endothelial cell leakage seen in HPS patients.
To address the question of whether VE-cadherin reduction could be seen in ANDV-infected endothelial cells in the absence of added VEGF, confluent primary human lung endothelial cells were infected with ANDV and cell lysates were analyzed by Western blotting for the expression of VE-cadherin at different time points up to 3 days postinfection. Controls included cells left untreated (mock) or cells treated with ANDV inactivated by gamma irradiation (Fig. 1). While gamma irradiation destroys virus infectivity, virion protein antigenicity remains unaltered (data not shown). ANDV-infected cells showed a significant decrease in VE-cadherin levels by 16 h postinfection, in comparison to mock- or inactivated virus-treated cells. Reduction of VE-cadherin in ANDV-infected cells was also observed at 24 h postinfection and was sustained up to 72 h. VE-cadherin reduction was specific to ANDV replication, as cells treated with inactivated ANDV showed no decrease in VE-cadherin, indicating that virus attachment and entry alone do not appear to play a significant role in this process.
FIG. 1.
Downregulation of VE-cadherin by ANDV and SNV infection. (Left and middle blots) Confluent monolayers of HMVEC-L cells were infected with ANDV at an MOI of 1, were treated with inactivated ANDV, or were left untreated (mock). Cell lysates were harvested at 12, 16, 24, or 72 h postinfection and analyzed. (Right blot) Cells infected with SNV at an MOI of 0.2, treated with inactivated SNV, of untreated were analyzed 72 h postinfection for expression of VE-cadherin, virus nucleocapsid, and cellular actin by Western blotting.
In a separate experiment, VE-cadherin reduction comparable with that seen with ANDV infection was also observed at 72 h postinfection in SNV-infected primary lung endothelial cells (Fig. 1). No VE-cadherin reduction was observed at 16 and 24 h postinfection with SNV (data not shown). Unfortunately, SNV grows more slowly and to lower titers than ANDV, and so the SNV experiments were performed using a multiplicity of infection (MOI) of 0.2, which was lower than that used with ANDV (1.0) and resulted in less of the cell monolayer being infected at early time points postinfection. The apparent difference between the ability of these two HPS-associated hantaviruses to induce reduced levels of VE-cadherin at early time points may be related to the lower MOI employed with SNV. While precise comparison of the two HPS-associated hantaviruses is not possible, the data do demonstrate that both ANDV and SNV induce decreases in VE-cadherin levels in infected lung endothelium in the absence of added exogenous factors.
Reduced VE-cadherin expression on the surface of ANDV-infected primary lung endothelial cells.
To examine whether the reduction seen in the total amounts of VE-cadherin during infection of primary human lung endothelial cells with ANDV could be visualized by immunofluorescence staining, a set of time course immunofluorescence experiments were performed. No apparent differences in the amounts of surface staining of VE-cadherin were observed at 12 h postinfection when comparing the mock-infected, inactivated virus-treated, and ANDV-infected cells (Fig. 2). Faint intracellular green staining at the 12-h time point was shown in a subsequent analysis to be merely bleed-over from the intense red staining of the virus antigen (data not shown). The endothelial cells exhibited the typical squamous shape, and VE-cadherin staining was continuous along the entire periphery of the cell. However, by 16 h post-ANDV infection, a disorganization and reduction of VE-cadherin could be observed (Fig. 2A). Increases in discontinuous adherens junctions and intercellular gaps were observed up to 72 h postinfection (Fig. 2A and B). In addition, starting at 24 h postinfection and becoming more prominent by 72 h, the cell morphology changed from the normal “cobblestone” appearance to a more elongated form (Fig. 2A and B). An increased number of endocytotic vesicles containing VE-cadherin was also observed at 72 h postinfection, indicating that VE-cadherin is endocytosed at higher rates in ANDV-infected cells (Fig. 2B, arrow).
FIG. 2.
Immunofluorescent staining of HMVEC-L cells infected with ANDV. HMVEC-L cells grown on coverslips were infected with ANDV at an MOI of 0.5. After 12, 16, or 24 h (A) or 72 h (B) postinfection, cells were fixed and triple stained with VE-cadherin-specific mouse monoclonal antibody (green), anti-ANDV rabbit polyclonal antibody (red), and 4′,6-diamidino-2-phenylindole (DAPI; blue). The arrow indicates VE-cadherin endocytosed at higher rates in the ANDV-infected cells. For controls, cells were either treated with inactivated ANDV or left untreated.
Neutralization of VEGF-R2 inhibits the VE-cadherin reduction induced by ANDV infection.
It is known that VEGF-R2 interacts with VE-cadherin, and together they maintain the endothelial cell barrier (26). To test whether the VE-cadherin reduction observed in the previous experiment was linked to VEGF-R2 activation, we examined the ability of a VEGF-R2-neutralizing antibody to block the reduction of VE-cadherin in ANDV-infected cells. The antibodies were added immediately following adsorption of the virus (time zero) and remained present during the 16- or 24-h course of the infection. At 16 and 24 h post-ANDV infection endothelial cells treated with VEGF-R2 antibody showed a dramatic blockage of the ANDV-induced reduction in the total amount of VE-cadherin, in comparison to controls treated with isotype-matched IgG (Fig. 3).
FIG. 3.
A VEGF-R2-neutralizing antibody inhibits ANDV-induced VE-cadherin downregulation. Confluent monolayers of HMVEC-L cells were infected with ANDV at an MOI of 1, were treated with inactivated ANDV, or were left untreated (mock). After 1 h of virus absorption, cells were either treated with a neutralizing monoclonal antibody to VEGF-R2 (500 ng/ml) or with control mouse IgG (500 ng/ml). Cell lysates were harvested at 16 or 24 h postinfection and analyzed for expression of VE-cadherin, virus nucleocapsid, and cellular actin by Western blotting.
The blockage of VE-cadherin reduction seen on treatment with VEGF-R2 only occurred in ANDV-infected cells and was absent in mock-infected cells or cells treated with inactivated ANDV. Furthermore, no change in virus nucleocapsid expression was observed on treatment with VEGF-R2 antibody in ANDV-infected cells compared to the isotype-matched control, indicating that the inhibitory effect on VE-cadherin degradation was not caused by an indirect effect on viral replication. Together, these results suggest that ANDV-induced reduction of VE-cadherin is dependent on the function of VEGF-R2.
VEGF secretion by ANDV-infected HMVEC-L cells.
It is known that VEGF-R2 is activated by its interaction with VEGF (59). To date, there have been no reports of induction of VEGF as a result of hantavirus infection of endothelial cells. Previously, we had failed to detect any increased amounts of secreted free VEGF in ANDV-infected HMVC-L cell supernatants when measured at 24 to 168 h postinfection (unpublished data). Because we had observed in this study that VE-cadherin was significantly reduced earlier than 24 h postinfection, we decided to analyze secreted free VEGF levels at early time points. HMVEC-L cells were infected with ANDV, and cell supernatants were collected at 12, 16, and 24 h postinfection. The free VEGF levels were measured in a quantitative ELISA. Increased levels of secreted free VEGF were seen from 12 to 16 h post-ANDV infection (Fig. 4). These levels peaked at 16 h and returned to baseline by the 24-h time point. These results clearly demonstrate that ANDV infection results in increased levels of secreted VEGF at early times postinfection, concomitant with the observed downregulation of VE-cadherin. The potential mechanisms that result in increased amounts of VEGF are complex and could include increased mRNA transcription, posttranscriptional modification, or release of preexisting stored VEGF (6, 18, 31). However, VEGF mRNA transcription was not increased by ANDV infection, suggesting that ANDV infection upregulates VEGF secretion by posttranscriptional mechanisms (data not shown). Similar but more modest trends could be seen with SNV-infected primary lung endothelial cells. For instance, an approximately 2-fold increase in free VEGF was observed at 16 h postinfection in the supernatant of SNV-infected primary lung endothelial cells (MOI of 0.2) (data not shown). However, the lower levels of secreted VEGF by SNV infection may reflect the low MOI used.
FIG. 4.
ANDV infection of HMVEC-L cells results in increased secretion of free VEGF. Confluent monolayers of HMVEC-L cells were infected with ANDV at an MOI of 1, were treated with inactivated ANDV, or were left untreated (mock). Supernatants were harvested at 12, 16, or 24 h postinfection, and the amount of free VEGF was determined by ELISA. The data represent the means and standard deviations of duplicate samples. Results shown are representative of five independent experiments.
Increased permeability of ANDV-infected HMVEC-L cells.
The reduced protein levels of VE-cadherin in conjunction with the increase in secreted VEGF observed in ANDV-infected endothelial cells suggested that ANDV infection alone may cause changes in endothelial cell permeability at early stages of infection. To examine this possibility, confluent HMVEC-L cells grown on transwell inserts were infected with ANDV, treated with inactivated ANDV, or left untreated (mock). Monolayer permeability was assayed by adding FITC-dextran to the upper chamber of the transwell and evaluating the migration of FITC-dextran to the lower chamber by fluorometry at 12, 16, 24, and 72 h post-ANDV infection. An ∼3-fold increase in permeability (relative to mock-infected cells) was detected in ANDV-infected cells at 16 h postinfection, and this further increased up to 4-fold at 24 h and ∼5-fold at 72 h postinfection (Fig. 5 A). No permeability increase was observed in endothelial cells treated with inactivated virus, indicating that the observed effect was specific to viral replication. These experimental results demonstrate that ANDV infection alone (without high levels of exogenously added VEGF) of human endothelial cells causes increased vascular permeability at the same early stages of infection at which decreased VE-cadherin and increased secreted VEGF are observed. In separate experiments, increased vascular permeability was also observed at 72 h postinfection in SNV-infected primary lung endothelial cells, although the increase was less (∼3-fold) than that observed in ANDV-infected cells (Fig. 5B).
FIG. 5.
ANDV infection increases endothelial cell permeability. (A) Confluent monolayers of HMVEC-L cells grown on transwell inserts were infected with ANDV at an MOI of 1, were treated with inactivated ANDV, or were left untreated. After 12, 16, 24, or 72 h postinfection, FITC-dextran was added to the upper chambers of transwell inserts. The passage of FITC-dextran to the lower chambers was assayed after 30 min of incubation at room temperature as described in Materials and Methods. The data represent the means and standard deviations of quadruplicate samples. (B) Cells were infected with SNV at an MOI of 0.2, treated with inactivated SNV, or left untreated and were assayed after 72 h postinfection as described for panel A.
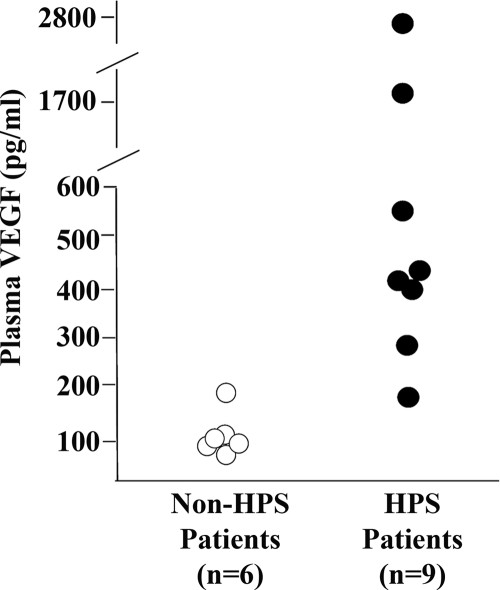
Increased VEGF levels in serum of HPS patients.
The data presented here showed increased secretion of VEGF, decreased cellular VE-cadherin, and increased permeability at early times post-ANDV infection of human lung endothelial cells. These observations raised the question of whether there was any evidence of increased VEGF in HPS patients. A limited collection of acute-phase sera collected in 1993 during the original HPS outbreak described in the southwestern United States was available at the CDC. These samples were all from hospitalized patients exhibiting adult respiratory distress syndrome (ARDS)-like symptoms. Nine of these samples were laboratory confirmed as HPS cases, while the other six infections were not hantavirus related. We found that HPS patient sera had much higher free VEGF levels than those seen in non-hantavirus-infected control patients (Fig. 6). While the number of patients analyzed was limited, these results suggest that the increased levels of VEGF seen in the sera of HPS patients relative to sera from patients with other severe respiratory infections correlates with the vascular leakage that is a hallmark of HPS.
FIG. 6.
Increased levels of free VEGF in sera from HPS patients. Acute-phase sera from hospitalized patients exhibiting ARDS-like symptoms were analyzed for free VEGF levels. Nine patients were laboratory confirmed as HPS cases, while the severe respiratory disease in the other six cases was undiagnosed but determined to be not hantavirus related.
DISCUSSION
There are two main characteristics of hantavirus infection in humans: endothelial cell tropism and increased vascular permeability (4, 35, 36, 57). The mechanism(s) by which hantaviruses cause vascular permeability is unknown. Several studies have indicated that hantavirus infection may exert changes in the barrier function of the vascular endothelium indirectly by inducing endothelial cell and leukocyte activation and triggering synthesis and release of proinflammatory lymphokines (1, 15, 27, 49, 57). Most of the in vitro studies examining hantavirus-induced vascular permeability have been performed with the addition of recombinant expressed factors, such as tumor necrosis factor alpha and VEGF (12, 22). Here, we showed for the first time that ANDV infection alone can directly increase the vascular permeability of human lung endothelial cells at early times postinfection.
VE-cadherin plays a prominent role in the establishment of the endothelial barrier (7, 8, 47). Disruption of VE-cadherin function has been shown to result in interstitial edema and accumulation of inflammatory cells in the heart and lung microcirculation (7, 44). Our observation that the enhanced permeability in endothelial cells resulting from ANDV infection correlated with downregulation of VE-cadherin suggests that disruption of cell-cell adhesion mediated by VE-cadherin is sufficient to induce interendothelial gap formation and hence play a controlling role in the perturbation of the endothelial barrier. VE-cadherin degradation is known to involve extensive internalization, ubiquitination, and proteosomal degradation (54).
Hantaviruses are not the only viruses that cause downregulation of VE-cadherin and increases in vascular permeability. Dengue virus (the cause of dengue hemorrhagic fever) and Karposi's sarcoma-associated herpesvirus (KSHV) have also been shown to downregulate VE-cadherin (21, 29, 37). In the case of KSHV it has been demonstrated that a viral protein which functions as a ubiquitin ligase targets VE-cadherin and causes its downregulation (29). The mechanism by which VE-cadherin is degraded by ANDV infection remains to be determined.
A previous study showed that infection with a pathogenic hantavirus sensitized cells to the addition of recombinant VEGF and suggested a role for VEGF in vascular leakage and disease (12). However, it was unclear what the source of VEGF might be during hantavirus infections. Here we showed detection of free secreted VEGF as a direct result of hantavirus infection of lung endothelial cells.
VEGF is a potent vascular permeability factor that has been shown to act directly on endothelial cells by binding to VEGF-R2 (26). VEGF can induce an increase in vascular permeability by a variety of means (49). In endothelial cells, VEGF-R2 associates with VE-cadherin and provides regulation of cell-cell junctional integrity (40). When VEGF binds to VEGF-R2, VEGF-R2 is activated and endocytosed and a signaling cascade is initiated, leading to the induction of VE-cadherin degradation and an increase in junctional permeability (10, 33, 40, 58, 59). The involvement of VEGF-R2 in VE-cadherin downregulation in ANDV-infected human lung endothelial cells is supported by the demonstration that a neutralizing antibody to VEGF-R2 (55) inhibits the virus-induced reduction of VE-cadherin. In further support, a very recent study demonstrated an increase in VEGF-R2 phosphorylation and internalization of VE-cadherin in hantavirus-infected cells treated with high levels of exogenous VEGF (14).
VEGF could also cause changes in the vascular permeability by the activation of integrins, which have been characterized as receptors for hantaviruses (5, 13, 28). However, this mechanism does not appear to play a major role, based on our observation that treatment of human lung endothelial cells with inactivated ANDV caused only a small transient increase in secreted VEGF and did not alter the total amounts of VE-cadherin or increase vascular permeability. Further studies are needed to precisely determine the mechanism of induction of the secreted VEGF in ANDV-infected cells.
Not only does hantavirus infection of endothelial cells result in secretion of VEGF (as shown here), but previous studies also demonstrated increases in permeability of these cells relative to uninfected endothelial cells in response to exogenously added VEGF (12). The synergy between VEGF production by hantavirus-infected endothelial cells and the sensitization of these cells to the effects of VEGF could result in an amplifying cascade that leads to enhanced endothelial cell leakage.
What happens in vivo is still poorly understood. The progression to HPS is possibly a multifactorial process with contributions from other elements of the immune response to the viral infection (19, 30). Is the induction of VEGF from the vascular endothelium enough to cause pulmonary edema? Pulmonary edema is a rapidly evolving process (24, 33). Studies of animal models of both high-permeability and cardiogenic pulmonary edema have shown increases in VEGF expression and its linkage to the vascular leakage (24). In one of the initial studies characterizing the role of VEGF in pulmonary edema, Kaner and colleagues demonstrated that overexpression of VEGF in mouse lungs induced high-permeability pulmonary edema (20). Here, we have shown that human primary lung endothelial cells infected with ANDV secrete VEGF at early times postinfection. We have also found increased secretion of VEGF from ANDV-infected primary human macrophages (unpublished data). Additional VEGF could be secreted by ANDV-activated T cells and platelets in vivo (2, 45, 48, 49). It is possible that VEGF from such sources would achieve high concentrations in the microvasculature of the lung. The increase in vascular permeability observed solely in response to virus infection is unlikely to be sufficient to be the whole explanation for HPS. However, virus infection per se will initiate vascular leakage and the cascade of cell signaling events which lead to hyperpermeability and disease.
Angiopoetin 1 is known to counteract induced increases in vascular permeability resulting from physiologic levels of VEGF (11). Interestingly, reduced expression of angiopoetin 1 has been recently reported in patients with HFRS (19). This may represent an additional means by which endothelial cell hyperpermeability could be induced during hantavirus infection in humans.
While the number of HPS cases analyzed in our study is limited, the finding that the HPS patient sera had much higher free VEGF levels than those seen in the non-hantavirus-infected control patients correlated well with our in vitro experimental results and suggested that the increased levels of VEGF seen in the sera of HPS patients may be linked to the vascular leakage typical of HPS cases. In dengue hemorrhagic fever, levels of free secreted VEGF and VEGF-R2 have been shown to correlate with the severity of the disease (41, 42, 50). Analysis of a greater number of HPS cases and more precise clinical records detailing the severity of disease will allow determination of whether a similar correlation exists for HPS. A complementary approach will be to analyze the significance of VEGF in disease development in the Syrian hamster animal model for HPS induced by ANDV infection (17, 32).
The novel aspect and importance of the study presented here is the demonstration of a direct virus effect on vascular endothelium which had been overlooked in previous studies. This observation modifies the established paradigm that HPS is solely an immune-modulated disease. We demonstrate for the first time that ANDV and to a lesser extent SNV infection of human primary lung endothelial cells directly results in the downregulation of VE-cadherin, and this downregulation correlates with increased secretion of VEGF and increased endothelial cell permeability. Our data showing that antibody neutralization of VEGF-R2 blocks virus-induced VE-cadherin downregulation implicates VEGF-R2 in this process. Identification of the precise steps leading to hantavirus-induced endothelial cell leakage will facilitate the development of novel immunotherapeutics for the treatment of patients with HPS.
Acknowledgments
We thank Eric Bergeron, Brian Bird, and Cesar Albarino for extensive discussions and helpful comments on the manuscript.
Footnotes
Published ahead of print on 1 September 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Abel Borges, A., and L. T. Figueiredo. 2008. Mechanisms of shock in hantavirus pulmonary syndrome. Curr. Opin. Infect. Dis. 21:293-297. [DOI] [PubMed] [Google Scholar]
- 2.Bambace, N. M., J. E. Levis, and C. E. Holmes. 2010. The effect of P2Y-mediated platelet activation on the release of VEGF and endostatin from platelets. Platelets 21:85-93. [DOI] [PubMed] [Google Scholar]
- 3.Borges, A. A., G. M. Campos, M. L. Moreli, R. L. Souza, V. H. Aquino, F. P. Saggioro, and L. T. Figueiredo. 2006. Hantavirus cardiopulmonary syndrome: immune response and pathogenesis. Microbes Infect. 8:2324-2330. [DOI] [PubMed] [Google Scholar]
- 4.Bustamante, E. A., H. Levy, and S. Q. Simpson. 1997. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest 112:1133-1136. [DOI] [PubMed] [Google Scholar]
- 5.Byzova, T. V., C. K. Goldman, N. Pampori, K. A. Thomas, A. Bett, S. J. Shattil, and E. F. Plow. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6:851-860. [PubMed] [Google Scholar]
- 6.Cao, J., N. Papadopoulou, D. Kempuraj, W. S. Boucher, K. Sugimoto, C. L. Cetrulo, and T. C. Theoharides. 2005. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 174:7665-7675. [DOI] [PubMed] [Google Scholar]
- 7.Corada, M., M. Mariotti, G. Thurston, K. Smith, R. Kunkel, M. Brockhaus, M. G. Lampugnani, I. Martin-Padura, A. Stoppacciaro, L. Ruco, D. M. McDonald, P. A. Ward, and E. Dejana. 1999. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. U. S. A. 96:9815-9820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejana, E., F. Orsenigo, and M. G. Lampugnani. 2008. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121:2115-2122. [DOI] [PubMed] [Google Scholar]
- 9.Duchin, J. S., F. T. Koster, C. J. Peters, G. L. Simpson, B. Tempest, S. R. Zaki, T. G. Ksiazek, P. E. Rollin, S. Nichol, E. T. Umland, et al. 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N. Engl. J. Med. 330:949-955. [DOI] [PubMed] [Google Scholar]
- 10.Gavard, J., and J. S. Gutkind. 2006. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8:1223-1234. [DOI] [PubMed] [Google Scholar]
- 11.Gavard, J., V. Patel, and J. S. Gutkind. 2008. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell 14:25-36. [DOI] [PubMed] [Google Scholar]
- 12.Gavrilovskaya, I. N., E. E. Gorbunova, N. A. Mackow, and E. R. Mackow. 2008. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 82:5797-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gavrilovskaya, I. N., M. Shepley, R. Shaw, M. H. Ginsberg, and E. R. Mackow. 1998. β3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. U. S. A. 95:7074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbunova, E., I. N. Gavrilovskaya, and E. R. Mackow. 2010. Pathogenic hantaviruses andes virus and hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J. Virol. 84:7405-7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayasaka, D., K. Maeda, F. A. Ennis, and M. Terajima. 2007. Increased permeability of human endothelial cell line EA.hy926 induced by hantavirus-specific cytotoxic T lymphocytes. Virus Res. 123:120-127. [DOI] [PubMed] [Google Scholar]
- 16.Hooper, J. 1999. Virus detection and identification with serological tests, p. 99-105. In C. C. H. W. Lee, and C. Schmaljohn (ed.), Manual of hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. WHO Collaborative Center for Virus Reference and Research (Hantaviruses). Asan Institute for Life Sciences, Seoul, Korea.
- 17.Hooper, J. W., T. Larsen, D. M. Custer, and C. S. Schmaljohn. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 289:6-14. [DOI] [PubMed] [Google Scholar]
- 18.Huez, I., L. Creancier, S. Audigier, M. C. Gensac, A. C. Prats, and H. Prats. 1998. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol. Cell. Biol. 18:6178-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang, J. Y., J. W. Park, S. Y. Hong, and H. S. Park. 2009. Reduced expression of angiopoietin-1 in Hantaan virus-infected human umbilical vein endothelial cell increases their permeability. Acta Virol. 53:7-13. [DOI] [PubMed] [Google Scholar]
- 20.Kaner, R. J., J. V. Ladetto, R. Singh, N. Fukuda, M. A. Matthay, and R. G. Crystal. 2000. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 22:657-664. [DOI] [PubMed] [Google Scholar]
- 21.Kanlaya, R., S. N. Pattanakitsakul, S. Sinchaikul, S. T. Chen, and V. Thongboonkerd. 2009. Alterations in actin cytoskeletal assembly and junctional protein complexes in human endothelial cells induced by dengue virus infection and mimicry of leukocyte transendothelial migration. J. Proteome Res. 8:2551-2562. [DOI] [PubMed] [Google Scholar]
- 22.Khaiboullina, S. F., D. M. Netski, P. Krumpe, and S. C. St. Jeor. 2000. Effects of tumor necrosis factor alpha on Sin Nombre virus infection in vitro. J. Virol. 74:11966-11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura, K., T. Hashiguchi, T. Deguchi, S. Horinouchi, T. Uto, H. Oku, S. Setoyama, I. Maruyama, M. Osame, and K. Arimura. 2007. Serum VEGF as a prognostic factor of atherosclerosis. Atherosclerosis 194:182-188. [DOI] [PubMed] [Google Scholar]
- 24.Kosmidou, I., D. Karmpaliotis, A. J. Kirtane, H. V. Barron, and C. M. Gibson. 2008. Vascular endothelial growth factors in pulmonary edema: an update. J. Thromb. Thrombolysis 25:259-264. [DOI] [PubMed] [Google Scholar]
- 25.Lampugnani, M. G., and E. Dejana. 2007. The control of endothelial cell functions by adherens junctions. Novartis Found. Symp. 283:4-13. [DOI] [PubMed] [Google Scholar]
- 26.Lampugnani, M. G., F. Orsenigo, M. C. Gagliani, C. Tacchetti, and E. Dejana. 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174:593-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linderholm, M., C. Ahlm, B. Settergren, A. Waage, and A. Tarnvik. 1996. Elevated plasma levels of tumor necrosis factor (TNF)-alpha, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 173:38-43. [DOI] [PubMed] [Google Scholar]
- 28.Mackow, E. R., and I. N. Gavrilovskaya. 2009. Hantavirus regulation of endothelial cell functions. Thromb. Haemost. 102:1030-1041. [DOI] [PubMed] [Google Scholar]
- 29.Mansouri, M., P. P. Rose, A. V. Moses, and K. Fruh. 2008. Remodeling of endothelial adherens junctions by Kaposi's sarcoma-associated herpesvirus. J. Virol. 82:9615-9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin, D., R. Galisteo, and J. S. Gutkind. 2009. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFκB through the CBM (Carma3/Bcl10/Malt1) complex. J. Biol. Chem. 284:6038-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuda, K., K. Abdelmohsen, and M. Gorospe. 2009. RNA-binding proteins implicated in the hypoxic response. J. Cell. Mol. Med. 13:2759-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milazzo, M. L., E. J. Eyzaguirre, C. P. Molina, and C. F. Fulhorst. 2002. Maporal viral infection in the Syrian golden hamster: a model of hantavirus pulmonary syndrome. J. Infect. Dis. 186:1390-1395. [DOI] [PubMed] [Google Scholar]
- 33.Monaghan-Benson, E., and K. Burridge. 2009. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J. Biol. Chem. 284:25602-25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nolte, K. B., R. M. Feddersen, K. Foucar, S. R. Zaki, F. T. Koster, D. Madar, T. L. Merlin, P. J. McFeeley, E. T. Umland, and R. E. Zumwalt. 1995. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathol. 26:110-120. [DOI] [PubMed] [Google Scholar]
- 35.Pensiero, M. N., J. B. Sharefkin, C. W. Dieffenbach, and J. Hay. 1992. Hantaan virus infection of human endothelial cells. J. Virol. 66:5929-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters, C. J., and S. R. Zaki. 2002. Role of the endothelium in viral hemorrhagic fevers. Crit. Care Med. 30:S268-S273. [DOI] [PubMed] [Google Scholar]
- 37.Qian, L. W., W. Greene, F. Ye, and S. J. Gao. 2008. Kaposi's sarcoma-associated herpesvirus disrupts adherens junctions and increases endothelial permeability by inducing degradation of VE-cadherin. J. Virol. 82:11902-11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmaljohn, C., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmaljohn, C. S., and S. T. Nichol. 2007. Bunyaviridae, p. 1741-1789. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott-Raven, Philadelphia, PA.
- 40.Shay-Salit, A., M. Shushy, E. Wolfovitz, H. Yahav, F. Breviario, E. Dejana, and N. Resnick. 2002. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 99:9462-9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srikiatkhachorn, A., C. Ajariyakhajorn, T. P. Endy, S. Kalayanarooj, D. H. Libraty, S. Green, F. A. Ennis, and A. L. Rothman. 2007. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J. Virol. 81:1592-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srikiatkhachorn, A., and S. Green. 2010. Markers of dengue disease severity. Curr. Top. Microbiol. Immunol. 338:67-82. [DOI] [PubMed] [Google Scholar]
- 43.Stefanini, M. O., F. T. Wu, F. Mac Gabhann, and A. S. Popel. 2008. A compartment model of VEGF distribution in blood, healthy and diseased tissues. BMC Syst. Biol. 2:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens, T., J. G. Garcia, D. M. Shasby, J. Bhattacharya, and A. B. Malik. 2000. Mechanisms regulating endothelial cell barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L419-L422. [DOI] [PubMed] [Google Scholar]
- 45.Suidan, G. L., J. W. Dickerson, Y. Chen, J. R. McDole, P. Tripathi, I. Pirko, K. B. Seroogy, and A. J. Johnson. 2010. CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. J. Immunol. 184:1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundstrom, J. B., L. K. McMullan, C. F. Spiropoulou, W. C. Hooper, A. A. Ansari, C. J. Peters, and P. E. Rollin. 2001. Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J. Virol. 75:6070-6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taddei, A., C. Giampietro, A. Conti, F. Orsenigo, F. Breviario, V. Pirazzoli, M. Potente, C. Daly, S. Dimmeler, and E. Dejana. 2008. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 10:923-934. [DOI] [PubMed] [Google Scholar]
- 48.Terajima, M., D. Hayasaka, K. Maeda, and F. A. Ennis. 2007. Immunopathogenesis of hantavirus pulmonary syndrome and hemorrhagic fever with renal syndrome: do CD8+ T cells trigger capillary leakage in viral hemorrhagic fevers? Immunol. Lett. 113:117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terajima, M., O. Vapalahti, H. L. Van Epps, A. Vaheri, and F. A. Ennis. 2004. Immune responses to Puumala virus infection and the pathogenesis of nephropathia epidemica. Microbes Infect. 6:238-245. [DOI] [PubMed] [Google Scholar]
- 50.Tseng, C. S., H. W. Lo, H. C. Teng, W. C. Lo, and C. G. Ker. 2005. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol. Med. Microbiol. 43:99-102. [DOI] [PubMed] [Google Scholar]
- 51.Venkiteswaran, K., K. Xiao, S. Summers, C. C. Calkins, P. A. Vincent, K. Pumiglia, and A. P. Kowalczyk. 2002. Regulation of endothelial barrier function and growth by VE-cadherin, plakoglobin, and beta-catenin. Am. J. Physiol. Cell Physiol. 283:C811-821. [DOI] [PubMed] [Google Scholar]
- 52.Vincent, P. A., K. Xiao, K. M. Buckley, and A. P. Kowalczyk. 2004. VE-cadherin: adhesion at arm's length. Am. J. Physiol. Cell Physiol. 286:C987-C997. [DOI] [PubMed] [Google Scholar]
- 53.Wahl-Jensen, V. M., T. A. Afanasieva, J. Seebach, U. Stroher, H. Feldmann, and H. J. Schnittler. 2005. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J. Virol. 79:10442-10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao, K., D. F. Allison, M. D. Kottke, S. Summers, G. P. Sorescu, V. Faundez, and A. P. Kowalczyk. 2003. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J. Biol. Chem. 278:19199-19208. [DOI] [PubMed] [Google Scholar]
- 55.Yang, X. H., X. Y. Man, S. Q. Cai, Y. G. Yao, Z. Y. Bu, and M. Zheng. 2006. Expression of VEGFR-2 on HaCaT cells is regulated by VEGF and plays an active role in mediating VEGF induced effects. Biochem. Biophys. Res. Commun. 349:31-38. [DOI] [PubMed] [Google Scholar]
- 56.Yang, Z. Y., H. J. Duckers, N. J. Sullivan, A. Sanchez, E. G. Nabel, and G. J. Nabel. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6:886-889. [DOI] [PubMed] [Google Scholar]
- 57.Zaki, S. R., P. W. Greer, L. M. Coffield, C. S. Goldsmith, K. B. Nolte, K. Foucar, R. M. Feddersen, R. E. Zumwalt, G. L. Miller, A. S. Khan, et al. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552-579. [PMC free article] [PubMed] [Google Scholar]
- 58.Zanetta, L., M. Corada, M. Grazia Lampugnani, A. Zanetti, F. Breviario, L. Moons, P. Carmeliet, M. S. Pepper, and E. Dejana. 2005. Downregulation of vascular endothelial-cadherin expression is associated with an increase in vascular tumor growth and hemorrhagic complications. Thromb. Haemost. 93:1041-1046. [DOI] [PubMed] [Google Scholar]
- 59.Zanetti, A., M. G. Lampugnani, G. Balconi, F. Breviario, M. Corada, L. Lanfrancone, and E. Dejana. 2002. Vascular endothelial growth factor induces SHC association with vascular endothelial cadherin: a potential feedback mechanism to control vascular endothelial growth factor receptor-2 signaling. Arterioscler Thromb Vasc Biol. 22:617-622. [DOI] [PubMed] [Google Scholar]