Abstract
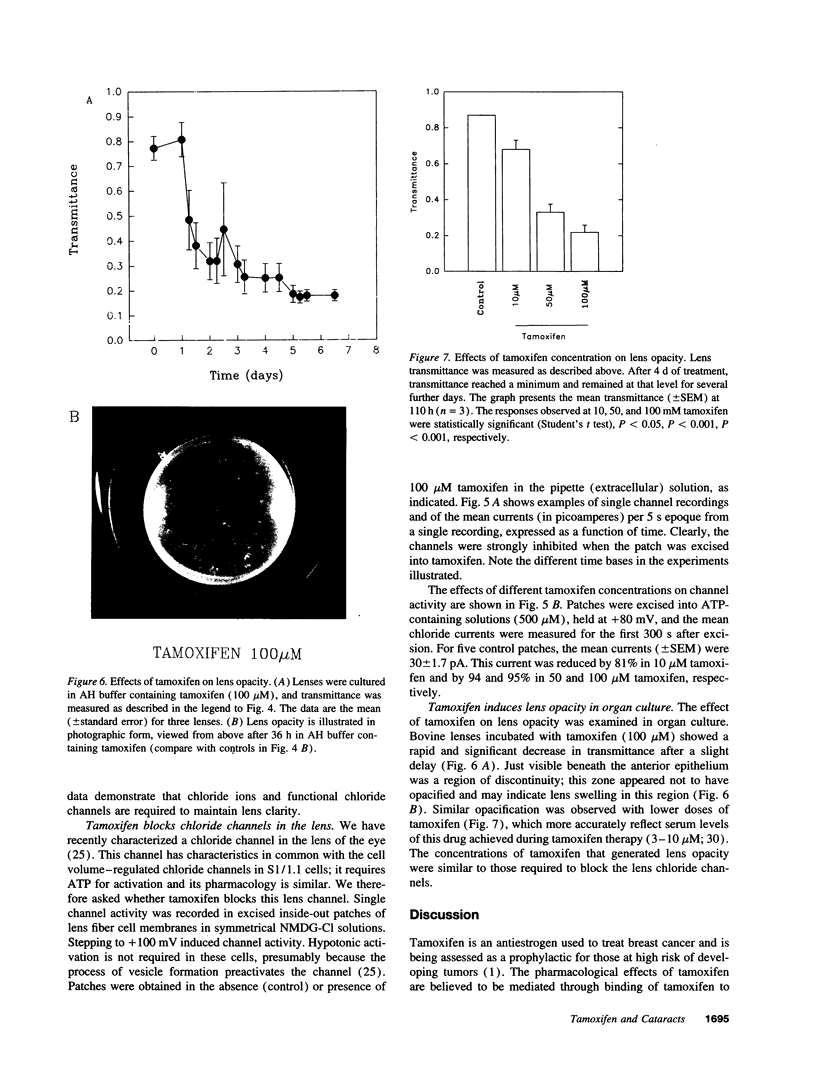
Tamoxifen is an antiestrogen frequently used in the treatment of breast cancer and is currently being assessed as a prophylactic for those at high risk of developing tumors. We have found that tamoxifen and its derivatives are high-affinity blockers of specific chloride channels. This blockade appears to be independent of the interaction of tamoxifen with the estrogen receptor and therefore reflects an alternative cellular target. One of the clinical side effects of tamoxifen is impaired vision and cataract. Chloride channels in the lens of the eye were shown to be essential for maintaining normal lens hydration and transmittance. These channels were blocked by tamoxifen and, in organ culture, tamoxifen led to lens opacity associated with cataracts at clinically relevant concentrations. These data suggest a molecular mechanism by which tamoxifen can cause cataract formation and have implications for the clinical use of tamoxifen and related antiestrogens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., Jongsma A. P., Broxterman H. J., Arceci R. J., Housman D., Scheffer G. L., Riethorst A., van Groenigen M., Nieuwint A. W., Joenje H. Non-P-glycoprotein mediated mechanism for multidrug resistance precedes P-glycoprotein expression during in vitro selection for doxorubicin resistance in a human lung cancer cell line. Cancer Res. 1990 Sep 1;50(17):5392–5398. [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Bercheme G., Ries F., Dicato M. La chémoprévention: une nouvelle stratégie de lutte contre le cancer du sein? Bull Soc Sci Med Grand Duche Luxemb. 1993;130(1):5–9. [PubMed] [Google Scholar]
- Berry M., Metzger D., Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990 Sep;9(9):2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGregorio M. W., Ford J. M., Benz C. C., Wiebe V. J. Toremifene: pharmacologic and pharmacokinetic basis of reversing multidrug resistance. J Clin Oncol. 1989 Sep;7(9):1359–1364. doi: 10.1200/JCO.1989.7.9.1359. [DOI] [PubMed] [Google Scholar]
- Dreinhöfer J., Gögelein H., Greger R. Blocking kinetics of Cl- channels in colonic carcinoma cells (HT29) as revealed by 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB). Biochim Biophys Acta. 1988 Dec 8;946(1):135–142. doi: 10.1016/0005-2736(88)90466-x. [DOI] [PubMed] [Google Scholar]
- Furr B. J., Jordan V. C. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Gerner E. W. Ocular toxicity of tamoxifen. Ann Ophthalmol. 1989 Nov;21(11):420–423. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Gögelein H. Chloride channels in the luminal membrane of the rectal gland of the dogfish (Squalus acanthias). Properties of the "larger" conductance channel. Pflugers Arch. 1987 Jun;409(1-2):114–121. doi: 10.1007/BF00584757. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Allen K. E., Dix C. J. Pharmacology of tamoxifen in laboratory animals. Cancer Treat Rep. 1980 Jun-Jul;64(6-7):745–759. [PubMed] [Google Scholar]
- Jordan V. C., Bain R. R., Brown R. R., Gosden B., Santos M. A. Determination and pharmacology of a new hydroxylated metabolite of tamoxifen observed in patient sera during therapy for advanced breast cancer. Cancer Res. 1983 Mar;43(3):1446–1450. [PubMed] [Google Scholar]
- Jordan V. C., Murphy C. S. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev. 1990 Nov;11(4):578–610. doi: 10.1210/edrv-11-4-578. [DOI] [PubMed] [Google Scholar]
- Jordan V. C. Overview from the International Conference on Long-Term Tamoxifen Therapy for Breast Cancer. J Natl Cancer Inst. 1992 Feb 19;84(4):231–234. doi: 10.1093/jnci/84.4.231. [DOI] [PubMed] [Google Scholar]
- Kangas L., Nieminen A. L., Blanco G., Grönroos M., Kallio S., Karjalainen A., Perilä M., Södervall M., Toivola R. A new triphenylethylene compound, Fc-1157a. II. Antitumor effects. Cancer Chemother Pharmacol. 1986;17(2):109–113. doi: 10.1007/BF00306737. [DOI] [PubMed] [Google Scholar]
- Kirk J., Houlbrook S., Stuart N. S., Stratford I. J., Harris A. L., Carmichael J. Differential modulation of doxorubicin toxicity to multidrug and intrinsically drug resistant cell lines by anti-oestrogens and their major metabolites. Br J Cancer. 1993 Jun;67(6):1189–1195. doi: 10.1038/bjc.1993.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J., Houlbrook S., Stuart N. S., Stratford I. J., Harris A. L., Carmichael J. Selective reversal of vinblastine resistance in multidrug-resistant cell lines by tamoxifen, toremifene and their metabolites. Eur J Cancer. 1993;29A(8):1152–1157. doi: 10.1016/s0959-8049(05)80306-5. [DOI] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., Parker M. G. Identification of two transactivation domains in the mouse oestrogen receptor. Nucleic Acids Res. 1989 Jul 25;17(14):5477–5488. doi: 10.1093/nar/17.14.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintenig G. M., Valverde M. A., Sepulveda F. V., Gill D. R., Hyde S. C., Kirk J., Higgins C. F. Specific inhibitors distinguish the chloride channel and drug transporter functions associated with the human multidrug resistance P-glycoprotein. Receptors Channels. 1993;1(4):305–313. [PubMed] [Google Scholar]
- Pastan I., Gottesman M. M., Ueda K., Lovelace E., Rutherford A. V., Willingham M. C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4486–4490. doi: 10.1073/pnas.85.12.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T. J., Tillyer C. R., Jones A. L., Ashley S. E., Treleaven J., Davey J. B., McKinna J. A. Prevention of breast cancer with tamoxifen--an update on the Royal Marsden Hospital pilot programme. Eur J Cancer. 1990;26(6):680–684. doi: 10.1016/0277-5379(90)90116-b. [DOI] [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Fuks Z. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by tamoxifen and other triparanol analogues. Cancer Res. 1984 Oct;44(10):4392–4395. [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Valverde M. A., Giraldez F., Sepúlveda F. V. Potassium currents of isolated Necturus enterocytes: a whole-cell patch-clamp study. J Physiol. 1991 Feb;433:663–676. doi: 10.1113/jphysiol.1991.sp018449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart N. S., Philip P., Harris A. L., Tonkin K., Houlbrook S., Kirk J., Lien E. A., Carmichael J. High-dose tamoxifen as an enhancer of etoposide cytotoxicity. Clinical effects and in vitro assessment in p-glycoprotein expressing cell lines. Br J Cancer. 1992 Nov;66(5):833–839. doi: 10.1038/bjc.1992.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Tachibana M., Kaneko A. Effects of glycine and GABA on isolated bipolar cells of the mouse retina. J Physiol. 1990 Feb;421:645–662. doi: 10.1113/jphysiol.1990.sp017967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Currier S. J., Bruggemann E. P., Ueda K., Germann U. A., Pastan I., Gottesman M. M. Use of recombinant P-glycoprotein fragments to produce antibodies to the multidrug transporter. Biochem Biophys Res Commun. 1990 Jan 15;166(1):180–186. doi: 10.1016/0006-291x(90)91928-l. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Díaz M., Sepúlveda F. V., Gill D. R., Hyde S. C., Higgins C. F. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992 Feb 27;355(6363):830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Mintenig G. M., Sepúlveda F. V. Differential effects of tamoxifen and I- on three distinguishable chloride currents activated in T84 intestinal cells. Pflugers Arch. 1993 Dec;425(5-6):552–554. doi: 10.1007/BF00374885. [DOI] [PubMed] [Google Scholar]
- Venglarik C. J., Singh A. K., Wang R., Bridges R. J. Trinitrophenyl-ATP blocks colonic Cl- channels in planar phospholipid bilayers. Evidence for two nucleotide binding sites. J Gen Physiol. 1993 Apr;101(4):545–569. doi: 10.1085/jgp.101.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]