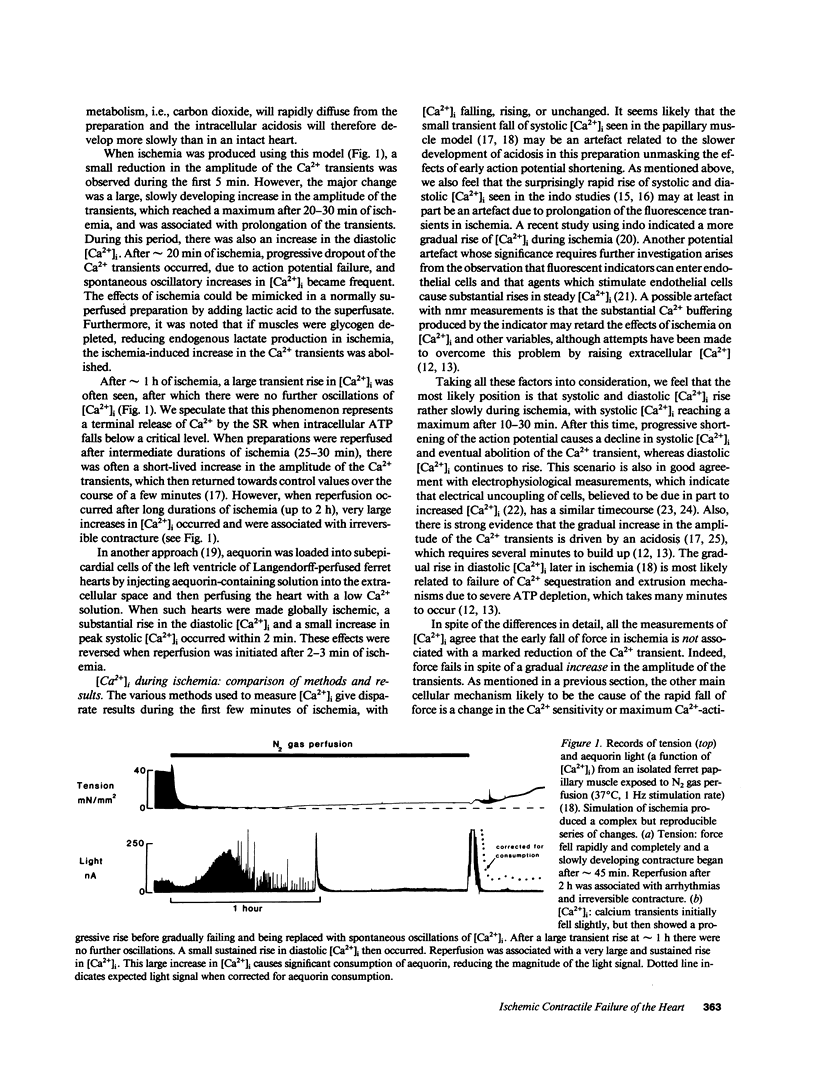
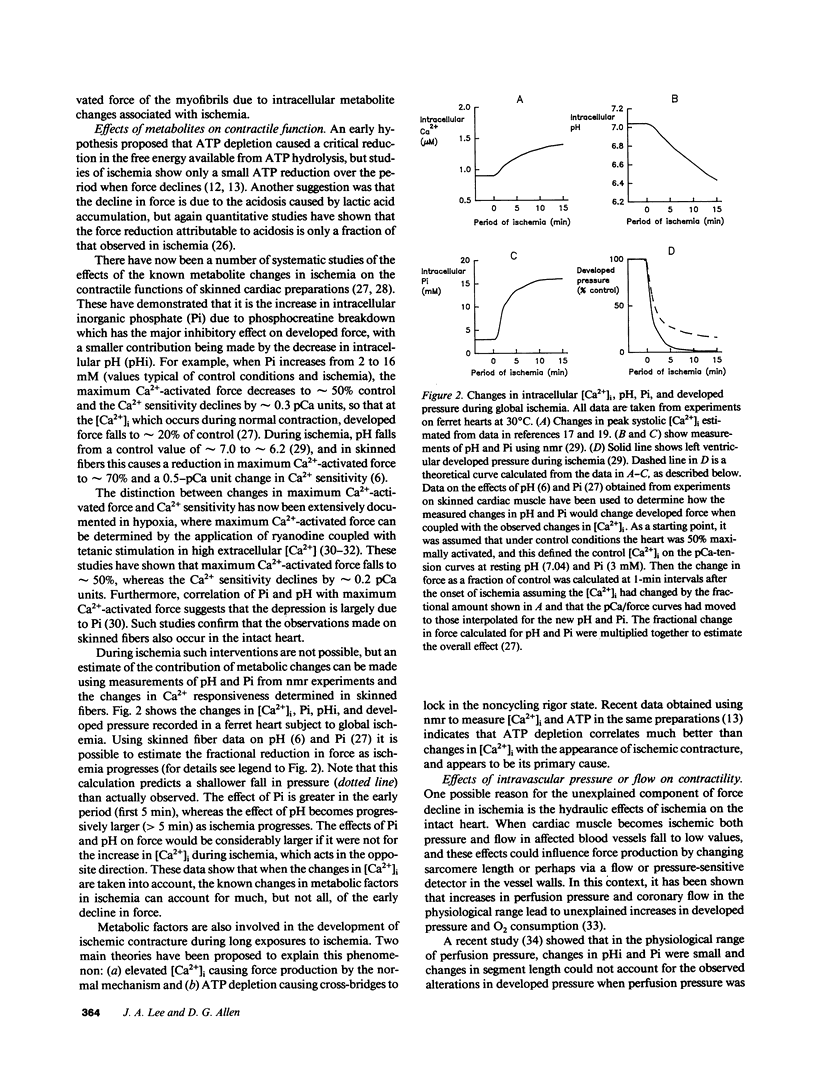
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Smith G. L. The consequences of simulated ischaemia on intracellular Ca2+ and tension in isolated ferret ventricular muscle. J Physiol. 1989 Mar;410:297–323. doi: 10.1113/jphysiol.1989.sp017534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Bourdillon P. D., Poole-Wilson P. A. Effects of ischaemia and reperfusion on calcium exchange and mechanical function in isolated rabbit myocardium. Cardiovasc Res. 1981 Mar;15(3):121–130. doi: 10.1093/cvr/15.3.121. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982 Dec;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Cascio W. E., Yan G. X., Kléber A. G. Passive electrical properties, mechanical activity, and extracellular potassium in arterially perfused and ischemic rabbit ventricular muscle. Effects of calcium entry blockade or hypocalcemia. Circ Res. 1990 Jun;66(6):1461–1473. doi: 10.1161/01.res.66.6.1461. [DOI] [PubMed] [Google Scholar]
- Clusin W. T., Buchbinder M., Ellis A. K., Kernoff R. S., Giacomini J. C., Harrison D. C. Reduction of ischemic depolarization by the calcium channel blocker diltiazem. Correlation with improvement of ventricular conduction and early arrhythmias in the dog. Circ Res. 1984 Jan;54(1):10–20. doi: 10.1161/01.res.54.1.10. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Effect of intracellular injection of calcium and strontium on cell communication in heart. J Physiol. 1975 Sep;250(2):231–245. doi: 10.1113/jphysiol.1975.sp011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey J. M. Free radicals and their involvement during long-term myocardial ischemia and reperfusion. Annu Rev Physiol. 1990;52:487–504. doi: 10.1146/annurev.ph.52.030190.002415. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar R. J., Gwathmey J. K. Direct evidence of changes in myofilament responsiveness to Ca2+ during hypoxia and reoxygenation in myocardium. Am J Physiol. 1990 Sep;259(3 Pt 2):H784–H795. doi: 10.1152/ajpheart.1990.259.3.H784. [DOI] [PubMed] [Google Scholar]
- Hill J. L., Gettes L. S. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980 Apr;61(4):768–778. doi: 10.1161/01.cir.61.4.768. [DOI] [PubMed] [Google Scholar]
- Janse M. J., Wit A. L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989 Oct;69(4):1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- Kantor P. F., Coetzee W. A., Carmeliet E. E., Dennis S. C., Opie L. H. Reduction of ischemic K+ loss and arrhythmias in rat hearts. Effect of glibenclamide, a sulfonylurea. Circ Res. 1990 Feb;66(2):478–485. doi: 10.1161/01.res.66.2.478. [DOI] [PubMed] [Google Scholar]
- Karmazyn M. Amiloride enhances postischemic ventricular recovery: possible role of Na+-H+ exchange. Am J Physiol. 1988 Sep;255(3 Pt 2):H608–H615. doi: 10.1152/ajpheart.1988.255.3.H608. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982 Jun;38(3):259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzung B. G., Hondeghem L. M., Grant A. O. Letter: Cardiac ventricular automaticity induced by current of injury. Pflugers Arch. 1975 Oct 28;360(2):193–197. doi: 10.1007/BF00580542. [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Aramendía P. Prevention of ventricular fibrillation induced by coronary ligation. J Pharmacol Exp Ther. 1968 Dec;164(2):326–332. [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y., Grossman W., Morgan J. P. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res. 1989 Oct;65(4):1029–1044. doi: 10.1161/01.res.65.4.1029. [DOI] [PubMed] [Google Scholar]
- Kitakaze M., Marban E. Cellular mechanism of the modulation of contractile function by coronary perfusion pressure in ferret hearts. J Physiol. 1989 Jul;414:455–472. doi: 10.1113/jphysiol.1989.sp017698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakaze M., Weisman H. F., Marban E. Contractile dysfunction and ATP depletion after transient calcium overload in perfused ferret hearts. Circulation. 1988 Mar;77(3):685–695. doi: 10.1161/01.cir.77.3.685. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Kléber A. G., Riegger C. B., Janse M. J. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res. 1987 Aug;61(2):271–279. doi: 10.1161/01.res.61.2.271. [DOI] [PubMed] [Google Scholar]
- Koretsune Y., Corretti M. C., Kusuoka H., Marban E. Mechanism of early ischemic contractile failure. Inexcitability, metabolite accumulation, or vascular collapse? Circ Res. 1991 Jan;68(1):255–262. doi: 10.1161/01.res.68.1.255. [DOI] [PubMed] [Google Scholar]
- Koretsune Y., Marban E. Mechanism of ischemic contracture in ferret hearts: relative roles of [Ca2+]i elevation and ATP depletion. Am J Physiol. 1990 Jan;258(1 Pt 2):H9–16. doi: 10.1152/ajpheart.1990.258.1.H9. [DOI] [PubMed] [Google Scholar]
- Kusuoka H., Koretsune Y., Chacko V. P., Weisfeldt M. L., Marban E. Excitation-contraction coupling in postischemic myocardium. Does failure of activator Ca2+ transients underlie stunning? Circ Res. 1990 May;66(5):1268–1276. doi: 10.1161/01.res.66.5.1268. [DOI] [PubMed] [Google Scholar]
- Kusuoka H., Weisfeldt M. L., Zweier J. L., Jacobus W. E., Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986 Sep;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr The effects of pH and temperature on fluorescent calcium indicators as determined with Chelex-100 and EDTA buffer systems. Biochem Biophys Res Commun. 1990 Aug 31;171(1):102–108. doi: 10.1016/0006-291x(90)91362-v. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Frelin C., Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol. 1985 Nov;17(11):1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Smith N., Mohabir R., Clusin W. T. Cytosolic calcium transients from the beating mammalian heart. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7793–7797. doi: 10.1073/pnas.84.21.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. Calcium sensitisers. BMJ. 1990 Mar 3;300(6724):551–552. doi: 10.1136/bmj.300.6724.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. The effects of repeated exposure to anoxia on intracellular calcium, glycogen and lactate in isolated ferret heart muscle. Pflugers Arch. 1988 Nov;413(1):83–89. doi: 10.1007/BF00581232. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Polansky E. W., Bianchi C. P., Narayan S. Influence of verapamil on cellular integrity and electrolyte concentrations of ischemic myocardial tissue in the cat. Basic Res Cardiol. 1979 Sep-Oct;74(5):555–567. doi: 10.1007/BF01907648. [DOI] [PubMed] [Google Scholar]
- Lorell B. H., Apstein C. S., Cunningham M. J., Schoen F. J., Weinberg E. O., Peeters G. A., Barry W. H. Contribution of endothelial cells to calcium-dependent fluorescence transients in rabbit hearts loaded with indo 1. Circ Res. 1990 Aug;67(2):415–425. doi: 10.1161/01.res.67.2.415. [DOI] [PubMed] [Google Scholar]
- Lucchesi B. R. Modulation of leukocyte-mediated myocardial reperfusion injury. Annu Rev Physiol. 1990;52:561–576. doi: 10.1146/annurev.ph.52.030190.003021. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Chacko V. P., Pike M. M. Ca2+ transients in perfused hearts revealed by gated 19F NMR spectroscopy. Circ Res. 1988 Sep;63(3):673–678. doi: 10.1161/01.res.63.3.673. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Koretsune Y., Yue D. T., Chacko V. P., Pike M. M. Quantification of [Ca2+]i in perfused hearts. Critical evaluation of the 5F-BAPTA and nuclear magnetic resonance method as applied to the study of ischemia and reperfusion. Circ Res. 1990 May;66(5):1255–1267. doi: 10.1161/01.res.66.5.1255. [DOI] [PubMed] [Google Scholar]
- Marban E., Kitakaze M., Kusuoka H., Porterfield J. K., Yue D. T., Chacko V. P. Intracellular free calcium concentration measured with 19F NMR spectroscopy in intact ferret hearts. Proc Natl Acad Sci U S A. 1987 Aug;84(16):6005–6009. doi: 10.1073/pnas.84.16.6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marban E., Kusuoka H. Maximal Ca2+-activated force and myofilament Ca2+ sensitivity in intact mammalian hearts. Differential effects of inorganic phosphate and hydrogen ions. J Gen Physiol. 1987 Nov;90(5):609–623. doi: 10.1085/jgp.90.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee E. E., Cheung J. Y., Rannels D. E., Morgan H. E. Measurement of the rate of protein synthesis and compartmentation of heart phenylalanine. J Biol Chem. 1978 Feb 25;253(4):1030–1040. [PubMed] [Google Scholar]
- Mellgren R. L. Canine cardiac calcium-dependent proteases: Resolution of two forms with different requirements for calcium. FEBS Lett. 1980 Jan 1;109(1):129–133. doi: 10.1016/0014-5793(80)81326-3. [DOI] [PubMed] [Google Scholar]
- Opie L. H. Coronary flow rate and perfusion pressure as determinants of mechanical function and oxidative metabolism of isolated perfused rat heart. J Physiol. 1965 Oct;180(3):529–541. doi: 10.1113/jphysiol.1965.sp007715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Eisner D. A., Allen D. G. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983 Aug 25;304(5928):735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Schwartz G. G., Schaefer S., Meyerhoff D. J., Gober J., Fochler P., Massie B., Weiner M. W. Dynamic relation between myocardial contractility and energy metabolism during and following brief coronary occlusion in the pig. Circ Res. 1990 Aug;67(2):490–500. doi: 10.1161/01.res.67.2.490. [DOI] [PubMed] [Google Scholar]
- Sharma A. D., Saffitz J. E., Lee B. I., Sobel B. E., Corr P. B. Alpha adrenergic-mediated accumulation of calcium in reperfused myocardium. J Clin Invest. 1983 Sep;72(3):802–818. doi: 10.1172/JCI111051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Steenbergen C., Murphy E., Levy L., London R. E. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987 May;60(5):700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Murphy E., Watts J. A., London R. E. Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart. Circ Res. 1990 Jan;66(1):135–146. doi: 10.1161/01.res.66.1.135. [DOI] [PubMed] [Google Scholar]
- Tani M. Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Annu Rev Physiol. 1990;52:543–559. doi: 10.1146/annurev.ph.52.030190.002551. [DOI] [PubMed] [Google Scholar]
- Tani M., Neely J. R. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ Res. 1989 Oct;65(4):1045–1056. doi: 10.1161/01.res.65.4.1045. [DOI] [PubMed] [Google Scholar]
- Thandroyen F. T., McCarthy J., Burton K. P., Opie L. H. Ryanodine and caffeine prevent ventricular arrhythmias during acute myocardial ischemia and reperfusion in rat heart. Circ Res. 1988 Feb;62(2):306–314. doi: 10.1161/01.res.62.2.306. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Cytoplasmic [Ca2+] in mammalian ventricle: dynamic control by cellular processes. Annu Rev Physiol. 1990;52:467–485. doi: 10.1146/annurev.ph.52.030190.002343. [DOI] [PubMed] [Google Scholar]
- Wilde A. A., Escande D., Schumacher C. A., Thuringer D., Mestre M., Fiolet J. W., Janse M. J. Potassium accumulation in the globally ischemic mammalian heart. A role for the ATP-sensitive potassium channel. Circ Res. 1990 Oct;67(4):835–843. doi: 10.1161/01.res.67.4.835. [DOI] [PubMed] [Google Scholar]


