Abstract
One type of RNA editing converts adenosines to inosines (A→I editing) in double-stranded RNA (dsRNA) substrates. A→I RNA editing is mediated by adenosine deaminase acting on RNA (ADAR) enzymes. A→I RNA editing of protein-coding sequences of a limited number of mammalian genes results in recoding and subsequent alterations of their functions. However, A→I RNA editing most frequently targets repetitive RNA sequences located within introns and 5′ and 3′ untranslated regions (UTRs). Although the biological significance of noncoding RNA editing remains largely unknown, several possibilities, including its role in the control of endogenous short interfering RNAs (esiRNAs), have been proposed. Furthermore, recent studies have revealed that the biogenesis and functions of certain microRNAs (miRNAs) are regulated by the editing of their precursors. Here, I review the recent findings that indicate new functions for A→I editing in the regulation of noncoding RNAs and for interactions between RNA editing and RNA interference mechanisms.
Keywords: double-stranded RNA, A→I RNA editing, noncoding RNA, RNA interference, microRNA, esiRNA
INTRODUCTION
With the number of genes much fewer than previously expected, the complexity of higher organisms largely depends on posttranscriptional and posttranslational mechanisms that create different gene products and the diversity required for complex structural, enzymatic, and regulatory functions (1). A primary RNA transcript of the gene undergoes various maturation processes, such as 5′ capping, splicing, 3′ processing, and polyadenylation (2). RNA editing is one of the posttranscriptional mechanisms that introduce changes in RNA sequences encoded by genome sequences.
The phenomenon of RNA editing was first discovered more than 20 years ago in kine-toplastid protozoa (3). In the mitochondrial mRNA of these trypanosomes, many uridine nucleotides were found to be inserted or deleted to generate functional proteins (3). Since then, many other types of RNA editing mechanisms have been identified (4). In the animal kingdom, the most prevalent type of RNA editing that alters one nucleotide to another is mediated by adenosine deaminase acting on RNA (ADAR) enzymes; ADAR converts adenosines to inosines (A→I editing) in double-stranded RNA (dsRNA) substrates. A→I RNA editing can lead to a codon change and consequent alterations of protein-coding sequences of selected genes, resulting in a diversification of their protein functions. However, the vast majority of A→I RNA editing sites are in noncoding sequences. 5′ and 3′ untranslated regions (UTRs) and intronic retrotransposon elements, such as Alu and long interspersed elements (LINEs), are frequently targeted. Although the biological significance of these repetitive RNAs remains largely unknown, the interesting possibility that they are involved in the control of endogenous short interfering RNAs (esiRNAs) has emerged.
Precursors of certain microRNAs (miRNAs) also undergo A→I RNA editing. Here, editing regulates processing of precursor miRNAs into mature miRNAs or leads to selection of new target genes for silencing by edited miRNAs. These recent studies reveal new functions for A→I editing in regulation of noncoding RNAs and modulation of RNA interference (RNAi) pathways (5).
In this review, I briefly introduce the A→I RNA editing system, ADARs, editing-site selectivity, and representative A→I RNA editing targets of some protein-coding genes and diversification of their functions. However, I put much emphasis on A→I RNA editing of non-coding RNAs and also on the interaction between RNA editing and RNAi pathways. I do not attempt to cover all aspects of A→I RNA editing. More comprehensive reviews are available (6–10).
A→I RNA EDITING AND ADAR GENES
A→I RNA editing is mediated by ADARs. In the following sections, the A→I deamination mechanism, different ADAR genes, domain structures of ADARs, editing-site selectivity, regulation of ADAR gene expression, and their localization are described.
Hydrolytic Deamination of Adenosine to Inosine by ADARs
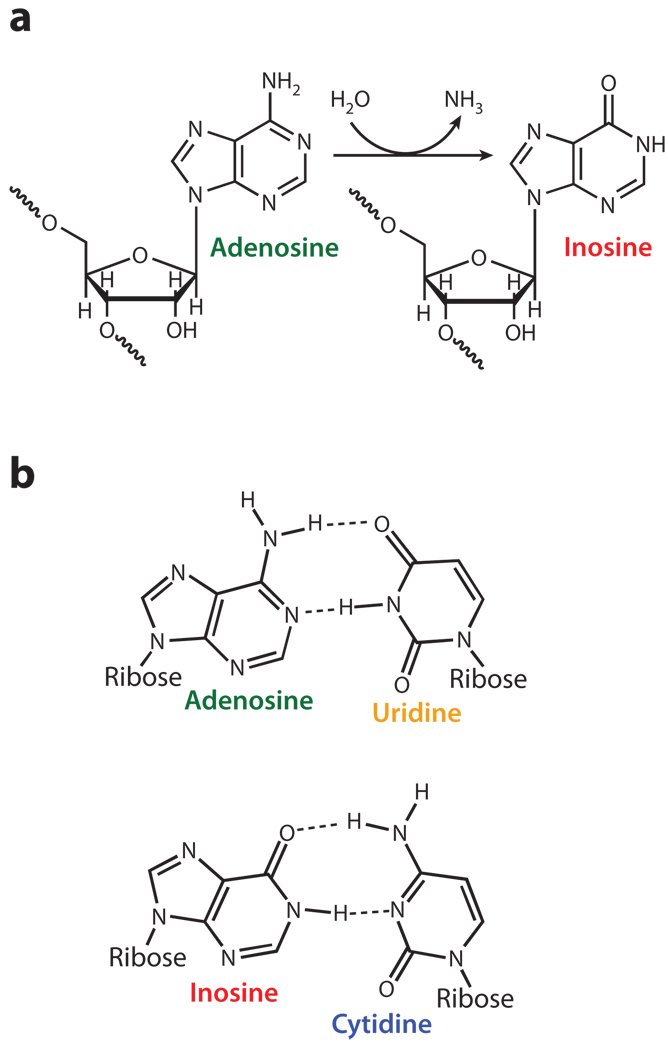
During A→I editing, adenosine is converted to inosine by hydrolytic deamination of the adenine base (Figure 1a) (11, 12). The A→I deamination reaction is catalyzed by ADARs (Figure 2a). ADARs were originally identified in Xenopus laevis eggs and embryos as a mysterious dsRNA-unwinding activity (13, 14). Soon after, however, it was revealed that this activity is a dsRNA-specific adenosine deaminase (11, 12). The first mammalian ADAR gene identified, human ADAR1, was cloned following biochemical purification (15, 16) and microsequencing of ADAR1 protein (17, 18), which led to identification of ADAR2 (19–21) and ADAR3 (Figure 2a) (22, 23). The enzymatic activity of ADAR1 and ADAR2 has been demonstrated (17, 19–21). Although ADAR3 activity has not been demonstrated, its functional domain features are conserved (22, 23). These three ADARs are highly conserved in vertebrates (24, 25).A single ADAR2-like gene, dADAR, is present in Drosophila melanogaster (26), whereas two ADAR genes, CeADR1 and CeADR2, exist in Caenorhabditis elegans (27). Two splicing isoforms of squid ADAR2, with high homology to human ADAR2, were also identified (Figure 2a) (28). Interestingly, recent screening of invertebrate genome databases identified ADAR1 and ADAR2 but not ADAR3 in sea urchin and sea anemones (29), indicating that ADAR1 and ADAR2 arose in early metazoan evolution, whereas ADAR3 might have evolved more recently by possible duplication of ADAR2 in vertebrates. Furthermore, ADAR1 or ADAR2 was lost in some species, such as insects and squid, during subsequent evolution. ADARs are absent in all protozoa, yeast, and plants (29).
Figure 1.
Deamination of adenosine to inosine by ADAR. (a) A hydrolytic deamination reaction converts adenosine to inosine. (b) Adenosine base pairs with uridine, whereas inosine base pairs, as if it were guanosine, in a Watson-Crick-bonding configuration with cytidine.
Figure 2.
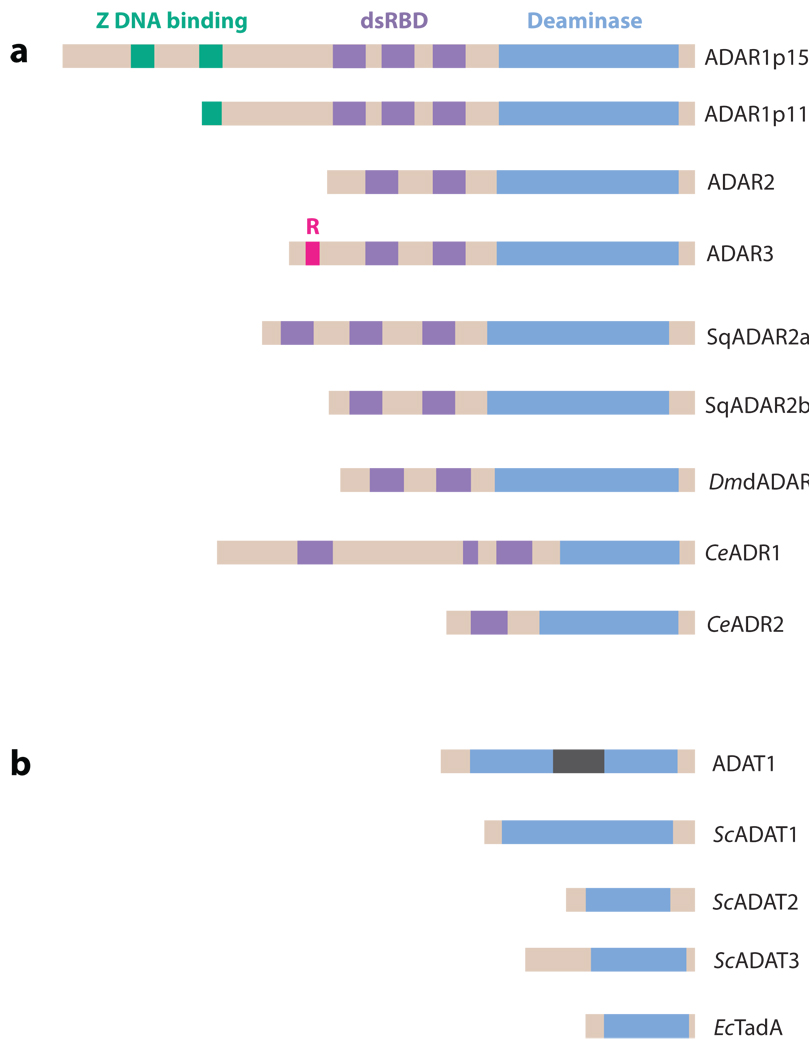
ADAR and ADAT family members. (a) The ADAR family is shown. Three vertebrate ADAR family members (ADAR1–3) are known. Two ADAR1 translation products (ADAR1p150 and ADAR1p110 isoforms) are known. Vertebrate ADARs, squid SqADAR2a and SqADAR2b (splicing isoforms), Drosophila melanogaster dADAR, and two C. elegans members (CeADR1 and CeADR2) share common functional domains: two to three repeats of the dsRNA-binding domain (dsRBD) and a catalytic deaminase domain. Certain structural features, such as Z-DNA-binding domains and the arginine-rich R domain, are unique to particular ADAR members. (b) The ADAT family is shown. Presented is the only known mammalian ADAT family member (ADAT1), its yeast homolog (ScADAT1), two additional yeast ADAT families (ScADAT2 and ScADAT3), and the only known bacterial ADAT family member (EcTadA). The unique mammalian ADAT1 sequence is located within the deaminase domain (gray bar). ADARs target dsRNA. ADATs target tRNA, even though they lack any known RNA-binding motifs. Yeast ScADAT2 and ScADAT3 form active heterodimers, whereas ADAR1 and ADAR2 form active homodimers.
In addition to the ADAR family, adenosine deaminases acting on tRNA (ADATs) have been identified because of their sequence homology to ADARs (Figure 2b). ADATs are involved in A→I editing of tRNAs at or near the anticodon position. The ADAT members are conserved in eukaryotes from yeast to man (30). Moreover, a bacterial ortholog of the ADAT family, tRNA adenosine deaminase (TadA), also exists, indicating conservation of this A→I editing function between prokaryotes and eukaryotes (31). ADATs have been hypothesized to be the evolutionary ancestors of ADARs. Interestingly, it is not the adenosine deaminase acting on mononucleotides (ADAs) but the cytidine deaminases acting on mononucleotides (CDAs) that are the likely predecessors to ADATs and consequently ADAR (17, 30).
ADAR Domain Structure and the A→I Editing Mechanism
Common domain structures are found among members of the ADAR gene family (Figure 2a). One to three repeats of the dsRNA-binding domain (dsRBD) (~65 amino acids), forming a highly conserved α-β-β-β-α configuration structure, are present among ADARs. The dsRBD makes direct contact with the dsRNA (32) and is required for dsRNA binding (33). Certain structural features are unique to particular ADAR members. For instance, ADAR1 contains two Z-DNA-binding domains, Zα and Zβ (34). The functional significance of Z domains remains largely unknown. ADAR3 contains an arginine-rich single-stranded RNA (ssRNA)-binding domain (R domain) in its N-terminal region (22). More recently, the presence of the R domain was detected also in a minor fraction of ADAR2 mRNAs, indicating the evolutionary conservation of this domain and thus its functional significance (35).
The C-terminal region of ADAR contains a catalytic domain consisting of amino acid residues that are conserved in several cytidine deaminases, including APOBEC1, that are involved in the C→U mRNA editing mechanism and are predicted to participate in the formation of the catalytic center containing a zinc ion (17, 36). The crystal structure of the catalytic domain of human ADAR2 reveals that histidine H394, glutamic acid E396, and two cysteine residues, C451 and C516, are involved in the coordination of a zinc atom and the formation of the catalytic center (37). Most interestingly, an inositol hexakisphosphate (IP6) moiety is buried within the enzyme core and likely stabilizes multiple arginine and lysine residues present in the catalytic pocket. IP6 is located very close to the catalytic center, strongly arguing that it plays a critical role during the hydrolytic deamination reaction (37).
Editing-Site Selectivity
A→I editing of dsRNA can be very specific, leading to deamination of select adenosine residues, or it can be almost random and lead to nonselective conversion of many adenosines. Both inter- and intramolecular dsRNAs of >20 base pair (bp) (two turns of the dsRNA helix) can serve as a substrate for ADAR (38). Many adenosine residues of long dsRNAs (>100 bp) are edited promiscuously, resulting in ~50% of all adenosine residues being converted to inosine. These are detected as so-called hypermutations of viral RNAs during replication and as a subsequent persistent infection with certain ss-RNA viruses, such as the measles virus (39), and they are also detected during extensive editing of sense-antisense RNA pairs made from transcripts of select genes, such as Drosophila 4f-rmp (40) and C. elegans eri-6 and eri-7 (41). By contrast, short dsRNAs (~20–30 bp) or long ds-RNAs with mismatched bases, bulges, and loops (imperfect dsRNAs) are edited selectively; only a few adenosines are specifically chosen, indicating that the secondary structure within ADAR substrates dictates editing-site selectivity (42).
For site-selective A→I editing of protein-coding sequences, an imperfect fold-back dsRNA structure is formed between the exon sequence surrounding the editing site(s) and a downstream, usually intronic complementary sequence termed editing-site complementary sequence (ECS). This is demonstrated for the pre-mRNAs of the glutamate receptor B (GluR-B) Q/R site (43); the GluR-B,-C,-D R/G sites (44); or the A–E sites of serotonin (5-HT) receptor 2C (5-HT2CR) (45, 46). However, the ECS of certain substrate RNAs is found within the exon sequence; for example, in the Drosophila Kv1.1 potassium ion channel (47); mammalian GABAA receptor α3 (Gabra-3) (48); and the self-editing site of dADAR (49). Furthermore, the dsRNA structure can be formed with the ECS through a complicated long-range pseudoknot, as shown for several recoding sites identified in Drosophila synaptotagmin I (50). Nonetheless, the ECS and the dsRNA structure are absolutely required for editing (8, 9).
Homodimerization of ADAR Is Required for Catalytic Activity
Some editing sites are preferentially edited only by ADAR1 or ADAR2, indicating a significant difference in their RNA-substrate interactions, possibly through their dsRBDs (51). ADAR1 or ADAR2 differ in the number of dsRBDs and in the spacing between the dsRBDs (51). In GluR-B pre-mRNA, the unique positioning of the second dsRBD of ADAR2, which is located close to two bulged bases adjacent to the R/G editing site, may contribute to selection of the adenine to be edited (51). The distinctive editing-site selectivity of ADAR1 and ADAR2 may also be mediated through functional interactions between the two monomers of ADAR1 and ADAR2, as such interactions possibly position specific adenosine residues relative to the catalytic center of ADAR (51, 52).
In vitro studies have revealed that the A→I editing activity of fly dADAR (53) and mammalian ADAR1 and ADAR2 (52) requires homodimerization. By contrast, ADAR3 does not dimerize at least in vitro, perhaps explaining the enzymatic inactivity of this ADAR family member (52). In vivo homodimerization of mammalian ADAR1 and ADAR2 was verified through studies using bioluminescence resonance energy transfer and fluorescence resonance energy transfer methods (54, 55). Whether ADAR dimerization requires RNA is the subject of debate (52–55). However, recent studies, using mutant ADAR1 and ADAR2 incapable of binding to dsRNA, indicate that dimerization is independent of RNA binding, suggesting that homodimer complex formation is mediated through protein-protein interactions between two monomers (33). Interestingly, a mutated ADAR subunit had a dominant-negative effect on dimer functions, indicating that the dsRBDs of the interacting monomers function cooperatively (33). The region involved in the dimerization of mammalian ADAR proteins remains to be established.
ADAR Regulation and Tissue and Cellular Distribution
Both ADAR1 and ADAR2 are present in many tissues, whereas ADAR3 is expressed only in brain (17, 19–23). Two isoforms of ADAR1, a full-length ADAR1p150 and a shorter, N-terminally truncated ADAR1p110, are known (Figure 2a) (56). One of the three promoters that drive transcription of the ADAR1 gene is interferon inducible, and the mRNA transcribed from this promoter directs translation of ADAR1p150 (57). Two other ADAR1 mRNAs, transcribed from constitutive promoters, direct the synthesis of ADAR1p110, which is initiated from a downstream methionine as the result of alternative splicing and skipping of the exon containing the upstream methionine (Figure 2a). ADAR2 expression is regulated by the transcriptional activator CREB (cyclic adenosine monophosphate response element-binding) protein during experimental induction of ischemia in rat brain (58). The regulatory mechanism for ADAR3 is currently unknown.
The developmentally regulated expression of ADAR1 and ADAR2, starting around E10.0, has been reported in mice (59, 60). Finally, ADAR1 is regulated by the miRNA-mediated RNAi mechanism. The expression of ADAR1 is downregulated by miRNA-1 (miR-1) (61). Interestingly, miR-1 plays a critical role in the development of embryonic heart (62). Moreover, the earliest and highest expression of ADAR1 is detected in mouse embryonic heart at E10.0 (63), suggesting an important function of ADAR1 in mouse heart development and its tight regulation by miR-1.
ADAR1p150 is detected mainly in the cytoplasm (56, 64, 65). The cellular distribution of ADAR1p150 suggests that its targets, possibly including a different class of dsRNA substrates (e.g., esiRNAs, see below), may be localized to the cytoplasm (66). A nuclear localization signal has been identified in the third dsRBD of ADAR1 (65, 67). In addition, a functional nuclear export signal has been identified in the N-terminal Zα domain in full-length ADAR1p150, which is exported to the cytoplasm by the CRM1-RanGTP-mediated mechanism (65). Recently, however, the nuclear cytoplasmic shuttling of ADAR1p110 that lacks this Zα domain has been reported in certain cell lines (68). Interaction with the protein exportin-5, which is mediated via dsRNA binding, may be responsible for the CRM1-RanGTP-independent nuclear export of ADAR1. Although binding of dsRNA to the third dsRBD appears to facilitate nuclear export of ADAR1, it inhibits nuclear import of the complex. Transportin-1, which binds to the third dsRBD, has been identified as the import receptor for ADAR1 (68).
ADAR1, together with RanGTP and exportin-5, seems to act as a nuclear export carrier of certain dsRNAs. Interestingly, pre-miRNAs, processed from primary transcripts of miRNAs (pri-miRNAs) by Drosha/DGCR8, are exported into the cytoplasm by the RanGTP/exportin-5 complex (69). Thus, it would be interesting to determine whether ADAR1 is involved in the nuclear export of pre-miRNAs (possibly edited pre-miRNAs).
Nuclear import of ADAR2 and ADAR3 appears to be controlled by importin α family members: ADAR2 by importin α4 and α5, whereas ADAR3 by importin α1, which recognizes the N-terminal R domain (70). The presence of the factor ADBP-1, which binds to CeADR2 and regulates the nuclear localization and activity of CeADR2, has also been reported (71).
Finally, ADAR1p110 and ADAR2 accumulate in the nucleolus (64, 72). The localization of ADAR1p110 and ADAR2 in this compartment, which is dependent on functional dsRBDs, has been proposed to occur through their binding to rRNA or to small nucleolar RNA. They may be stored temporarily in the nucleolus but move out to the nucleoplasm as substrate dsRNAs appear (64, 72). However, the significance of the nucleolar localization of ADAR1p110 and ADAR2 remains largely unknown.
PHYSIOLOGY OF EDITING
Editing of coding sequences can result in dramatic alterations of the target gene functions. Deficiency in editing created in animal model systems revealed the importance of editing in vivo. Furthermore, the presence of human diseases related to A→I RNA editing has recently become known.
Diversification of Protein-Coding Gene Functions
The translation machinery reads an inosine as if it were guanosine (Figure 1b), which could lead to codon changes. Despite the initial expectation, only a limited number of cellular genes (~30, mostly neurotransmitter receptors and ion channels) that are subjected to site-selective A→I RNA editing within their coding sequences have been identified. These include mammalian GluR (43), 5-HT2CR (46), and potassium channel Kv1.1 (73), squid Kv1.1A (74), and Drosophila Na+ channel (75) gene transcripts as well as the hepatitis delta virus antigen gene (76).
In most cases, RNA editing of protein-coding genes results in generation of protein isoforms and diversification of protein functions. For instance, the coding regions of receptors for several GluR ion channel subunits contain a total of eight A→I RNA editing sites (8, 43). One of these sites, the Q/R site, which is located within the channel pore-loop domain of the GluR-B subunit, plays a critical role in ion channel function. A→I editing (CAG glutamine → CIG arginine, thus Q/R site) of this site changes the tetrameric channel protein so that the channel becomes impermeable to Ca2+ (8, 43). Another example is the combinatorial editing of five sites (A-E sites) located within the second intracellular loop or G protein– coupling domain of 5-HT2CR. Editing of these sites changes three codons—AUA (isoleucine), AAU (asparagine), and AUU (isoleucine)—to possibly six different amino acid residues (45, 46, 77). The result is that up to 24 receptor isoforms are expressed with substantially altered G protein–coupling functions, affecting 5-HT potency and ligand-binding affinity (45, 46, 77).
Among the 14 editing sites identified in the squid Kv1.1 channel, some sites affect the rate of deactivation, whereas other sites located within the N terminus inhibit tetramerization of individual subunits (74). However, the functional significance of most protein-recoding editing sites remains to be established (7). This includes sites recently found in mammalian Gabra-3 (48) as well as numerous sites in Drosophila neurotransmitters and ion channels identified by the comparative genomic method (73). The reader may refer to more comprehensive reviews on these protein-recoding type A→I editing targets and their biological significance (7, 8, 10, 73).
Advantage of RNA Editing over Gene Mutations
RNA editing has several advantages over gene mutations. As with alternative splicing, the extent of RNA editing can be differentially regulated (ranging from no editing to nearly 100% editing) and can be spatiotemporally controlled. By contrast, because of the lack of such control, gene mutations convey permanent, hardwired changes in the genome. This advantage of RNA editing is reflected in the developmental regulation of GluRs (8), 5-HT2CR (46), and Gabra-3 mRNA editing (48, 78, 79), as well as in editing targets containing multiple editing sites, such as GluR-B (8), 5-HT2CR (46), and fly Ca2+ channel α1-subunit (80). Combinatorial editing of the latter can generate numerous editing isoforms that are distributed differentially in various brain subregions, contributing to a complicated regulation of multiple, functionally diversified gene products. Interestingly, certain editing sites, such as the GluR-B Q/R site (81) and Gabra-3 (48), are hardwired in the fish and frog genomes (which contain guanosine instead of adenosine at sites subject to A→I editing in other species), indicating that the G→A mutation of this site and control of the codon through A→I editing must have occurred more recently for an evolutionary advantage.
RNA-Editing Deficiencies and Mutants with Altered Editing
Inactivation of ADAR gene family members has significant physiological consequences, as seen in phenotypic alterations of ADAR gene mutants created in various species. Mutant flies with a homozygous deletion in the dADAR gene exhibit brain-related changes, such as temperature-sensitive paralysis, uncoordinated locomotion, and age-dependent neurodegeneration, presumably resulting from a lack of editing of important dADAR target genes such as Na+ (para), Ca2+ (Dmca1A), and glutamate-gated Cl− channels (DrosGluCl-α) (26). C. elegans strains that contain homozygous deletions of both CeADR1 and CeADR2 display defective chemotaxis (27), although much reduced penetration of this phenotype has been reported by a separate group (71). Mice with a homozygous ADAR2 null mutation die several weeks after birth. These mice experience repeated episodes of epileptic seizures that originate from excess influx of Ca2+ and consequent neuronal death caused by underediting of the GluR-B Q/R site (82), which is a major target of ADAR2. However, ADAR2−/− mice can be rescued by introducing the genomic GluR-BR mutation, which restores expression of GluR-B “edited” at the Q/R site (82). ADAR3−/− mice are viable and appear to be normal, although the possibility that ADAR1 and/or ADAR2 activity compensates for loss of ADAR3 function has not been excluded (M. Higuchi & P.H. Seeburg, personal communication). By contrast, the inactivation of ADAR1 leads to an embryonic lethal phenotype because of widespread apoptosis (60, 63, 83). Thus, at least ADAR1 is absolutely required for life in mammals.
Recent studies using conditional inactivation of ADAR1 revealed that ADAR1 also plays a critical role in the maintenance of hematopoietic stem cells, perhaps by preventing inappropriate induction of interferon signaling pathways and apoptosis of hematopoietic progenitor cells (84). Mutant mice overexpressing ADAR2 display adult-onset hyperphagia and consequent obesity (85). Interestingly, this obese phenotype was reproduced with a separate mutant mouse line overexpressing catalytically inactive ADAR2, indicating the possibility that hyperphagia and obese phenotype are caused by other ADAR2 functions, perhaps through its binding to a currently unknown dsRNA substrate (85).
Mutant animal model systems with permanently altered editing patterns of specific substrate RNAs have also been created. Phenotypes of these mutants generated important and often surprising insight into the in vivo significance of A→I editing of particular substrate RNAs. Self-editing of an ADAR2 intronic site creates a proximal 3′ splice site containing a noncanonical adenosine-inosine dinucleoside. This leads to alternative splicing and consequent loss of functional ADAR2 protein expression owing to premature translation termination (86). The mutant mice, in which this ADAR2 intronic self-editing is eliminated by removing the ECS sequence, lose ADAR2 autoediting and subsequent alternative splicing. ADAR2 protein expression is substantially increased in the mutant mice, confirming the function of this intronic editing site in a negative feedback regulatory mechanism (87).
Drosophila dADAR undergoes developmentally regulated self-editing of its own mRNA, which changes a conserved Ser (AGU) residue to Gly (IGU) in the catalytic domain and generates an isoform with much reduced editing activities (88). Ubiquitous expression of only the unedited isoform dADAR in embryos and in larvae of mutant flies is lethal because of the excess editing activities of the unedited enzyme (49).
The importance of GluR-B Q/R site editing was clearly demonstrated in heterozygous mice GluR-B+/ΔECS harboring an editing-incompetent GluR-B allele that lacks the ECS essential for editing (89). The unedited GluR-B subunit, although having relatively low expression levels (~25%), increased Ca2+ permeability in neurons and led to epileptic seizures and premature death by 3 weeks of age (89).
Mutant mouse lines harboring knockin 5-HT2CR-VGV or 5-HT2CR-INI alleles have been created, resulting in the sole expression of the fully edited 5-HT2CR-VGV isoform or the unedited (editing-blocked) 5-HT2CR-INI isoform (90). Although INI mice grew normally, VGV mice had drastically reduced fat mass, in spite of compensatory hyperphagia. Constitutive activation of the sympathetic nervous system and consequent increase in energy expenditure were detected in VGV mice. These studies revealed the presence of a previously unknown mechanism, mediated via 5-HT2CR mRNA editing, that regulates energy expenditure and fat metabolism (90). This discovery raised the possibility that substantial variations in metabolic rate and obesity among individuals of different ages and ethnic backgrounds may be related, at least in part, to differences in the editing efficacy of 5-HT2CR mRNAs (90).
RNA Editing in Human Diseases and Pathophysiology
Dysfunction of the A→I RNA editing mechanism can cause human diseases or pathophysiology (91, 92). Heterozygosity for functional null mutations in the ADAR1 gene—a total of 41 sites have been discovered—results in dyschromatosis symmetrica hereditaria, an autosomal dominant human pigmentary genodermatosis (93). Although the target dsRNA, as well as the mechanism underlying the pathophysiology, of this genetic disease is unknown, a longer isoform of ADAR1 (p150) has been given particular importance (91).
RNA-editing deficiencies also underlie disorders of the central nervous system. Underediting of the Q/R site of GluR-B pre-mRNA has been implicated in the death of motor neurons of sporadic amyotrophic lateral sclerosis (ALS) patients (94). Deficiency in the Q/R site of GluR-B pre-mRNA editing has also been proposed to be responsible for the apoptotic death of neurons during ischemia resulting from cardiac arrest and disruption of blood flow to the brain (58). RNA editing of 5-HT2CR might be relevant to the cause of certain neuropsychiatric disorders, such as depression and schizophrenia (92). Also, the editing pattern of 5-HT2CR mRNA is significantly altered in the prefrontal cortex of suicide victims (92, 95, 96).
Underediting of the GluR-B Q/R site (ADAR2 site) and reduced ADAR2 activities have been reported in human gliomas. Increased Ca2+ influx through GluRs with unedited GluR-B was proposed to underlie aggressive growth of tumor cells (97, 98). Furthermore, the loss of ADAR2 editing activity in pediatric astrocytomas has been reported (99). Results of a global bioinformatic survey of inverted Alu sequence editing also suggest that significant hypoediting occurs in various types of human cancers, due at least in part to simultaneous downregulation of ADAR1, ADAR2, and ADAR3 (100). This suggests that reduced A→I editing in general may be involved in the patho-genesis of cancer (100). However, the causative relevance of the reduction in A→I editing activities to the malignant transformation of cancers remains to be established.
A→I EDITING OF REPETITIVE NONCODING RNA
Physiologically important editing sites, such as those for GluR-B and 5-HT2CR, and the consequent alterations of their protein functions, were discovered serendipitously. Identification of many more A→I RNA editing target genes was anticipated because a substantial amount of inosine was detected in rat brain poly(A)+ RNA (101). Accordingly, bioinformatics strategies for globally searching for A→I editing sites have been developed, leading to the identification of a large number of new sites, which are mainly within noncoding regions.
Bioinformatics Screening and Identification of A→I Editing Sites in Repeat Sequences
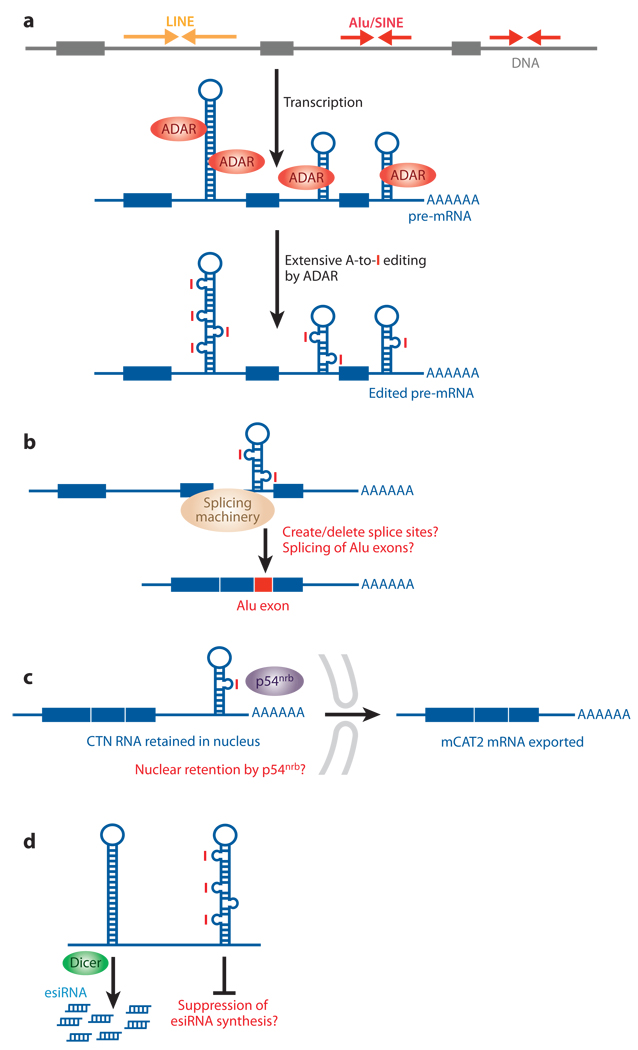
Reverse transcriptase recognizes inosine as guanosine; and therefore, A→I editing is detected as an A→G change in the cDNA sequence or expressed sequence tag (EST). Accordingly, several groups have developed computational methods for genome-wide identification of A→I editing sites in cDNA (EST) sequencing databases (102–105). The screening strategy consists of an algorithm that aligns a cluster of A→G mismatches within cDNAs or ESTs to the genome sequence. A large number of human A→I RNA editing sites (~15,000 sites mapped in ~2,000 different genes) have been identified in noncoding regions that consist of inversely oriented repetitive elements (Figure 3a), mostly within Alu elements and some LINE (102–105). It is predicted that more than 85% of pre-mRNAs may be edited, with the vast majority being targeted in introns and UTRs (102).
Figure 3.
Possible regulatory functions for noncoding RNA editing. (a) Extensive A→I editing of an RNA duplex structure that consists of inverted Alu or LINE repeats. The inverted Alu or LINE repeats in introns and untranslated regions (UTRs) form intramolecular RNA duplexes genome wide, which are then subjected to A→I RNA editing by ADAR. (b) Splicing machinery interprets an inosine as a guanosine. Therefore, splice sites might be created or deleted by A→I editing of intronic Alu fold-back dsRNAs, leading to the inclusion or exclusion of Alu exons (102). (c) A→I editing of a short interspersed element (SINE) fold-back dsRNA present within the 3′ UTR of cationic amino acid transporter 2 (CAT2) nuclear (CTN) RNA. Its binding to p54nrb might be involved in the regulatory mechanism that retains this RNA within nuclear speckles (118). When cells are placed under stress, CTN-RNA is cleaved and de novo polyadenylated at an alternative site to release the protein-coding mCAT2 mRNA, which is then translated for CAT2 proteins (118). (d) A→I editing of Alu or LINE fold-back dsRNAs might suppress the generation of esiRNAs and consequent RNAi-mediated silencing of retrotransposon activities and/or genes (mRNAs) harboring these sequences within UTRs in trans.
Editing depends on formation of RNA fold-back structures formed between intramolecular inverted Alu (LINE) repeats. It turns out that the distance between an Alu and the closest inverted element is a critical determinant for the likelihood that a given element is targeted by the editing machinery (102, 103). Alu repeats are short interspersed elements (SINEs) that are unique to primates. Interestingly, bioinformatics screening for A→I editing sites in mouse, rat, and chicken transcriptomes revealed that noncoding repeat sequences are also major targets in these species, but the editing frequency is much lower than that observed in human transcriptomes (104, 106, 107). The substantial reduction in frequency is most likely a result of the differences in repeat length (e.g., ~300 bp versus ~150 bp for human Alu and mouse SINE, respectively) and of the higher sequence homogeneity among human Alus as compared to more divergent SINEs of other species (104, 106, 107).
Rare Editing of Protein-Coding Sequences
Combined bioinformatics and comparative genomics screening restricted to coding regions resulted in the identification of only a few editing target genes (108, 109). Application of a similar bioinformatics screening of Drosophila cDNA and EST databases identified 27 new protein-coding targets, raising the possibility that the insect editing system may more efficiently utilize recoding type A→I editing (110). Moreover, it became clear that some single-nucleotide polymorphism (SNP) databases included many A→I RNA editing sites. However, screening of a human SNP database identified only a few editing sites that result in recoding of protein sequences (111, 112). Finally, most recently, an unbiased screening method (the padlock approach) was developed that allows amplification and deep sequencing of a large number of human exons simultaneously. By focusing on the exons containing potential A→I RNA editing sites (A→G changes in cDNA and EST), 53 new recoding type editing sites were identified, increasing the repertoire of recoding type A→I RNA editing sites substantially (113). Nonetheless, these results together indicate that the most common targets of ADARs are the noncoding sequences of transcriptomes and also that protein recoding as a result of A→I RNA editing is relatively rare.
FUNCTIONAL IMPLICATIONS OF REPETITIVE RNA EDITING
The biological significance of widespread global A→I editing of noncoding, repetitive sequences remains largely a mystery (Figure 3a). The editing of an A:U pair results in creation of an I·U wobble base pair and destabilization of the dsRNA structure. Some of the editing sites are at an A·C mismatched pair, with editing resulting in an I:C Watson-Crick pair and stabilization of the dsRNA structure. This change in the local and overall stability of the dsRNA structure and of inosines converted from adenosines seems to be recognized by several mechanisms. Accordingly, several possible functions of repeat element editing have been proposed.
Creation and Elimination of Splicing Sites?
The splicing machinery interprets an inosine as a guanosine. A→I editing could therefore create or delete splice donor and acceptor sites (Figure 3b). For example, a highly conserved canonical 5′ splice site dinucleotide recognition sequence GU (AU→IU = GU) or a 3′ splice acceptor site AG (AA→ AI = AG) can be created by editing. Similarly, editing of a 3′ splice site AG (AG→IG = GG) can destroy the site (114). Indeed, several examples of inclusion and exclusion of the Alu exon due to editing of the Alu fold-back dsRNA sequence have been identified through analysis of human cDNA sequences (102). More recently, a series of exonizations of the intronic sequence of the human nuclear prelamin A recognition factor resulting in coding differences has been reported (115, 116). The A→I editing of the Alu sequence and exonization of the noncoding sequence may be one important evolutionary means for generating a new variant protein with a novel function (102, 115, 116).
Nuclear Retention of Inosine-Containing RNAs?
A nuclear-localized multifunctional protein called p54nrb may play a role in the retention of extensively edited RNAs (I-RNA) within the nucleus (Figure 3c) (117). It has been proposed that A→I editing of a dsRNA formed on inverted repeats of SINEs present within the 3′ UTR of mouse cationic amino acid transporter 2 (CAT2) nuclear RNA (CTN-RNA) and its binding to p54nrb is involved in the mechanism that traps CTN-RNA within nuclear speckles (also known as interchromatin granule clusters) (118). During stress, CTN RNA is posttranscriptionally cleaved and de novo polyadenylated at an alternative site to produce protein-coding mCAT2 mRNA, which is then translated for CAT2 proteins (118). Whether the A→I editing of inverted Alu repeats in the 3′ UTR affects the nuclear retention mechanism was evaluated in human cells with an EGFP reporter system (119). Silencing of the reporter resulted from mRNA retention in the nucleus and was correlated with the extent of A→I editing of inverted Alu repeats as well as by its association with p54nrb. However, more recent studies on reporter RNAs, as well as on endogenous C. elegans and human mRNAs with an inverted repeat dsRNA structure, revealed that the presence of the dsRNA structure or A→I editing of the dsRNA region plays no role in the nuclear retention of these mRNAs (120). In fact, these dsRNA-containing mRNAs are exported into the cytoplasm, integrated into polysomes, and translated efficiently, independent of their editing status (120). This raises doubt on whether A→I editing of inversely oriented repeat elements does indeed play a role in the nuclear retention mechanism (120).
Control of esiRNA Synthesis?
The concentration of editing sites in repeat elements brings up the question of whether A→I editing acts as an antitransposon mechanism by altering the element sequence and inhibiting the integration of transcribed Alu and LINE sequences back to the genome (102, 104). Alternatively, editing may protect dsRNAs derived from inverted repeats of these retrotransposons from entering into the RNAi pathway (5). Recently, generation of esiRNAs from long hairpin dsRNAs (hpRNAs) has been reported in insect somatic cells (121) and mouse oocytes (122, 123). Processing of founder dsRNAs into esiRNAs proceeds in a processive fashion, producing esiRNAs with a ~21-nucleotide periodicity. The vast majority of esiRNAs originate from long terminal repeats of transposons and retrotransposons, such as SINEs and LINEs, whereas a substantial number of the remaining esiRNAs arise from pseudogenes or protein-coding genes arranged in reverse orientations (124). These esiRNAs are generated by Dicer and are involved in the silencing of transposon activities and founder genes of the pseudogenes (122, 123). Most interestingly, some esiRNAs contain a limited number of A→G changes, indicating that the founder dsRNAs of these esiRNAs have undergone A→I RNA editing (125). dsRNA that is extensively edited in vitro by ADAR becomes resistant to Dicer (see the next section) (126). Therefore, generation of esiRNAs may be suppressed through extensive A→I editing of the fold-back dsRNA in cells and in tissues expressing high levels of ADARs (Figure 3d).
INTERACTION OF RNA EDITING AND RNAi PATHWAYS
Accumulating evidence suggests that A→I RNA editing and RNAi pathways interact. In fact, the major function and evolutional reason for development of A→I RNA editing system may be to modulate the efficacy of RNAi. In following sections, the studies related to this particular aspect of A→I RNA editing are described.
Antagonistic Effects of A→I RNA Editing on RNAi
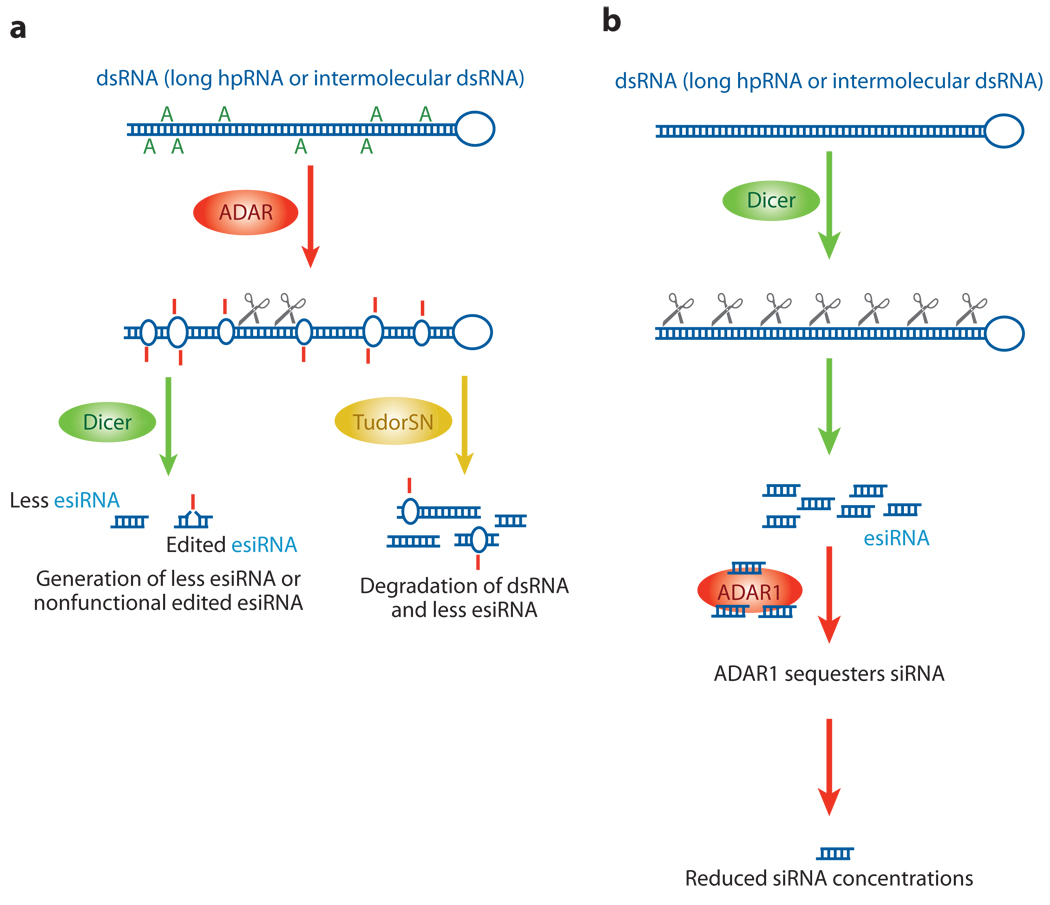
The A→I editing mechanism may interact with the RNAi pathway by competing for shared dsRNA substrates and by antagonizing RNAi efficacy (5, 127). Dicer seems to distinguish dsRNAs that contain I·U wobble base pairs from dsRNAs that contain only Watson-Crick base pairs. Increasing deamination of long dsR-NAs by ADAR progressively reduces the production of small interfering RNA (siRNA) by Dicer. Indeed, dsRNA that is extensively edited (~50% of the adenosines are converted to inosines) in vitro by ADAR becomes completely resistant to Dicer (126). By contrast, moderately deaminated dsRNA can be processed in vitro to siRNAs containing up to one I·U base pair per siRNA (126, 128). However, the substitution of even a single I·U for A:U base pair between the edited siRNAs and target mRNA could reduce the efficacy of RNAi (126). As already described in the previous section, a large proportion of esiRNAs containing a single A→G change has been identified recently in insect somatic cells (125). These results suggest that founder dsRNAs consisting of long hpRNAs of repetitive sequences are subject to A→I editing. Furthermore, they suggest that processing of the extensively edited founder dsRNAs by Dicer may be prohibited. Taken together, A→I RNA editing of long dsRNAs, such as those to be processed to esiRNAs, certainly can antagonize siRNA-mediated gene-silencing efficacy (Figure 4a).
Figure 4.
Interaction between RNA editing and RNAi pathways. Two possible ways in which RNA editing and RNAi pathways may interact. (a) The introduction of many I·U base pairs and the alteration of the dsRNA structure by ADAR leads to the generation of fewer esiRNAs by Dicer, because dsRNAs that contain many I·U base pairs become resistant to Dicer cleavage. Alternatively, extensively edited dsRNAs with multiple I·U base pairs may be rapidly degraded by Tudor staphylococcal nuclease (Tudor-SN), resulting in the generation of fewer esiRNAs. (b) In addition, a fraction of already processed siRNAs might be sequestered by certain ADAR gene family members, reducing the effective siRNA concentration. For instance, cytoplasmic ADAR1p150 binds siRNA tightly. Gene silencing by siRNA is significantly more effective in the absence of ADAR1, indicating that ADAR1p150 is a cellular factor that limits siRNA potency in mammalian cells by decreasing the effective siRNA concentration and its incorporation into RNA-induced silencing complex.
Degradation of Edited dsRNAs by Tudor Staphylococcal Nuclease
Extensive A→I editing of dsRNA may also result in degradation, consequently reducing the expression of esiRNAs. A ribonuclease activity that specifically cleaves inosine-containing dsRNA (I-dsRNA) has been reported (126). This ribonuclease preferentially cleaves dsRNA that contains multiple I·U base pairs (I-dsRNA) (126). Interestingly, Tudor staphylococcal nuclease (Tudor-SN), an RNA-induced silencing complex (RISC)-associated component that lacks an assigned function in the RNAi mechanism, has been identified as a potential I-dsRNA-specific ribonuclease or at least as an essential cofactor of ribonuclease activity (129). Extensive A→I editing might therefore lead to the degradation of dsRNAs by Tudor-SN, which in turn results in reduced esiRNA expression levels (Figure 4a).
Sequestration of siRNA by ADAR1p150
In mammalian cells, ADAR1p150 might quench the function of siRNAs that have already been processed from long dsRNA by Dicer. In vitro studies revealed that cytoplasmic ADAR1p150 binds siRNA very tightly, and gene silencing by siRNA is significantly more effective in mouse fibroblasts that are homozygous for an ADAR1 null mutation than in wild-type cells (66). These findings implicate ADAR1p150 as a cellular factor that limits siRNA potency in mammalian cells by decreasing the effective siRNA concentration (66). Interestingly, induced ADAR1 gene expression is observed in mice injected with high doses of nonspecific siRNA (130), indicating that ADAR1 is involved in a cellular feedback mechanism in response to siRNA (Figure 4b).
RNAi-Dependent Phenotypes of ADAR null Worms
C. elegans strains that contain homozygous deletions of both CeADR1 and CeADR2 genes have defective chemotaxis (Figure 2a) (131). These phenotypic alterations, however, can be reverted in RNAi-defective worms, indicating that the ADAR null phenotype is RNAi dependent (131). Expression of a gene involved in the chemotaxis mechanism seems to be controlled by the balance between A→I editing and RNAi on dsRNA derived from the chemotaxis gene (Figure 4a). Overly enhanced RNAi effects and suppression of the chemotaxis gene likely result in ADAR null worm phenotypes, but the identity of the chemotaxis gene as well as details of the interaction between RNA editing and the RNAi pathway remain unknown.
In addition, studies of ADAR null worms indicate that ADAR is involved in the mechanism that regulates the expression of transgenes. In C. elegans, A→I RNA editing of dsRNAs derived from inverted repeats of transgenes seems to prevent silencing of the transgenes by RNAi (132). RNAi-mediated transgene or viral gene silencing (cosuppression) is very efficient in plants and fungi that lack ADAR genes and the A→I RNA editing system (133). Thus, ADAR gene families and the A→I RNA editing system might have evolved to counteract or regulate RNAi in the animal kingdom.
EDITING OF MIRNA AND ITS BIOLOGICAL SIGNIFICANCE
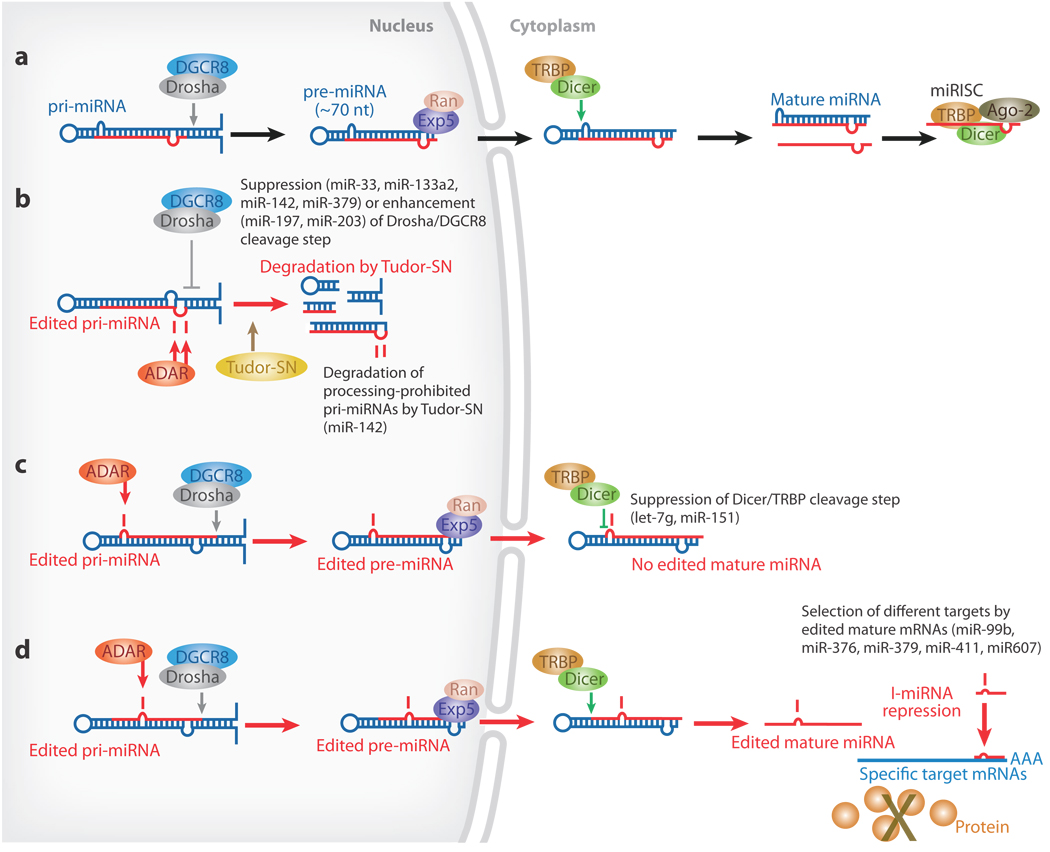
miRNA-mediated gene silencing controls many processes, such as development, differentiation, and apoptosis (133, 134). pri-miRNAs are processed sequentially by Drosha and Dicer (69). Nuclear Drosha, together with its partner, DGCR8, cleaves pri-miRNAs, releasing 60- to 70-nt pre-miRNAs. Correctly processed pre-miRNAs are recognized and exported from the nucleus by exportin-5 and RanGTP. Cytoplasmic Dicer together with the dsRNA-binding protein, transactivating response RNA-binding protein (TRBP), then cleaves pre-miRNAs into 20- to 22-nt siRNA-like duplexes. One or both strands of the duplex may serve as the mature miRNA. Following integration into miRNA-induced silencing complex (miRISC), miRNAs block the translation of partially complementary targets located in the 3′ UTR of specific mRNAs or guide the degradation of target mRNAs as do siRNAs (Figure 5a) (69, 134). Although certain pri-miRNAs have been reported to undergo A→I RNA editing in vivo (135–137), the biological significance of pri-miRNA remains unknown. Recent studies revealed that A→I RNA editing modulates processing and function of miRNAs.
Figure 5.
Regulation of miRNA processing and expression by RNA editing. (a) The Drosha/DGCR8 complex cleaves pri-miRNAs in the nucleus, producing ~70-nt pre-miRNA intermediates, which are exported by exportin-5 (Exp5) and RanGTP (Ran) into the cytoplasm. The Dicer/TRBP complex executes a second cleavage, generating mature miRNAs. (b) Drosha cleavage of pri- to pre-miRNA is suppressed by A→ editing of certain sites, such as the +4 and +5 sites of pri-miRNA-142 (miR-142). In addition, highly edited pri-miRNA-142 is degraded by Tudor staphylococcal nuclease (Tudor-SN) (138). (c) A→I editing of certain sites might lead to inhibition of the Dicer/TRBP cleavage step, as shown for pri-miR-151 editing. (d) Editing of pri-miRNAs at certain sites might lead to the expression of edited mature miRNAs and silencing of a different set of target genes owing to “seed” sequence alterations, as shown for miR-376.
Editing of Pri-miRNA Inhibits Drosha Cleavage
Several adenosine residues of pri-miR-142 are highly edited by ADAR1 and/or ADAR2. In vitro processing assays using Drosha/DGCR8 and Dicer/TRBP complexes revealed that editing inhibits Drosha cleavage of pri-miR-142 and that sites most inhibitory for processing of pri- to pre-miRNA-142 RNAs are the +4 and +5 sites located in the fold-back dsRNA stem near the Drosha cleavage site (Figure 5b). As expected, miR-142-5p and miR-142-3p expression levels are significantly higher in spleens of B-cell-lineage-specific ADAR1−/− mice and ADAR2−/− mice than in spleens of wild-type control mice (138).
In view of the suppressive effects of A→I editing on the processing of pri-miR-142 and on the expression of mature miR-142 RNAs, detection of a substantial level of highly edited pri-miR-142 RNA sequences in wild-type mouse spleen is anticipated. However, only a relatively low level of edited pri-miR-142 RNAs can be detected. Highly edited pri-miR-142 RNAs (prohibited from cleavage by Drosha) appear to be rapidly degraded by Tudor-SN, a ribonuclease specific to I-dsRNAs (Figure 5b) (129). Taken together, these findings suggest that steady-state levels of edited pri-miR-142 RNAs are regulated by editing frequency and Tudor-SN activity (138). However, some edited pri-miRNAs are stable, and perhaps only some edited pri-miRNAs with multiple I·U pairs are selected for degradation by Tudor-SN following A→I RNA editing.
Editing of Pri-miRNA Inhibits Dicer Cleavage
A major editing site (+3) and a minor site (−1) of pri-miR-151 were identified on its antisense strand and near its end loop (139). Analysis of ADAR1−/− embryos revealed that editing of the −1 and +3 sites is carried out by ADAR1 (139). No edited mature miR-151-3p RNAs were detected, but all pre-miR-151 molecules detected were completely edited at the +3 site. An in vitro pri-miRNA processing assay revealed that the A→I substitution at the −1 or +3 site had no effect on Drosha cleavage but inhibited cleavage of the edited pre-miR-151 by the Dicer/TRBP complex. These studies demonstrated that Dicer cleavage is the step inhibited by the editing of pri-miR-151 at the major +3 and minor −1 sites, resulting in accumulation of edited pre-miR-151 RNAs in the cytoplasm (139). Interestingly, in vitro studies showed that the editing efficiency at the +3 site is much higher with pre-miR-151 than with pri-miR-151. It is possible that certain pri-miRNAs, not edited in the nucleus, may be edited only after processing to pre-miRNAs in the cytoplasm, most likely by the only cytoplasmic ADAR, ADAR1p150 (139).
Redirection of Silencing Target Genes by Edited miRNAs
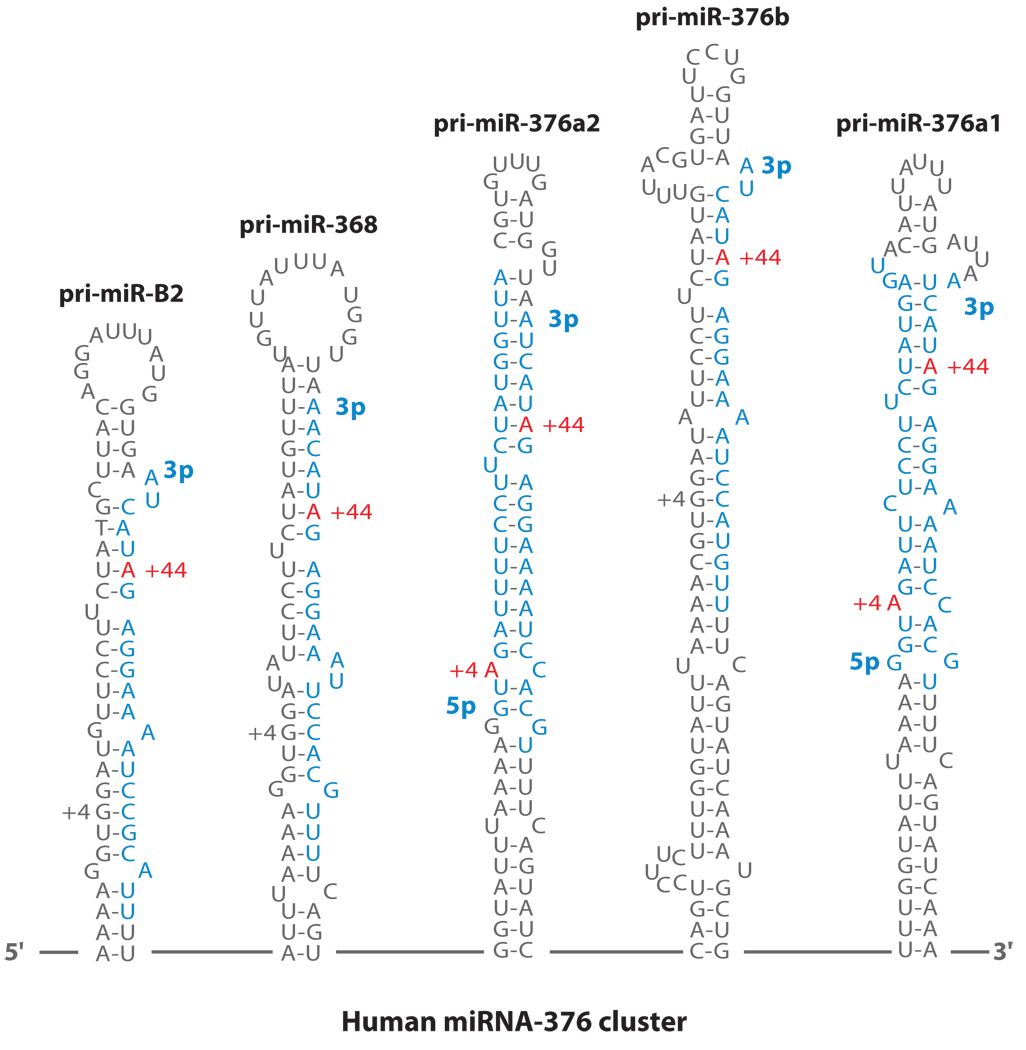
A→I editing of miR-376 cluster transcripts can lead to the silencing of specific sets of genes, resulting in stringent and tissue-specific regulation of certain gene products. Six human and three mouse miR-376 family genes are included within the maternally imprinted loci. Most miR-376 cluster members undergo extensive and simultaneous A→I editing at two specific sites (+4 and +44 sites) in select human and mouse tissues and specific subregions of the brain (Figure 6) (140). The +4 site is edited by ADAR2, whereas the +44 site is selectively edited by ADAR1. Identification of the edited forms of mature miR-376 RNAs in certain tissues, such as brain, revealed that, unlike the case of pri-miR-142 and pri-miR-151, editing of pri-miR-376 RNAs at the two sites does not affect the Drosha and Dicer cleavage steps. Both of the two editing sites in pri-miR-376 RNAs (+4 and +44) are located within the functionally critical 5′ proximal “seed” sequences of miRNA-376-5p and −3p strands. Two different sets of the potential target genes for unedited and edited miR-376a-5p (~80 each) were predicted in silico. Only a few overlapping genes were predicted to be targeted by both unedited and edited miR-376a-5p. In vitro reporter verification experiments for select unedited-version targets and edited-version targets confirmed that a single A→I base change is sufficient to redirect silencing miRNAs to a new set of targets.
Figure 6.
A→I RNA editing of pri-miR-376 RNAs. Hairpin structures of five human pri-miR-376 cluster member RNAs that are frequently edited in brain and other tissues. Regions processed into mature miRNAs are highlighted in blue. The two most highly edited adenosines (+4 and +44 sites) are highlighted in red. Editing sites that are numbered with the 5′ end of the human miR-376a1–5p sequence are counted as +1.
Endogenous expression levels of one of the edited-version miR-376a-5p targets, pyrophosphate synthetase 1 (PRPS1), were assessed in wild-type and ADAR2−/− mouse cortices. The +4 site of miR-376a-5p is edited exclusively by ADAR2. No edited mature miR-376a-5p is expressed in ADAR2−/− mice, whereas both unedited and edited miR-376a-5p are expressed in brain cortex of wild-type mice. Only unedited mature miR-376a-5p RNAs were detected in liver of wild-type mice because of a nearly total lack of pri-miR376a RNA editing in this tissue. The levels of PRPS1 were almost twofold lower in wild-type mouse cortex than in ADAR2−/− mouse cortex. By contrast, no difference in PRPS1 expression was detected between wild-type and ADAR2−/− liver, revealing that the edited miR-376a-5p does repress this target gene in a tissue-specific manner. PRPS1 is involved in purine metabolism and the uric acid synthesis pathway. Analysis of uric acid levels confirmed that tissue-specific repression of PRPS1 is indeed reflected in a twofold increase in uric acid levels in ADAR2−/− cortex. Thus, editing of miR-376a appears to be one of the mechanisms that ensure tight regulation of uric acid levels in select tissues such as brain cortex (140).
Frequency and Fate of miRNA Editing
A large-scale survey of pri-miRNAs subject to A→I editing identified 86 A→I editing sites in 47 pri-miRNAs, which are highly edited in human brain (141). Statistical analysis revealed the most preferred sequence and structure surrounding pri-miRNA editing sites. The adenosine residue of the UAG triplet with a partner nucleotide of cytidine appears to be the most frequently and highly edited (141), consistent with the sequence preference of the highly edited adenosine residues found previously by analysis of synthetic dsRNAs (6). In vitro processing assays conducted on select pri-miRNAs (pri-let7-g, pri-miR-33, pri-miR-133a2, pri-miR-197, pri-miR-203, and pri-miR-379) suggest that the majority of pri-miRNA editing events suppresses or enhances miRNA processing steps (Figure 5b,c). Additional edited mature miRNAs (miR-99b-3p, miR-379-5p, miR-411-5p, and miR-607) with altered seed sequences are also detected. These edited mature miRNAs are predicted to repress a set of genes that differ from those targeted by the unedited miRNAs (Figure 5d). Approximately 16% of human pri-miRNAs are edited in brain, and thus the expression of a large number of genes is expected to be affected globally by A→I editing of miRNAs (141). Conversely, ADARs may have affected development of mammalian miRNAs during evolution because of a selective force imposed on miRNA sequences by A→I editing.
Although this is yet to be demonstrated, A→I editing of certain pri-miRNAs at specific sites may suppress their export from the nucleus by RanGTP/exportin-5 (Figure 5a). A→I RNA editing is expected to affect the local stability of the duplex. The selection of the “effective” miRNA strand that is loaded onto miRISC and that guides the miRNA to the target mRNA depends on the local stability of the sense-antisense miRNA duplex (Figure 5a) (133, 134, 142). Thus, editing may also affect the selection of the effective miRNA strand.
Modulation of the miRNA Target Sites by A→I Editing?
The possibility that editing of miRNA target sites may affect miRNA-mediated gene silencing has been proposed (143). Because A→I editing occurs frequently within 3′ UTRs, which are also common miRNA-binding (target) sites, A→I editing of 3′ UTRs may create or eliminate the miRNA target site. However, computational screening for the frequency of editing at sites that contain potential miRNA target sites revealed that RNA editing tends to avoid miRNA target sites (143).
INVOLVEMENT OF ADAR AND I-RNA IN OTHER FUNCTIONS
Several reports have implicated ADAR and/or inosine-containing RNAs (edited RNA molecules) in functions that are currently not thoroughly understood. Nonetheless, these reports may indicate important future directions of the field.
Function of I-dsRNA
dsRNAs containing tandem repeats of I·U base pairs have a unique geometry and reduced stability compared to those containing A:U or G·U pairs (129). Moreover, they appear to be recognized by certain cellular proteins, e.g., Tudor-SN (129). Hyperedited I-dsRNA may have a unique function (144). I-dsRNAs specifically bind a complex that consists of proteins previously characterized as components of stress granules. During stress, a subset of cellular mRNAs are translationally silenced by sequestration into stress granules. Apparently, I-dsRNAs complexed with stress granule component proteins downregulate gene expression by inhibiting the translation initiation step (144). One of the I-dsRNAs tested for its translation suppression activity was the edited pri-miR-142 (Figure 5b) (138). The presence of edited pri-miR-142 substantially reduces the expression of a luciferase reporter, indicating that edited miRNAs in general may have the potential for regulating gene expression in trans (144).
Involvement of ADAR in Other RNA Processing Pathways?
A→I RNA editing machinery appears to interact with several posttranscriptional RNA processing pathways. Involvement of the intron sequence in the formation of the dsRNA structure essential for editing as well as identification of many intronic editing sites predicted that interactions occur between splicing machinery and editing enzymes. Both ADAR1 and ADAR2 associate with spliceosomal component proteins (Sm and SR) within large nuclear ribonucleoprotein particles (145). Indeed, coordination of editing and splicing has been noted for the editing of GluR-B pre-mRNA Editing at the GluR-B Q/R site, 24 bases upstream of the intron 5′ splice donor site, stimulates splicing (146). Furthermore, RNA editing and the nonsense-mediated mRNA decay (NMD) mechanism also reportedly interact: The NMD-associated protein Upf1 associates with ADAR1, leading to the proposed degradation of pre-mRNAs containing edited Alu repeats by the NMD mechanism with the aid of Upf1 (147).
Heterochromatic Silencing?
The possible involvement of A→I RNA editing in the heterochromatic silencing mechanism was proposed following the identification of vigilin as a cellular factor that binds I-RNAs (148). Vigilin localized to heterochromatin is a component of a complex that contains ADAR1, the Ku86/Ku70 heterodimer (DNA-binding proteins involved in the DNA repair mechanism); RNA helicase A (RHA), the histone variant H2AX; and the heterochromatin protein HP1α (148). RHA has various proposed functions, such as unwinding a dsRNA structure formed around the exon-intron of the D. melanogaster Na+ channel gene, which is also an A→I RNA editing target (75). The vigilin/ADAR1/Ku heterodimer/RHA complex recruits the DNA-protein kinase DNA-PKcs enzyme, which phosphorylates a set of target proteins including HP1α and H2AX and also plays a major role in the chromatin silencing mechanism (148). The presence of ADAR1 in this complex suggests its involvement and a role for A→I RNA editing or edited RNAs (I-RNAs) in the heterochromatic gene-silencing mechanism (148). Vigilin supposedly recognizes the genomic loci producing dsRNAs derived from repetitive sequences, such as retrotransposons, through binding to hyperedited I-RNAs and recruits other factors (such as histone methylase SUV39HZ) essential for transformation of the region into heterochromatin (149).
ADAR1 Function in Antiviral Mechanisms and Immunity?
Interferons induce the upregulation of ADAR1 (56), thus raising the possibility that ADAR1 functions in host defense mechanisms against viral infection and inflammation. For instance, ADAR1 edits the hepatitis C virus RNA genome and inhibits its replication (150). Inosine-containing mRNAs increase in T lymphocytes and macrophages stimulated with a variety of inflammatory mediators, including tumor necrosis factor-α and interferon-γ (151). Furthermore, ADAR1 interacts with nuclear factor 90 (NF90) family proteins, also known as interleukin enhancer-binding factor 3 (ILF3), and affects the NF90-mediated gene regulatory mechanism (152). NF90 is a known regulator that stimulates the activation of the cellular antiviral expression cascade and activates interferon-β (152). Suppression of the interferon activation pathway through the ADAR1-NF90 interaction is important for protecting hematopoietic progenitor cells from apoptosis (84). Finally, the inhibitory effects of ADAR1 on interferon-β induction of the cellular response to DNA treatment have been reported, supporting ADAR1’s function in DNA-mediated immune responses (153).
CONCLUSIONS AND PERSPECTIVES
Nearly two decades ago, ADARs were originally discovered as a mysterious dsRNA-unwinding activity. Soon thereafter, ADARs were identified as dsRNA-specific adenosine deaminase enzymes that are essential for the recoding type A→I editing of important mammalian genes, such as GluRs and 5-HT2CRs. Since then, great progress has been made with regard to identifying new RNA editing targets, the ADAR enzyme structure, and the site-selective editing mechanism. However, many questions remain to be answered.
One of the most important issues to address is the biological significance of A→I editing of noncoding RNAs, especially in relation to RNAi-mediated gene silencing.We are now beginning to realize that A→I editing and ADAR functions are more global, affecting expression of many more genes than previously anticipated. It appears that the primordial function of ADAR may be to deal with noncoding dsR-NAs, such as those made from repetitive elements of transposons and retrotransposons as well as pri-miRNAs, and to counterbalance the efficacy of RNAi in the animal kingdom, possibly along with the expansion of repeat elements in the genome. Editing of protein-coding sites may have appeared more recently, perhaps by chance at the beginning, but were retained because of their extreme advantage. The Functional Annotation of Mouse (FANTOM) project has revealed that mouse retrotransposons are much more transcriptionally active than expected (154). The expression of these SINE, LINE, and LTR elements is tightly regulated in a tissue-specific manner (154). It would be interesting to determine whether A→I RNA editing is involved in the regulation of the retrotransposon transcriptome.
The inactivation of ADAR1 leads to an embryonic-lethal phenotype, which is caused by widespread apoptosis (60, 63, 83). Editing of currently unknown target dsRNA(s) appears to protect developing embryos from massive apoptosis. It is tempting to speculate that the critical function of ADAR1 may be to regulate the expression of noncoding RNAs, perhaps a select set of miRNAs or the founder dsRNAs of esiRNAs.
Apart from further investigation into RNAi and RNA editing interactions, studies in other areas are needed also. Studies on the interaction of ADAR with other proteins and factors (e.g., vigilin) revealed the involvement of A→I RNA editing in previously unexpected areas (e.g., heterochromatic gene silencing). Investigations into additional proteins and machinery that interact with ADAR are anticipated to reveal new functions of A→I RNA editing. ADAR expression levels in certain tissues and/or developing embryos do not necessarily correlate with A→I RNA editing activities, indicating the presence of a posttranslational regulatory mechanism (79). Almost no investigations exist on the posttranslational modification of ADARs, except for one report on sumoylation of ADAR1 and downregulation of its activity (155). Furthermore, a better understanding of the mechanism that controls the cellular distribution of ADARs (nuclear import and export) seems to be essential. Investigation into such mechanisms—e.g., identification of regulatory factors, control of ADAR cellular distribution, and posttranslational modifications—is needed to better understand the mechanism regulating ADAR activity.
SUMMARY POINTS.
A→I RNA editing is catalyzed by adenosine deaminases acting on RNA (ADARs).
Three mammalian ADAR genes (ADAR1–3) with common functional domains have been identified.
ADARs edit protein-coding sequences of a limited number of genes, such as glutamate receptor GluR-B and serotonin receptor 2C, resulting in dramatic alterations of protein functions.
Deficiencies in the A→I RNA editing mechanism cause human diseases and pathophysiology.
Bioinformatics studies have identified numerous A→I RNA editing sites genome wide in Alu and LINE-repetitive RNA sequences located within introns and untranslated regions (UTRs).
Although the biological significance of A→I editing of repeat RNAs remains mostly unknown, one possibility is to control the synthesis of esiRNAs derived from retrotransposons.
A→I RNA editing and RNAi mechanisms appear to interact and compete for common substrate dsRNAs.
The biogenesis and function of certain miRNAs are regulated by the editing of their precursors.
FUTURE ISSUES.
The physiological significance of recoding editing of newly discovered protein-coding genes needs to be identified.
Studies on mechanisms that regulate ADAR enzymatic activities, including posttranslational modification and nuclear cytoplasmic transport control, and that identify regulatory factors are necessary.
Structural studies on full-length ADAR proteins in complex with their RNA substrates are essential for understanding of the site-selective editing mechanism.
We need to better understand the significance of the editing of noncoding repetitive RNAs of transposons and retrotransposons.
Comprehensive identification of editing sites of pri-miRNAs and edited miRNAs and determination of their significance are required.
The interaction between RNAi and RNA editing pathways needs to be further investigated.
The RNA substrate(s) critical for apoptosis-prone ADAR1−/− mouse embryos must be identified.
ACKNOWLEDGMENTS
I am grateful to members of my laboratory—Hiromitsu Ota, Boris Zinshteyn, and Bjørn-Erik Wulff—for their comments and suggestions. This work was supported in part by grants from the U.S. National Institutes of Health, the Ellison Medical Foundation, and the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
Glossary
- Adenosine deaminase acting on RNA (ADAR)
ADAR catalyzes an RNA editing reaction whereby an adenosine is converted to an inosine
- Retrotransposons
genetic elements containing retroviruses and transposons are replicated through an intermediate RNA stage
- Alu
a dispersed, middle-repetitive DNA sequence (~1.4 million copies) found in the human genome
- LINE
a long interspersed element sequence that is typically used for non-long terminal repeat retrotransposons
- Endogenous siRNA (esiRNA)
repeat-associated siRNAs derived from endogenous repetitive sequences such as Alu or LINE retrotransposon elements
- MicroRNA (miRNA)
a small (19–23 nucleotides) single-stranded RNA that is processed from a precursor that consists of a short dsRNA region, bulges, and a loop
- Noncoding RNA
RNA transcribed from DNA but not translated into protein
- RNA interference (RNAi)
a posttranscriptional gene-silencing process in which dsRNA triggers the degradation of homologous mRNA induced by siRNAs
- Double-stranded RNA-binding domain (dsRBD)
each dsRBD forms a domain (~65 amino acids) with α-β-β-β-α structures and makes direct contact with dsRNA
- Z-DNA
left-handed DNA that differs from A- and B-DNA and that is believed to be involved in specific biological functions
- Expressed sequence tag (EST)
a single-pass, short read of complementary DNA that is generated from a transcribed region of the genome
- SINE
a short interspersed element of repetitive sequences, such as Alu elements, generated by retrotransposons
- Single-nucleotide polymorphism (SNP)
SNPs are base-pair substitutions that are the most common forms of genetic polymorphism SNPs are typically biallelic
- Wobble base pair
non-Watson-Crick pairing such as the thermodynamically less stable G·U and I·U pairing
- Small interfering RNA (siRNA)
a small (19–23 bp) noncoding dsRNA that is processed from a longer dsRNA by Dicer
- miRNA-induced silencing complex (miRISC)
the miRNA-mediated RNAi machinery that contains miRNAs and other protein factors, such as Ago-2
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Baltimore D. Our genome unveiled. Nature. 2001;409:814–816. doi: 10.1038/35057267. [DOI] [PubMed] [Google Scholar]
- 2.Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- 3.Benne R, Van den Burg J, Brakenhoff JP, Sloof P, Van Boom JH, Tromp MC. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986;46:819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- 4.Gott JM, Emeson RB. Functions and mechanisms of RNA editing. Annu. Rev. Genet. 2000;34:499–531. doi: 10.1146/annurev.genet.34.1.499. [DOI] [PubMed] [Google Scholar]
- 5.Nishikura K. Editor meets silencer: crosstalk between RNA editing and RNA interference. Nat. Rev. Mol. Cell Biol. 2006;7:919–931. doi: 10.1038/nrm2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu. Rev. Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jepson JE, Reenan RA. RNA editing in regulating gene expression in the brain. Biochim. Biophys. Acta. 2008;1779:459–470. doi: 10.1016/j.bbagrm.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Seeburg PH, Hartner J. Regulation of ion channel/neurotransmitter receptor function by RNA editing. Curr. Opin. Neurobiol. 2003;13:279–283. doi: 10.1016/s0959-4388(03)00062-x. [DOI] [PubMed] [Google Scholar]
- 9.Reenan RA. The RNA world meets behavior: A→I pre-mRNA editing in animals. Trends Genet. 2001;17:53–56. doi: 10.1016/s0168-9525(00)02169-7. [DOI] [PubMed] [Google Scholar]
- 10.Keegan LP, Gallo A, O’Connell MA. The many roles of an RNA editor. Nat. Rev. Genet. 2001;2:869–878. doi: 10.1038/35098584. [DOI] [PubMed] [Google Scholar]
- 11.Bass BL, Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988;55:1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- 12.Wagner RW, Smith JE, Cooperman BS, Nishikura K. A double-stranded RNA unwinding activity introduces structural alterations by means of adenosine to inosine conversions in mammalian cells and Xenopus eggs. Proc. Natl. Acad. Sci. USA. 1989;86:2647–2651. doi: 10.1073/pnas.86.8.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bass BL, Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987;48:607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- 14.Rebagliati MR, Melton DA. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987;48:599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim U, Garner TL, Sanford T, Speicher D, Murray JM, Nishikura K. Purification and characterization of double-stranded RNA adenosine deaminase from bovine nuclear extracts. J. Biol. Chem. 1994;269:13480–13489. [PubMed] [Google Scholar]
- 16.O’Connell MA, Keller W. Purification and properties of double-stranded RNA-specific adenosine deaminase from calf thymus. Proc. Natl. Acad. Sci. USA. 1994;91:10596–10600. doi: 10.1073/pnas.91.22.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc. Natl. Acad. Sci. USA. 1994;91:11457–11461. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, et al. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol. Cell. Biol. 1995;15:1389–1397. doi: 10.1128/mcb.15.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber A, O’Connell MA, Keller W. Two forms of human double-stranded RNA-specific editase 1 (hRED1) generated by the insertion of an Alu cassette. RNA. 1997;3:453–463. [PMC free article] [PubMed] [Google Scholar]
- 20.Lai F, Chen CX, Carter KC, Nishikura K. Editing of glutamate receptor B subunit ion channel RNAs by four alternatively spliced DRADA2 double-stranded RNA adenosine deaminases. Mol. Cell. Biol. 1997;17:2413–2424. doi: 10.1128/mcb.17.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- 22.Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA. 2000;6:755–767. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melcher T, Maas S, Herb A, Sprengel R, Higuchi M, Seeburg PH. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J. Biol. Chem. 1996;271:31795–31798. doi: 10.1074/jbc.271.50.31795. [DOI] [PubMed] [Google Scholar]
- 24.Slavov D, Clark M, Gardiner K. Comparative analysis of the RED1 and RED2 A-to-I RNA editing genes from mammals, pufferfish and zebrafish. Gene. 2000;250:41–51. doi: 10.1016/s0378-1119(00)00174-8. [DOI] [PubMed] [Google Scholar]
- 25.Slavov D, Crnogorac-Jurcevic T, Clark M, Gardiner K. Comparative analysis of the DRADA A-to-I RNA editing gene from mammals, pufferfish and zebrafish. Gene. 2000;250:53–60. doi: 10.1016/s0378-1119(00)00175-x. [DOI] [PubMed] [Google Scholar]
- 26.Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. A-to-I pre-mRNA editing in Drosophila is primarily involved in adult nervous system function and integrity. Cell. 2000;102:437–449. doi: 10.1016/s0092-8674(00)00049-0. [DOI] [PubMed] [Google Scholar]
- 27.Tonkin LA, Saccomanno L, Morse DP, Brodigan T, Krause M, Bass BL. RNA editing by ADARs is important for normal behavior in Caenorhabditis elegans. EMBO J. 2002;21:6025–6035. doi: 10.1093/emboj/cdf607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palavicini JP, O’Connell MA, Rosenthal JJ. An extra double-stranded RNA binding domain confers high activity to a squid RNA editing enzyme. RNA. 2009;15:1208–1218. doi: 10.1261/rna.1471209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Y, Zhang W, Li Q. Origins and evolution of ADAR-mediated RNA editing. IUBMB Life. 2009;61:572–578. doi: 10.1002/iub.207. [DOI] [PubMed] [Google Scholar]
- 30.Gerber AP, Keller W. RNA editing by base deamination: more enzymes, more targets, new mysteries. Trends Biochem. Sci. 2001;26:376–384. doi: 10.1016/s0968-0004(01)01827-8. [DOI] [PubMed] [Google Scholar]
- 31.Wolf J, Gerber AP, Keller W. tadA, an essential tRNA-specific adenosine deaminase from Escherichia coli. EMBO J. 2002;21:3841–3851. doi: 10.1093/emboj/cdf362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valente L, Nishikura K. RNA binding-independent dimerization of adenosine deaminases acting on RNA and dominant negative effects of nonfunctional subunits on dimer functions. J. Biol. Chem. 2007;282:16054–16061. doi: 10.1074/jbc.M611392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc. Natl. Acad. Sci. USA. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maas S, Gommans WM. Novel exon of mammalian ADAR2 extends open reading frame. PLoS ONE. 2009;4:e4225. doi: 10.1371/journal.pone.0004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai F, Drakas R, Nishikura K. Mutagenic analysis of double-stranded RNA adenosine deaminase, a candidate enzyme for RNA editing of glutamate-gated ion channel transcripts. J. Biol. Chem. 1995;270:17098–17105. doi: 10.1074/jbc.270.29.17098. [DOI] [PubMed] [Google Scholar]
- 37.Macbeth MR, Schubert HL, VanDemark AP, Lingam AT, Hill CP, Bass BL. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science. 2005;309:1534–1539. doi: 10.1126/science.1113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikura K, Yoo C, Kim U, Murray JM, Estes PA, et al. Substrate specificity of the dsRNA unwinding/modifying activity. EMBO J. 1991;10:3523–3532. doi: 10.1002/j.1460-2075.1991.tb04916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter MA. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters NT, Rohrbach JA, Zalewski BA, Byrkett CM, Vaughn JC. RNA editing and regulation of Drosophila 4f–rnp expression by sas-10 antisense readthrough mRNA transcripts. RNA. 2003;9:698–710. doi: 10.1261/rna.2120703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer SE, Butler MD, Pan Q, Ruvkun G. Trans-splicing in C. elegans generates the negative RNAi regulator ERI-6/7. Nature. 2008;455:491–496. doi: 10.1038/nature07274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehmann KA, Bass BL. The importance of internal loops within RNA substrates of ADAR1. J. Mol. Biol. 1999;291:1–13. doi: 10.1006/jmbi.1999.2914. [DOI] [PubMed] [Google Scholar]
- 43.Higuchi M, Single FN, Kohler M, Sommer B, Sprengel R, Seeburg PH. RNA editing of AMPA receptor subunit GluR-B: A base-paired intron-exon structure determines position and efficiency. Cell. 1993;75:1361–1370. doi: 10.1016/0092-8674(93)90622-w. [DOI] [PubMed] [Google Scholar]
- 44.Lomeli H, Mosbacher J, Melcher T, Hoger T, Geiger JR, et al. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science. 1994;266:1709–1713. doi: 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, O’Brien PJ, Chen CX, Cho DS, Murray JM, Nishikura K. Altered G protein–coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J. Neurochem. 2000;74:1290–1300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- 46.Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 47.Bhalla T, Rosenthal JJ, Holmgren M, Reenan R. Control of human potassium channel inactivation by editing of a small mRNA hairpin. Nat. Struct. Mol. Biol. 2004;11:950–956. doi: 10.1038/nsmb825. [DOI] [PubMed] [Google Scholar]
- 48.Ohlson J, Pedersen JS, Haussler D, Ohman M. Editing modifies the GABAA receptor subunit α3. RNA. 2007;13:698–703. doi: 10.1261/rna.349107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keegan LP, Brindle J, Gallo A, Leroy A, Reenan RA, O’Connell MA. Tuning of RNA editing by ADAR is required in Drosophila. EMBO J. 2005;24:2183–2193. doi: 10.1038/sj.emboj.7600691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reenan RA. Molecular determinants and guided evolution of species-specific RNA editing. Nature. 2005;434:409–413. doi: 10.1038/nature03364. [DOI] [PubMed] [Google Scholar]
- 51.Stefl R, Xu M, Skrisovska L, Emeson RB, Allain FH. Structure and specific RNA binding of ADAR2 double-stranded RNA binding motifs. Structure. 2006;14:345–355. doi: 10.1016/j.str.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Cho DS, Yang W, Lee JT, Shiekhattar R, Murray JM, Nishikura K. Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA. J. Biol. Chem. 2003;278:17093–17102. doi: 10.1074/jbc.M213127200. [DOI] [PubMed] [Google Scholar]
- 53.Gallo A, Keegan LP, Ring GM, O’Connell MA. An ADAR that edits transcripts encoding ion channel subunits functions as a dimer. EMBO J. 2003;22:3421–3430. doi: 10.1093/emboj/cdg327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chilibeck KA, Wu T, Liang C, Schellenberg MJ, Gesner EM, et al. FRET analysis of in vivo dimerization by RNA-editing enzymes. J. Biol. Chem. 2006;281:16530–16535. doi: 10.1074/jbc.M511831200. [DOI] [PubMed] [Google Scholar]
- 55.Poulsen H, Jorgensen R, Heding A, Nielsen FC, Bonven B, Egebjerg J. Dimerization of ADAR2 is mediated by the double-stranded RNA binding domain. RNA. 2006;12:1350–1360. doi: 10.1261/rna.2314406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol. Cell. Biol. 1995;15:5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawakubo K, Samuel CE. Human RNA-specific adenosine deaminase (ADAR1) gene specifies transcripts that initiate from a constitutively active alternative promoter. Gene. 2000;258:165–172. doi: 10.1016/s0378-1119(00)00368-1. [DOI] [PubMed] [Google Scholar]
- 58.Peng PL, Zhong X, Tu W, Soundarapandian MM, Molner P, et al. ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 determines vulnerability of neurons in forebrain ischemia. Neuron. 2006;49:719–733. doi: 10.1016/j.neuron.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 59.Reymond A, Marigo V, Yaylaoglu MB, Leoni A, Ucla C, et al. Human chromosome 21 gene expression atlas in the mouse. Nature. 2002;420:582–586. doi: 10.1038/nature01178. [DOI] [PubMed] [Google Scholar]
- 60.Wang Q, Khillan J, Gadue P, Nishikura K. Requirement of the RNA editing deaminase ADAR1 gene for embryonic erythropoiesis. Science. 2000;290:1765–1768. doi: 10.1126/science.290.5497.1765. [DOI] [PubMed] [Google Scholar]
- 61.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Miyakoda M, Yang W, Khillan J, Stachura DL, et al. Stress-induced apoptosis associated with null mutation of ADAR1 RNA editing deaminase gene. J. Biol. Chem. 2004;279:4952–4961. doi: 10.1074/jbc.M310162200. [DOI] [PubMed] [Google Scholar]
- 64.Desterro JM, Keegan LP, Lafarga M, Berciano MT, O’Connell M, Carmo-Fonseca M. Dynamic association of RNA-editing enzymes with the nucleolus. J. Cell Sci. 2003;116:1805–18018. doi: 10.1242/jcs.00371. [DOI] [PubMed] [Google Scholar]
- 65.Poulsen H, Nilsson J, Damgaard CK, Egebjerg J, Kjems J. CRM1 mediates the export of ADAR1 through a nuclear export signal within the Z-DNA binding domain. Mol. Cell. Biol. 2001;21:7862–7871. doi: 10.1128/MCB.21.22.7862-7871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang W, Wang Q, Howell KL, Lee JT, Cho DS, et al. ADAR1 RNA deaminase limits short interfering RNA efficacy in mammalian cells. J. Biol. Chem. 2005;280:3946–3953. doi: 10.1074/jbc.M407876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eckmann CR, Neunteufl A, Pfaffstetter L, Jantsch MF. The human but not the Xenopus RNA-editing enzyme ADAR1 has an atypical nuclear localization signal and displays the characteristics of a shuttling protein. Mol. Biol. Cell. 2001;12:1911–1924. doi: 10.1091/mbc.12.7.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fritz J, Strehblow A, Taschner A, Schopoff S, Pasierbek P, Jantsch MF. RNA-regulated interaction of transportin-1 and exportin-5 with the double-stranded RNA-binding domain regulates nucleocyto-plasmic shuttling of ADAR1. Mol. Cell. Biol. 2009;29:1487–1497. doi: 10.1128/MCB.01519-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 70.Maas S, Gommans WM. Identification of a selective nuclear import signal in adenosine deaminases acting on RNA. Nucleic Acids Res. 2009;37:5822–5829. doi: 10.1093/nar/gkp599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohta H, Fujiwara M, Ohshima Y, Ishihara T. ADBP-1 regulates an ADAR RNA-editing enzyme to antagonize RNA-interference-mediated gene silencing in Caenorhabditis elegans. Genetics. 2008;180:785–796. doi: 10.1534/genetics.108.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sansam CL, Wells KS, Emeson RB. Modulation of RNA editing by functional nucleolar sequestration of ADAR2. Proc. Natl. Acad. Sci. USA. 2003;100:14018–14023. doi: 10.1073/pnas.2336131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoopengardner B, Bhalla T, Staber C, Reenan R. Nervous system targets of RNA editing identified by comparative genomics. Science. 2003;301:832–836. doi: 10.1126/science.1086763. [DOI] [PubMed] [Google Scholar]
- 74.Rosenthal JJ, Bezanilla F. Extensive editing of mRNAs for the squid delayed rectifier K+ channel regulates subunit tetramerization. Neuron. 2002;34:743–757. doi: 10.1016/s0896-6273(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 75.Reenan RA, Hanrahan CJ, Ganetzky B. The mlenapts RNA helicase mutation in Drosophila results in a splicing catastrophe of the para Na+ channel transcript in a region of RNA editing. Neuron. 2000;25:139–149. doi: 10.1016/s0896-6273(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 76.Polson AG, Bass BL, Casey JL. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature. 1996;380:454–456. doi: 10.1038/380454a0. [DOI] [PubMed] [Google Scholar]
- 77.Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J. Biol. Chem. 1999;274:9472–9478. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- 78.Rula EY, Lagrange AH, Jacobs MM, Hu N, Macdonald RL, Emeson RB. Developmental modulation of GABAA receptor function by RNA editing. J. Neurosci. 2008;28:6196–6201. doi: 10.1523/JNEUROSCI.0443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wahlstedt H, Daniel C, Ensterö M, Öhman M. Large-scale mRNA sequencing determines global regulation of RNA editing during brain development. Genome Res. 2009;19:978–986. doi: 10.1101/gr.089409.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith LA, Peixoto AA, Hall JC. RNA editing in the Drosophila Dmca1A calcium-channel alpha 1 subunit transcript. J. Neurogenet. 1998;12:227–240. doi: 10.3109/01677069809108560. [DOI] [PubMed] [Google Scholar]
- 81.Wu YM, Kung SS, Chen J, Chow WY. Molecular analysis of cDNA molecules encoding glutamate receptor subunits, fGluR1 alpha and fGluR1 beta, of Oreochromis sp. DNA Cell Biol. 1996;15:717–725. doi: 10.1089/dna.1996.15.717. [DOI] [PubMed] [Google Scholar]
- 82.Higuchi M, Maas S, Single FN, Hartner J, Rozov A, et al. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 83.Hartner JC, Schmittwolf C, Kispert A, Muller AM, Higuchi M, Seeburg PH. Liver disintegration in the mouse embryo caused by deficiency in the RNA-editing enzyme ADAR1. J. Biol. Chem. 2004;279:4894–4902. doi: 10.1074/jbc.M311347200. [DOI] [PubMed] [Google Scholar]
- 84.Hartner JC, Walkley CR, Lu J, Orkin SH. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat. Immunol. 2009;10:109–115. doi: 10.1038/ni.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh M, Kesterson RA, Jacobs MM, Joers JM, Gore JC, Emeson RB. Hyperphagia-mediated obesity in transgenic mice misexpressing the RNA-editing enzyme ADAR2. J. Biol. Chem. 2007;282:22448–22459. doi: 10.1074/jbc.M700265200. [DOI] [PubMed] [Google Scholar]
- 86.Rueter SM, Dawson TR, Emeson RB. Regulation of alternative splicing by RNA editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- 87.Feng Y, Sansam CL, Singh M, Emeson RB. Altered RNA editing in mice lacking ADAR2 autoregulation. Mol. Cell. Biol. 2006;26:480–488. doi: 10.1128/MCB.26.2.480-488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palladino MJ, Keegan LP, O’Connell MA, Reenan RA. dADAR, a Drosophila double-stranded RNA-specific adenosine deaminase is highly developmentally regulated and is itself a target for RNA editing. RNA. 2000;6:1004–1018. doi: 10.1017/s1355838200000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, et al. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- 90.Kawahara Y, Grimberg A, Teegarden S, Mombereau C, Liu S, et al. Dysregulated editing of serotonin 2C receptor mRNAs results in energy dissipation and loss of fat mass. J. Neurosci. 2008;28:12834–12844. doi: 10.1523/JNEUROSCI.3896-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maas S, Kawahara Y, Tamburro KM, Nishikura K. A-to-I RNA editing and human disease. RNA Biol. 2006;3:1–9. doi: 10.4161/rna.3.1.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schmauss C. Regulation of serotonin 2C receptor pre-mRNA editing by serotonin. Int. Rev. Neurobiol. 2005;63:83–100. doi: 10.1016/S0074-7742(05)63004-8. [DOI] [PubMed] [Google Scholar]
- 93.Miyamura Y, Suzuki T, Kono M, Inagaki K, Ito S, et al. Mutations of the RNA-specific adenosine deaminase gene (DSRAD) are involved in dyschromatosis symmetrica hereditaria. Am. J. Hum. Genet. 2003;73:693–699. doi: 10.1086/378209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawahara Y, Ito K, Sun H, Aizawa H, Kanazawa I, Kwak S. Glutamate receptors: RNA editing and death of motor neurons. Nature. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- 95.Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- 96.Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, et al. RNA editing of the human serotonin 5-HT2C receptor: alterations in suicide and implications for serotonergic pharma-cotherapy. Neuropsychopharmacology. 2001;24:478–491. doi: 10.1016/S0893-133X(00)00223-2. [DOI] [PubMed] [Google Scholar]
- 97.Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada N, Hagimura N, et al. Blockage of Ca2+-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat. Med. 2002;8:971–978. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- 98.Maas S, Patt S, Schrey M, Rich A. Underediting of glutamate receptor GluR-B mRNA in malignant gliomas. Proc. Natl. Acad. Sci. USA. 2001;98:14687–14692. doi: 10.1073/pnas.251531398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cenci C, Barzotti R, Galeano F, Corbelli S, Rota R, et al. Down-regulation of RNA editing in pediatric astrocytomas: ADAR2 editing activity inhibits cell migration and proliferation. J. Biol. Chem. 2008;283:7251–7260. doi: 10.1074/jbc.M708316200. [DOI] [PubMed] [Google Scholar]
- 100.Paz N, Levanon EY, Amariglio N, Heimberger AB, Ram Z, et al. Altered adenosine-to-inosine RNA editing in human cancer. Genome Res. 2007;17:1586–1595. doi: 10.1101/gr.6493107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paul MS, Bass BL. Inosine exists in mRNA at tissue-specific levels and is most abundant in brain mRNA. EMBO J. 1998;17:1120–1127. doi: 10.1093/emboj/17.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2004;2:e391. doi: 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blow M, Futreal PA, Wooster R, Stratton MR. A survey of RNA editing in human brain. Genome Res. 2004;14:2379–2387. doi: 10.1101/gr.2951204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim DDY, Kim TTY, Walsh T, Kobayashi Y, Matise TC, et al. Widespread RNA editing of embedded Alu elements in the human transcriptome. Genome Res. 2004;14:1719–1725. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levanon EY, Eisenberg E, Yelin R, Nemzer S, Hallegger M, et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat. Biotechnol. 2004;22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- 106.Eisenberg E, Nemzer S, Kinar Y, Sorek R, Rechavi G, Levanon EY. Is abundant A-to-I RNA editing primate-specific? Trends Genet. 2005;21:77–81. doi: 10.1016/j.tig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 107.Neeman Y, Levanon EY, Jantsch MF, Eisenberg E. RNA editing level in the mouse is determined by the genomic repeat repertoire. RNA. 2006;12:1802–1809. doi: 10.1261/rna.165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clutterbuck DR, Leroy A, O’Connell MA, Semple CA. A bioinformatic screen for novel A–I RNA editing sites reveals recoding editing in BC10. Bioinformatics. 2005;21:2590–2595. doi: 10.1093/bioinformatics/bti411. [DOI] [PubMed] [Google Scholar]
- 109.Levanon EY, Hallegger M, Kinar Y, Shemesh R, Djinovic-Carugo K, et al. Evolutionarily conserved human targets of adenosine to inosine RNA editing. Nucleic Acids Res. 2005;33:1162–1168. doi: 10.1093/nar/gki239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stapleton M, Carlson JW, Celniker SE. RNA editing in Drosophila melanogaster: new targets and functional consequences. RNA. 2006;12:1922–1932. doi: 10.1261/rna.254306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gommans WM, Tatalias NE, Sie CP, Dupuis D, Vendetti N, et al. Screening of human SNP database identifies recoding sites of A-to-I RNA editing. RNA. 2008;14:2074–2085. doi: 10.1261/rna.816908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eisenberg E, Adamsky K, Cohen L, Amariglio N, Hirshberg A, et al. Identification of RNA editing sites in the SNP database. Nucleic Acids Res. 2005;33:4612–4617. doi: 10.1093/nar/gki771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li JB, Levanon EY, Yoon JK, Aach J, Xie B, et al. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science. 2009;324:1210–1213. doi: 10.1126/science.1170995. [DOI] [PubMed] [Google Scholar]
- 114.Valente L, Nishikura K. ADAR gene family and A-to-I RNA editing: diverse roles in posttran-scriptional gene regulation. Prog. Nucleic Acid Res. Mol. Biol. 2005;79:299–338. doi: 10.1016/S0079-6603(04)79006-6. [DOI] [PubMed] [Google Scholar]
- 115.Lev-Maor G, Sorek R, Levanon EY, Paz N, Eisenberg E, Ast G. RNA-editing-mediated exon evolution. Genome Biol. 2007;8:R29. doi: 10.1186/gb-2007-8-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moller-Krull M, Zemann A, Roos C, Brosius J, Schmitz J. Beyond DNA: RNA editing and steps toward Alu exonization in primates. J. Mol. Biol. 2008;382:601–609. doi: 10.1016/j.jmb.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Z, Carmichael GG. The fate of dsRNA in the nucleus: a p54nrb-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell. 2001;106:465–475. doi: 10.1016/s0092-8674(01)00466-4. [DOI] [PubMed] [Google Scholar]
- 118.Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, et al. Regulating gene expression through RNA nuclear retention. Cell. 2005;123:249–263. doi: 10.1016/j.cell.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 119.Chen LL, DeCerbo JN, Carmichael GG. Alu element-mediated gene silencing. EMBO J. 2008;27:1694–1705. doi: 10.1038/emboj.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hundley HA, Krauchuk AA, Bass BL. C. elegans and H. sapiens mRNAs with edited 3′ UTRs are present on polysomes. RNA. 2008;14:2050–2060. doi: 10.1261/rna.1165008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, et al. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 124.Okamura K, Chung WJ, Ruby JG, Guo H, Bartel DP, Lai EC. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature. 2008;453:803–806. doi: 10.1038/nature07015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kawamura Y, Saito K, Kin T, Ono Y, Asai K, et al. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature. 2008;453:793–797. doi: 10.1038/nature06938. [DOI] [PubMed] [Google Scholar]
- 126.Scadden AD, Smith CW. RNAi is antagonized by A→ I hyper-editing. EMBO Rep. 2001;2:1107–1111. doi: 10.1093/embo-reports/kve244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bass BL. Double-stranded RNA as a template for gene silencing. Cell. 2000;101:235–238. doi: 10.1016/s0092-8674(02)71133-1. [DOI] [PubMed] [Google Scholar]
- 128.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of Mrna at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 129.Scadden AD. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA and promotes its cleavage. Nat. Struct. Mol. Biol. 2005;12:489–496. doi: 10.1038/nsmb936. [DOI] [PubMed] [Google Scholar]
- 130.Hong J, Qian Z, Shen S, Min T, Tan C, et al. High doses of siRNAs induce eri-1 and adar-1 gene expression and reduce the efficiency of RNA interference in the mouse. Biochem. J. 2005;390:675–679. doi: 10.1042/BJ20050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tonkin LA, Bass BL. Mutations in RNAi rescue aberrant chemotaxis of ADAR mutants. Science. 2003;302:1725. doi: 10.1126/science.1091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Knight SW, Bass BL. The role of RNA editing by ADARs in RNAi. Mol. Cell. 2002;10:809–817. doi: 10.1016/s1097-2765(02)00649-4. [DOI] [PubMed] [Google Scholar]
- 133.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 134.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 135.Blow MJ, Grocock RJ, van Dongen S, Enright AJ, Dicks E, et al. RNA editing of human microRNAs. Genome Biol. 2006;7:R27.1–R27.8. doi: 10.1186/gb-2006-7-4-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Luciano DJ, Mirsky H, Vendetti NJ, Maas S. RNA editing of a miRNA precursor. RNA. 2004;10:1174–1177. doi: 10.1261/rna.7350304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, et al. Identification of microRNAs of the herpesvirus family. Nat. Methods. 2005;2:269–276. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- 138.Yang W, Chendrimada TP, Wang Q, Higuchi M, Seeburg PH, et al. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat. Struct. Mol. Biol. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kawahara Y, Zinshteyn B, Chendrimada TP, Shiekhattar R, Nishikura K. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO Rep. 2007;8:763–769. doi: 10.1038/sj.embor.7401011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kawahara Y, Zinshteyn B, Sethupathy P, Iizasa H, Hatzigeorgiou AG, Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137–1140. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kawahara Y, Megraw M, Kreider E, Iizasa H, Valente L, et al. Frequency and fate of microRNA editing in human brain. Nucleic Acids Res. 2008;36:5270–5280. doi: 10.1093/nar/gkn479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–4652. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 143.Liang H, Landweber LF. Hypothesis: RNA editing of microRNA target sites in humans? RNA. 2007;13:463–467. doi: 10.1261/rna.296407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scadden AD. Inosine-containing dsRNA binds a stress-granule-like complex and downregulates gene expression in trans. Mol. Cell. 2007;28:491–500. doi: 10.1016/j.molcel.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Raitskin O, Cho DS, Sperling J, Nishikura K, Sperling R. RNA editing activity is associated with splicing factors in lnRNP particles: the nuclear pre-mRNA processing machinery. Proc. Natl. Acad. Sci. USA. 2001;98:6571–6576. doi: 10.1073/pnas.111153798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ryman K, Fong N, Bratt E, Bentley DL, Ohman M. The C-terminal domain of RNA Pol II helps ensure that editing precedes splicing of the GluR-B transcript. RNA. 2007;13:1071–1078. doi: 10.1261/rna.404407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Agranat L, Raitskin O, Sperling J, Sperling R. The editing enzyme ADAR1 and the mRNA surveillance protein hUpf1 interact in the cell nucleus. Proc. Natl. Acad. Sci. USA. 2008;105:5028–5033. doi: 10.1073/pnas.0710576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Q, Zhang Z, Blackwell K, Carmichael GG. Vigilins bind to promiscuously A-to-I-edited RNAs and are involved in the formation of heterochromatin. Curr. Biol. 2005;15:384–391. doi: 10.1016/j.cub.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 149.Zhou J, Wang Q, Chen LL, Carmichael GG. On the mechanism of induction of heterochromatin by the RNA-binding protein vigilin. RNA. 2008;14:1773–1781. doi: 10.1261/rna.1036308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taylor DR, Puig M, Darnell ME, Mihalik K, Feinstone SM. New antiviral pathway that mediates hepatitis C virus replicon interferon sensitivity through ADAR1. J. Virol. 2005;79:6291–6298. doi: 10.1128/JVI.79.10.6291-6298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang JH, Luo X, Nie Y, Su Y, Zhao Q, et al. Widespread inosine-containing mRNA in lymphocytes regulated by ADAR1 in response to inflammation. Immunology. 2003;109:15–23. doi: 10.1046/j.1365-2567.2003.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nie Y, Ding L, Kao PN, Braun R, Yang JH. ADAR1 interacts with NF90 through double-stranded RNA and regulates NF90-mediated gene expression independently of RNA editing. Mol. Cell. Biol. 2005;25:6956–6963. doi: 10.1128/MCB.25.16.6956-6963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Z, Choi MK, Ban T, Yanai H, Negishi H, et al. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc. Natl. Acad. Sci. USA. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Faulkner GJ, Kimura Y, Daub CO, Wani S, Plessy C, et al. The regulated retrotransposon tran-scriptome of mammalian cells. Nat. Genet. 2009;41:563–571. doi: 10.1038/ng.368. [DOI] [PubMed] [Google Scholar]
- 155.Desterro JM, Keegan LP, Jaffray E, Hay RT, O’Connell MA, Carmo-Fonseca M. SUMO-1 modification alters ADAR1 editing activity. Mol. Biol. Cell. 2005;16:5115–5126. doi: 10.1091/mbc.E05-06-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]