Abstract
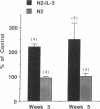
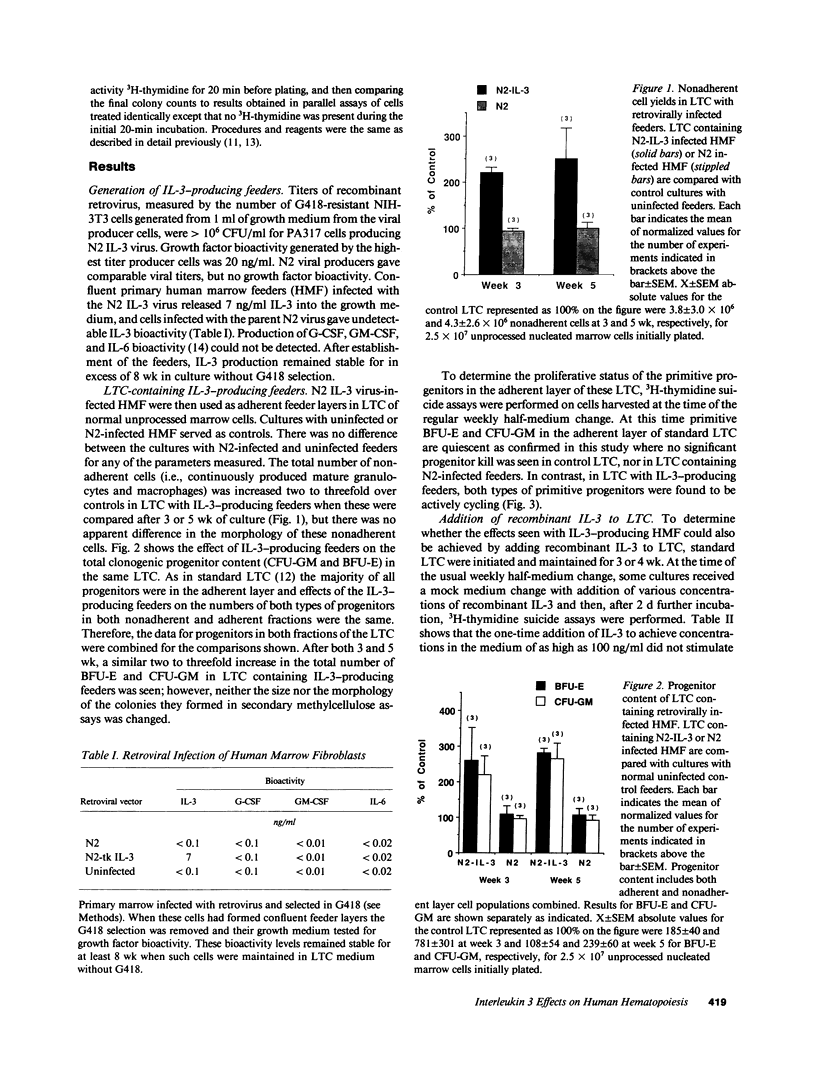
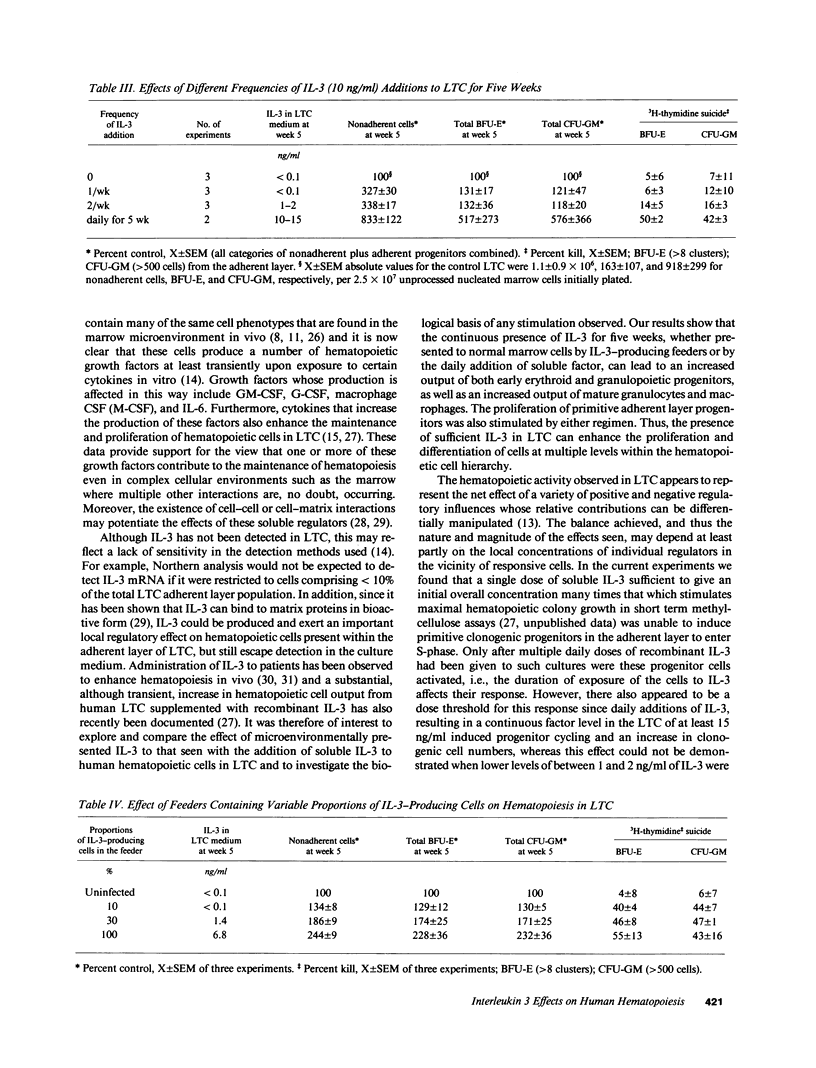
The effect of IL-3 on hematopoiesis in long-term culture (LTC) was studied by cocultivating normal human marrow cells with human marrow fibroblast feeders engineered to constitutively produce IL-3 and by adding soluble IL-3 to LTC according to a variety of dose-time schedules. Feeders stably producing 7 ng/ml IL-3, or LTC to which 10 ng/ml IL-3 was added daily for 5 wk, but not once or twice weekly for the same time period, increased the output of mature nonadherent cells and progenitors from LTC as compared to control cultures. At the time of the weekly half-medium change, when primitive clonogenic progenitors in the adherent layer of standard LTC are quiescent, such cells were actively cycling in cultures containing a continuous source of an adequate dose of IL-3. In LTC, where the proportion of IL-3-producing cells in the feeder layer was diluted to 10% and no IL-3 was detectable in culture medium, primitive adherent layer progenitors were, nevertheless, maintained as a population of continuously proliferating cells. Thus, the presence of IL-3 in LTC can enhance the proliferation and differentiation of very early human hematopoietic cells, but the concentration, duration of exposure, and method of IL-3 presentation are important determinants of the ultimate effects observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avanzi G. C., Lista P., Giovinazzo B., Miniero R., Saglio G., Benetton G., Coda R., Cattoretti G., Pegoraro L. Selective growth response to IL-3 of a human leukaemic cell line with megakaryoblastic features. Br J Haematol. 1988 Jul;69(3):359–366. doi: 10.1111/j.1365-2141.1988.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Bernstein S. E. Tissue transplantation as an analytic and therapeutic tool in hereditary anemias. Am J Surg. 1970 Apr;119(4):448–451. doi: 10.1016/0002-9610(70)90148-0. [DOI] [PubMed] [Google Scholar]
- Cashman J. D., Eaves A. C., Raines E. W., Ross R., Eaves C. J. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. I. Stimulatory role of a variety of mesenchymal cell activators and inhibitory role of TGF-beta. Blood. 1990 Jan 1;75(1):96–101. [PubMed] [Google Scholar]
- Cashman J., Eaves A. C., Eaves C. J. Regulated proliferation of primitive hematopoietic progenitor cells in long-term human marrow cultures. Blood. 1985 Oct;66(4):1002–1005. [PubMed] [Google Scholar]
- Chan S. H., Metcalf D. Local and systemic control of granuloctic and macrophage progenitor cell regeneration after irradiation. Cell Tissue Kinet. 1973 Mar;6(2):185–197. doi: 10.1111/j.1365-2184.1973.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Coutinho L. H., Will A., Radford J., Schiró R., Testa N. G., Dexter T. M. Effects of recombinant human granulocyte colony-stimulating factor (CSF), human granulocyte macrophage-CSF, and gibbon interleukin-3 on hematopoiesis in human long-term bone marrow culture. Blood. 1990 Jun 1;75(11):2118–2129. [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E., Toksoz D., Lajtha L. G. The role of cells and their products in the regulation of in vitro stem cell proliferation and granulocyte development. J Supramol Struct. 1980;13(4):513–524. doi: 10.1002/jss.400130410. [DOI] [PubMed] [Google Scholar]
- Eaves A. C., Cashman J. D., Gaboury L. A., Eaves C. J. Clinical significance of long-term cultures of myeloid blood cells. Crit Rev Oncol Hematol. 1987;7(2):125–138. doi: 10.1016/s1040-8428(87)80022-7. [DOI] [PubMed] [Google Scholar]
- Eaves A. C., Cashman J. D., Gaboury L. A., Kalousek D. K., Eaves C. J. Unregulated proliferation of primitive chronic myeloid leukemia progenitors in the presence of normal marrow adherent cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5306–5310. doi: 10.1073/pnas.83.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Eliason J. F., Thorens B., Kindler V., Vassalli P. The roles of granulocyte-macrophage colony-stimulating factor and interleukin 3 in stromal cell-mediated hemopoiesis in vivo. Exp Hematol. 1988 May;16(4):307–312. [PubMed] [Google Scholar]
- Ganser A., Lindemann A., Seipelt G., Ottmann O. G., Eder M., Falk S., Herrmann F., Kaltwasser J. P., Meusers P., Klausmann M. Effects of recombinant human interleukin-3 in aplastic anemia. Blood. 1990 Oct 1;76(7):1287–1292. [PubMed] [Google Scholar]
- Gidali J., Lajtha L. G. Regulation of haemopoietic stem cell turnover in partially irradiated mice. Cell Tissue Kinet. 1972 Mar;5(2):147–157. doi: 10.1111/j.1365-2184.1972.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Hogge D. E., Cashman J. D., Humphries R. K., Eaves C. J. Differential and synergistic effects of human granulocyte-macrophage colony-stimulating factor and human granulocyte colony-stimulating factor on hematopoiesis in human long-term marrow cultures. Blood. 1991 Feb 1;77(3):493–499. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Kannourakis G., Johnson G. R. Proliferative properties of unfractionated, purified, and single cell human progenitor populations stimulated by recombinant human interleukin-3. Blood. 1990 Jan 15;75(2):370–377. [PubMed] [Google Scholar]
- Kay R., Dougherty G. J., Humphries R. K. Heterogeneous modifications of the 114/A10 protein of interleukin-3-dependent cells are concentrated in a highly repetitive amino-terminal domain. J Biol Chem. 1990 Mar 25;265(9):4962–4968. [PubMed] [Google Scholar]
- Lansdorp P. M., Aarden L. A., Calafat J., Zeiljemaker W. P. A growth-factor dependent B-cell hybridoma. Curr Top Microbiol Immunol. 1986;132:105–113. doi: 10.1007/978-3-642-71562-4_14. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottmann O. G., Ganser A., Seipelt G., Eder M., Schulz G., Hoelzer D. Effects of recombinant human interleukin-3 on human hematopoietic progenitor and precursor cells in vivo. Blood. 1990 Oct 15;76(8):1494–1502. [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Santoli D., Yang Y. C., Clark S. C., Kreider B. L., Caracciolo D., Rovera G. Synergistic and antagonistic effects of recombinant human interleukin (IL) 3, IL-1 alpha, granulocyte and macrophage colony-stimulating factors (G-CSF and M-CSF) on the growth of GM-CSF-dependent leukemic cell lines. J Immunol. 1987 Nov 15;139(10):3348–3354. [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Eaves A. C., Dragowska W., Lansdorp P. M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989 Oct;74(5):1563–1570. [PubMed] [Google Scholar]
- Sutherland H. J., Lansdorp P. M., Henkelman D. H., Eaves A. C., Eaves C. J. Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc Natl Acad Sci U S A. 1990 May;87(9):3584–3588. doi: 10.1073/pnas.87.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein Y., Ihle J. N., Lavu S., Reddy E. P. Truncation of the c-myb gene by a retroviral integration in an interleukin 3-dependent myeloid leukemia cell line. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5010–5014. doi: 10.1073/pnas.83.14.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]