Abstract
Cellular interactions in lymphoid organs initiate the immune response and determine its outcome. Using two-photon microscopy in the lymph node, several groups have begun to investigate the motility characteristics and interactions among T lymphocytes, B lymphocytes, and dendritic cells (DC) in lymphoid organs. In the first “close encounter”, T cells of a particular antigen specificity interact with antigen-bearing dendritic cells and begin to activate. Activation of both CD4+ and CD8+ T cells evolves through several stages; from transient interactions to stable clusters and later to dissociation and proliferation of T cells (clonal expansion). The second “close encounter” requires that antigen-engaged B cells become accessible to T cells by directed migration to the edge of the follicle. T cells and B cells then pair up and waltz together for an extended period, while helper T cells provide signals for B cells to differentiate into plasma cells. In this topical review, we compare the activation choreography of CD4+ T cells interacting first with dendritic cells, and then with B cells, during initiation of the humoral immune response.
Keywords: Two-photon microscopy, T lymphocyte, Dendritic cell, B lymphocyte, Motility, Lymph node
1. Introduction
To mount an effective immune response, lymphocytes must home to lymphoid organs; recognize specific antigens presented by antigen-presenting cells (APC); proliferate (enabling clonal expansion in response to a specific antigen); differentiate (to anergic, apoptotic, or effector cells); exit the organ; and finally migrate to tissues to mediate a variety of effector functions. Effector responses depend upon secretion of lymphokines, chemotactic agents, perforins, or antibodies, depending upon the lymphoid subset. All these processes involve cell recognition dynamics and intracellular Ca2+ signaling that take place hidden from view within lymphoid organs.
A key component in the normal course of an immune response to an infectious agent or immunization is the formation of an ‘immunological synapse’; a structure enabling signaling between different immune cells. The concept of the immunological synapse encompasses a wide variety of immune cell interactions that trigger intracellular signaling by promoting local clustering of receptors, adapter molecules, cytoskeletal elements, and kinases in the region of contact [1-3]. CD4+ helper T cells initially form immunological synapses with dendritic cells (DC) and subsequently with B cells that have encountered antigen. These are the first and second ‘close encounters’ that take place within the lymph node and lead to a humoral immune response.
Fig. 1 illustrates some of the main events that take place within the lymph node during an immune response. Our understanding of these events derives largely from histological studies of fixed tissues, which provide only a ‘snapshot’ view and cannot convey the single-cell dynamics that are clearly essential for functional responses. However, the recent application of two-photon microscopy for immunoimaging [4,5] has allowed visualization within intact lymphoid organs of the dynamic aspects of many processes that initiate an adaptive immune response. Each process involves cell motility and cell-cell recognition events that we are now beginning to elucidate. In this review, we focus on two distinct types of cell recognition events that lead to a humoral immune response: T cell-DC interactions that initiate an antigen response; and T cell-B cell interactions that lead to secretion of antibodies.
Fig. 1.
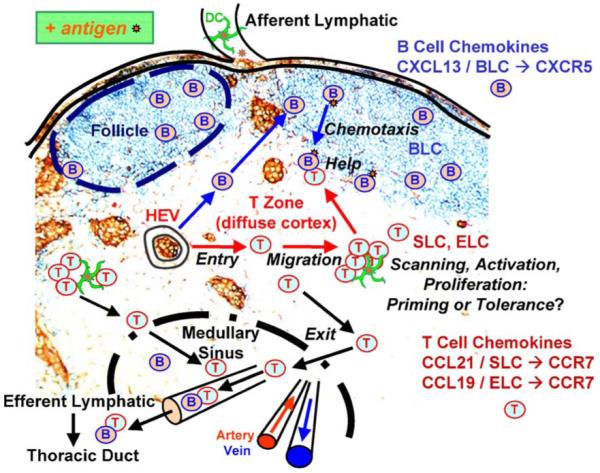
Cell migration and interactions within the lymph node. T-cell homing from high endothelial venules (HEV) constitutes the entry step of lymphocytes moving into the lymph node. The migration of rapidly moving T cells and slower B cells within the diffuse cortex and follicles, respectively, consists of an amoeboid random walk. Antigen scanning is accomplished by random encounters between motile näive T cells and actively probing dendritic cells (DC). The activation choreography of T cells and antigen-bearing dendritic cells evolves in stages during a specific antigen response for both CD4+ and CD8+ T cells. T-cell clusters form differently during immune response priming or tolerance. The proliferation of activated antigen-specific T cells takes place after the first day in a priming response. Antigen-engaged B cells migrate to the follicle edge by chemotaxis and then encounter CD4+ T cells that provide help for antibody production at the start of the humoral immune response.
2. Functional immunoimaging
2.1. Two-photon microscopy
Two-photon microscopy offers a nearly ideal technique for studying live cell behaviors within their native environments. In contrast to other optical imaging technologies (such as epifluorescence and confocal microscopy), two-photon imaging has unique advantages that enable imaging at much greater depths within biological tissue while causing minimal photodamage. Moreover, it provides a spatial and temporal resolution more than adequate to monitor the motility and interactions of single living cells deep within intact organs and even inside the living animal. An individual two-photon image provides an optical ‘section’ into tissue with a thickness typically <1 μm, and by acquiring a sequence of images at differing focal depths (z-stack) it is possible to reconstruct a three-dimensional volume within a lymph node or other tissue. Off-line measurement using multi-dimensional image analysis software then allows quantitative tracking of cells to derive velocities and motility coefficients, as well as measurements of cell-cell contact durations and surface areas.
To visualize different immune cell types, most imaging experiments have utilized adoptive transfer of cells labeled with fluorescent cell tracker dyes into the tail vein of a mouse. By adjusting the number of injected cells and the time of imaging following homing, an appropriate density of labeled cells can later be imaged either in organ explant preparations or by intravital microscopy [4]. Alternatively, cells can be visualized by making them express fluorescent proteins [6] although relatively high expression levels of GFP or YFP are required to obtain signals as bright as those readily obtained with fluorescent dyes. DC may also be labeled in vivo [7], simply by including a cell tracker dye (CFSE) in an alum adjuvant mixture injected under the skin along with cytokines to promote DC maturation and trafficking.
A secondary advantage of two-photon excitation is that excitation spectra of many fluorophores are broader than with conventional (one-photon) excitation. Thus, a femtosecond laser tuned to a fixed wavelength can be used to simultaneously excite three (or, in principle, even more) dyes having distinctly different emission peaks (e.g. blue, green, and red). Three photomultiplier detectors then permit simultaneous and independent tracking of different cell types labeled with different dyes—for example, T and B cells together; or control and experimental groups of cells treated with drugs in vitro; or control and transgenic or knockout donor animals; or ratiometric [Ca2+]i images together with a second cell type.
2.2. Applications of immunoimaging
Apart from the sheer pleasure in being able to see living cells and their responses to antigen inside lymphoid organs, and even in the living animal, several important insights have emerged from immunoimaging that were previously unsuspected or merely surmised from ‘snapshot’ immunology of fixed tissue. For example, two-photon microscopy has provided the means to investigate living T cells, B cells, and DC within intact lymphoid tissue, thereby linking results from in vitro and in vivo immunological approaches, and changing the way many immunologists think about how an adaptive immune response is initiated and regulated. Immunoimaging techniques have now been applied to isolated organ and tissue preparations, including lymph node [6-13], thymus [14-17], spleen [18], intestinal tissue [19], skin [20], and in brain and spinal cord slices [21,22]. In addition, for the first time, immune cells have been imaged in living, anesthetized mice, using intravital preparations of lymph nodes [5,23,24].
Two-photon microscopy is not limited to mere visualization, but further permits quantitative analysis and testing of specific mechanisms. Moreover, there is no doubt that the largely phenomenological aspect of current immunoimaging studies will soon be enhanced by pharmacological approaches, and by [Ca2+]i imaging in combination with knockout and transgenic mice to gain further mechanistic insights at a molecular level. As a specific example, thymocytes in thymic slices undergo [Ca2+]i oscillations in response to antigen encounter [15]. The Ca2+ signal stops thymocytes in their tracks and promotes prolonged interactions with stromal cells that are important for positive selection and differentiation to become mature T cells. Such [Ca2+]i oscillations may result from a stable T cell-APC interaction and immunological synapse or from a T cell-APC union whose stability is in dynamic competition with the mechanical forces of its integrin-rich cellular environment. To characterize early antigen presentation and associated activation events, it will be important to image [Ca2+]i within the lymph node in conjunction with genetic deletion, reporter gene expression, and specific pharmacological agents as independent experimental approaches to elucidate signaling pathways in the natural tissue environment.
We have barely scratched the surface of what has now become possible through immunoimaging. The field is now poised to use functional imaging to investigate the molecular events that underlie lymphocyte homing, motility, and antigen recognition in the living animal.
3. Initiation of the immune response by direct T cell-DC contact: close encounters of the first kind
The initiation of an immune response first requires that antigen-presenting dendritic cells (DCs) physically contact antigen-specific T cells within the complex environment of the lymph node. Although DCs are remarkably efficient in evoking T-cell responses with few antigen-MHC complexes (1-100 per DC) [25-27], they must first encounter a T cell with appropriate antigen specificity (one in 105-106). This presents a ‘needle in a haystack’ problem, in that DCs must rapidly scan a large portion of the T-cell repertoire to establish rare cognate interactions. The mechanisms by which this is accomplished are only now beginning to be understood. Chemotaxis has been proposed to guide T cells toward DCs, thereby increasing the likelihood of productive interactions [28-30]. However, recent observations by live-cell imaging indicate that naïve T cells exhibit random migration in vivo, suggesting that antigen recognition may arise, instead, through a stochastic mechanism [31,32].
Once T cells encounter APC, questions then arise as to the nature of the cell-cell interactions that lead to T-cell activation. Two competing models have been proposed [33]. In the stable interaction model based on studies utilizing 2-D surfaces in liquid culture systems and in lipid bilayers, one or more naïve T cells remain in sustained close contact with a single antigen-presenting cell (APC) to form an immunological synapse [34,35], and become activated over the course of hours [36-39]. Synapse formation is initiated by interaction of adhesion molecules LFA-1 on T cells with ICAM-1 on APC upon contact, thus encouraging sampling between the cells. In the presence of cognate antigen, these complexes move to the periphery of the contact area to form the pSMAC (peripheral supramolecular activation cluster) while MHC-peptide and TCR migrate together with other molecules to form the cSMAC and complete the mature synapse [34,40]. In contrast, the serial encounter model downplays the importance of a stable immunological synapse for cells in a more physiologically relevant three-dimensional fibrillar environment; contending that stable synapses may arise only in an environment lacking in competing integrin interactions [3,41]. Instead, short-lived, transient exchanges between T cells and dendritic cells (DCs) are proposed to enhance antigen sampling, and integrate signaling events generated by serial productive encounters until a threshold for commitment to activation is reached. In support of this hypothesis, transient rises in [Ca2+]i were observed during sequential encounters with one or more APC in 3-D collagen gels, and such short-lived serial interactions were sufficient to cause T-cell activation [41].
The real test of such models must come from imaging the motility and interactions of lymphocytes and APC under physiological conditions within their native environment. As described below, recent studies employing two-photon microscopy to visualize deep inside intact lymphoid organs are now beginning to cast light on these processes.
3.1. Lymphocyte motility in the excised lymph node
Miller et al. [8] described the first application of two-photon laser microscopy to image the dynamic behavior of individual living lymphocytes within an intact lymphoid organ. T and B cells in excised lymph nodes were observed to move rapidly and in an amoeboid manner in their native environment. The cells crawl over each other and contact fibrous elements in the lymph node, pausing every few minutes before continuing their journey. When actively moving, T cells are elongated and move with velocities that exceed 20 μm/min, averaging 10-12 μm/min. For a cell that has a diameter of 7 μm when rounded up, the motion of T cells is very rapid indeed.
By comparison, B cells within the follicle move more slowly, averaging 6 μm/min. Tracking the cells in three spatial dimensions revealed that both T and B cells meander in a random walk through, respectively, the diffuse cortex and in follicles, without indication of directed migration. The net displacement from any given position is proportional to the square root of time—analogous to Brownian motion and diffusion—enabling a “motility coefficient” to be defined.
3.2. Intravital imaging of lymphocyte motility: in vivo veritas
The study by Miller et al. [8] described results obtained in explanted lymph nodes that, despite being warmed to 35 °C and superfused with medium bubbled with 95% O2/5% CO2, were necessarily deprived of their normal blood and lymphatic circulations. What is truly important is what happens in a living animal. Are lymphocytes highly motile in vivo, or are they not? To address this question, the authors went on to image the locomotion and trafficking of näive CD4+ T cells in the inguinal lymph nodes of anesthetized mice [5]. That study provided the first look at single lymphocytes in a living animal. Intravital imaging deep within the inguinal lymph node revealed T cells flowing rapidly in the microvasculature, and captured individual homing events. After extravasation into the diffuse cortex, T cells display robust motility with an average velocity of 11 μm/min and cycle between states of low and high motility roughly every 2 min, achieving peak velocities >25 μm/min (Fig. 2A and B)—values remarkably close to those obtained in lymph node explants. In particular, T-cell migration again revealed a default trafficking program analogous to a random walk (Fig. 2C). Thus, näive T cells within the cortical T-cell zone do not migrate collectively (Fig. 2D), as they might under the direction of pervasive chemokine gradients, and their mean displacement from an initial starting point increases as a square-root function of time (Fig. 2E). Subsequent intravital imaging studies confirmed the robust basal motility of T cells in vivo [23,24].
Fig. 2.
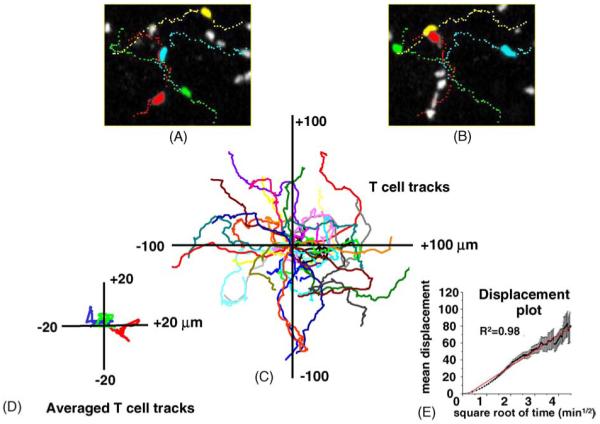
Random walk motility of T cells within the cortical T-cell zone of an inguinal lymph node in vivo. (A and B) Panels show ‘snapshots’ of fluorescently labeled CD4+ T cells captured 5 min after one another. Each image represents a z-axis compression (top view). Four selected T cells are shown in different colors, and the dotted lines trace out their tracks. (C) Superimposed tracks of several T cells within the same imaging field (different colors) after normalizing their starting positions. The tracks follow random walk patterns, without any preferred direction of motion. (D) T-cell populations show no bulk motion. Traces show mean coordinates of three populations of T cells. (E) Plot shows the mean absolute displacement of individual T cells away from their origin as a function of square root of time. A random walk process is expected to yield a straight line on this transformed scale, and the red line shows a linear regression. Data are reproduced with permission [31].
T cells appear to migrate as autonomous agents, each taking an independent, apparently random trafficking path. These findings call into question the role of chemokine gradients for basal T-cell trafficking within T-cell areas, and suggest that antigen detection may result from a stochastic process in which the robustly motile random walk of T cells facilitates contact with antigen-presenting dendritic cells.
3.3. Dendritic cell gesticulations and motility
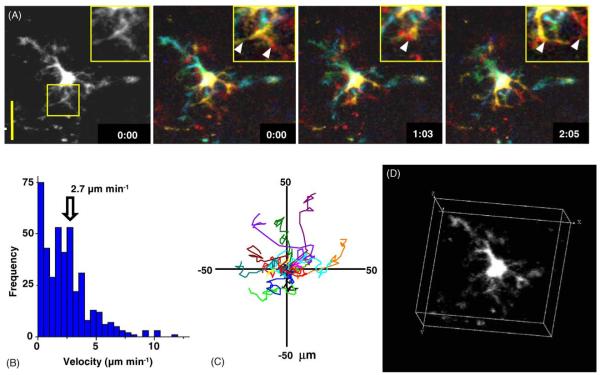
After initial reports describing imaging of T and B cells, methods were developed to fluorescently label dendritic cells for visualization by confocal microscopy [42] or two-photon microscopy [6,7,12,13,23]. DCs initiate the adaptive immune response by capturing antigen in the periphery—for example at a site of immunization, injury, or infection—and then transporting the antigen into lymph nodes and presenting antigenic peptides bound to MHC along with co-stimulatory interactions to specific T cells. Inclusion of a green cell-tracker dye (CFSE) in the adjuvant mix was found to efficiently label DCs that expressed appropriate surface markers, and these DC trafficked into the node where they clustered near high endothelial venules outside B-cell follicles, ideally situated for interaction with newly homed T cells [7]. Viewed within the lymph node, living dendritic cells were observed for the first time to extend and retract long processes (Fig. 3A; Video 1). Thus, although DCs migrate only slowly (mean velocity 2.7 μm/min; Fig. 3B and C), the rapid and extensive ‘gesticulations’ of their fine dendritic processes (Fig. 3D) greatly expand the effective swept volume within which migrating T cells may contact, and thereby likely enhance scanning of the T-cell repertoire.
Fig. 3.
Dendritic cell gesticulations and motility. (A) High-resolution monochrome and color depth-encoded z-projections of a DC, illustrating elaborate dendritic morphology, shown at indicated times (in min:s); note rapid changes in morphology. Insets show enlarged views of the selected region. Scale bar = 25 μm. (B) Histogram of instantaneous DC velocities derived by tracking the center of fluorescence of 15 DCs in the x-y plane. (C) Tracks of 15 DCs in the x-y plane, normalized to their starting positions. (D) Three-dimensional representation of a DC, illustrating complex dendritic morphology. See also Video 1. Data are reproduced with permission from Miller et al. [5].
3.4. Interactions between näive T cells and DC: stochastic repertoire scanning
Simultaneous two-photon visualization of T cells together with DC was enabled by labeling each with differently colored fluorescent cell tracker dyes [7]. T-cell migration with respect to DCs demonstrated that the initial encounter between a näive T cell and a DC is a stochastic event resulting from a random walk by the T cell. Specifically, it appears not to be guided by a simple chemotactic process as there was no evidence for preferential motion of T cells toward DCs. Moreover, DCs are clearly active partners in initiating and terminating contacts with T cells, as they vigorously extend and sweep their dendrites in all directions. Encounters occur preferentially on dendritic processes many microns from the DC body, thereby increasing the surface area of DC membrane available for interaction and minimizing steric hindrance among T cells.
In the absence of specific antigen, these chance encounters result in interactions lasting only about 3 min before T cells resume their autonomous migration pattern and leave DCs free to interact with other T cells (Fig. 4A and F (left panel)). Each DC makes ~20 T-cell contacts per hour individual contacts lasting about 3 min, so that on average a single labeled T cell is in contact with a DC at any given time. Because labeled T cells in those experiments comprised only ~0.4% of the total number of T cells in the node, each DC would be in contact with about 250 T cells at any instant—a number that is likely to be limited simply by the available membrane surface of DCs. A single DC may thus turn over about 5000 T cells per hour (an order of magnitude larger than derived in another study that used CD8+ T cells and in vitro-derived dendritic cells [12]) at a rate limited primarily by contact duration. Assuming that a node contains 100 antigen-bearing DCs, this scanning rate would give a 95% probability of contacting an antigen-specific T cell present at a frequency of 1 in 106 within ~6 h. This admittedly over-simplified calculation assumes an even distribution of T cells that can each be sampled more than once, and verifies that a stochastic scanning mechanism is compatible with the kinetics of immune response initiation in vivo. Recently, resident DC have been observed to localize near sites of T-cell homing at high endothelial venules (HEV) [43]; this would enhance the efficiency of stochastic scanning, since DC would be more likely to encounter newly arrived T cells than resident T cells that may have already been scanned.
Fig. 4.
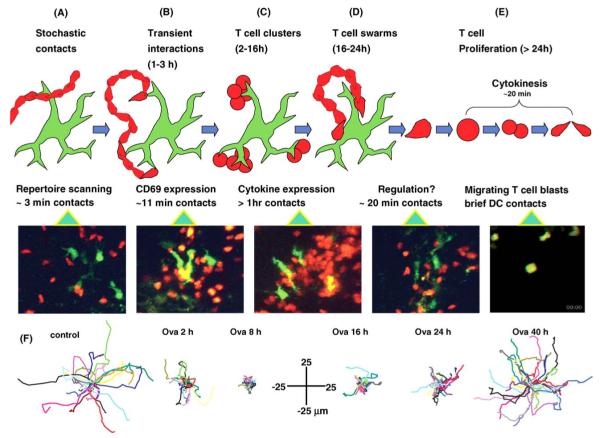
Multiple phases of T-cell behavior during initiation of the immune response. (A-E) Each panel shows a schematic diagram characterizing the nature of the interactions between T cells (red) and a DC (green) during different phases together with a representative two-photon ‘snapshot’. (A) In the absence of specific antigen, T cells follow random paths and make only brief (ca. 3 min duration) contacts with dendrites of DC. (B-E) Cell behaviors at different times after adoptive transfer of T cells in the presence of specific antigen (ovalbumin). (B) After 1-3 h, T cell-DC contacts are still transient, but are longer on average (ca. 11 min) than in the absence of antigen. These contacts are sufficient to result in CD69 upregulation. (C) Long-lived (ca. 1 h) dense clustering of T cells around DC, associated with cytokine expression. See also Video 2. (D) After 16-24 h, T-cell clusters dissociate, but T cells continue to ‘swarm’ around DC, making contacts lasting for ca. 20 min. (E) After about 24 h, T cells begin to resume normal motility, and undergo multiple rounds of cytokinesis. (F) Displacement plots showing representative T-cell tracks (normalized to their starting positions) in the absence of antigen (left), and at different times following adoptive transfer in the presence of ovalbumin. Data are reproduced with permission from Miller et al. [9].
Even though T-cell motions are random, and must incur a substantial energy cost, stochastic encounters allow efficient scanning of the T-cell repertoire. The rate-limiting step, instead, appears to be the turnover of T cells as they sample the DC surface for cognate antigen. Collectively, the behavior of T cells and DCs emerges as an efficient search strategy of stochastic repertoire scanning to identify foreign antigen and to assure that T-cell responses are rapidly initiated during the early stages of infection.
3.5. Kinetic stages of an immune response
Prior to the development of in situ live cell imaging technologies, little was known regarding the cell-cell interactions during initiation of the immune response. In liquid culture systems, T cells and APC make contact and remain together for hours, forming the immunological synapse at the zone of contact [40,44,45]. The initial study of T cell-dendritic cell interactions in lymph node captured confocal images of antigen-pulsed dendritic cells associated with T cells primarily in a one-to-one ratio and showed molecular evidence of synapse formation in situ using live imaging [42]. A more complex picture of the evolution of cell-cell interactions now emerges from two independent two-photon imaging studies of CD4+ T cells [9] and CD8+ T cells [23] in the lymph node. T cells and DCs were found to display an elaborately choreographed sequence of interactions during antigen activation leading to a robust T-cell response. The presence of antigen, brought to the draining lymph node by dendritic cells from the adjuvant injection site under the skin, triggers a sequence of behavioral changes in CD4+ T cells. Instead of immediately forming stable interactions as seen in liquid culture systems, antigen activation evolves in distinct stages (Fig. 4). After homing to the lymph node, CD4+ T cells in the vicinity of antigen-bearing dendritic cells initially make contact intermittently with several partners. As the immune response runs its course, interaction times lengthen and tracks become more confined. In the first 2 h after adoptive transfer, T cells make brief, serial interactions lasting 11-12 min (Fig. 4B). The mode of interaction then switches to stable clusters consisting of about a dozen T cells firmly attached to dendritic cells for 1 h or longer (Fig. 4C; Video 2). Still later during the activation sequence, T cells dissociate from dendritic cells and begin to swarm locally (Fig. 4D). After >24 h of activation, and having already detached from dendritic cells, T cells round up and begin to divide, the daughter cells continuing their rapid migration immediately thereafter. Over the next few days, T cells divide five to eight times and then exit the lymph node to take up residence in distant lymphoid organs as central memory cells.
These observations suggest that the immunological synapse in native tissues is remarkably fluid during the first hours of an immune response, and that stable synapses form only at specific stages of antigen presentation to T cells. Furthermore, the serial nature of these interactions implies that T cells activate by way of multiple antigen recognition events. The initial period of scanning for specific antigen interactions may be optimized by serial interactions with several DCs during the early phase of T-cell activation.
3.6. Tolerance and priming responses
Recognition of antigen may result in either priming or tolerization; that is to say, the respective enhancement or diminution of a subsequent response to antigen, respectively. Three recent studies have imaged antigen-specific T cells in real-time within lymph nodes to determine how their behavior during initial exposure to antigen may influence the decision between oral priming versus oral tolerance. In the first [13], the engagements between CD8+ T cells and DC differed depending upon whether the DC were treated in a tolerogenic regimen or were primed to elicit the immune response. During a priming response, T cells stopped moving and formed stable clusters, while in a tolerizing response they continued to move and failed to form clusters, establishing only brief serial contacts with dendritic cells. The authors concluded that stable DC-T cell interactions occur during the induction of priming, whereas brief contacts may contribute to the induction of T-cell tolerance. In contrast, two subsequent studies reported only subtle differences between priming and tolerizing stimuli within the first day. Zinselmeyer et al. [46] administered oral ovalbumin in the presence or absence of cholera-toxin adjuvant to induce priming or tolerance, respectively, and then imaged mucosal and systemic lymph nodes at early and late time points after feeding antigen. As described in the preceding section, there were marked differences in behaviors between näive CD4+ T cells and those exposed to antigen, but differences between priming and tolerizing responses were less obvious. In both cases, stable clusters formed after several hours. However, during priming T cells formed larger and longer lived clusters within both mucosal and peripheral lymph nodes at later time points (20 h), suggesting that delayed events may regulate the commitment to tolerance or priming. In the third study, Shakhar et al. [24] compared priming and tolerizing responses of CD4+ T cells and could not distinguish any significant differences in T-cell behavior; in both cases T cells arrested in stable contacts with dendritic cells.
Reasons for the discrepancies between these studies remain to be resolved, and may include differences in experimental procedures and immunization regimens and possible differences in the way CD4+ and CD8+ T cells become tolerized. In particular, it will be important to correlate activation signals with cell behavior.
4. T-cell interactions with antigen-engaged B lymphocytes: close encounters of the second kind
A second critical antigen-specific interaction within the lymph node occurs when helper T cells interact with antigen-engaged B cells of the same specificity to provide “help” for these B cells to differentiate into antibody-producing plasma cells. B cells are initially confined to follicles within which they migrate by a random walk. So how do the T and B cells locate each other, and then what happens? A recent study [10] employed two-photon microscopy to determine how B cells migrate to the follicle edge and how antigen-specific T and B cells interact there. The findings provide the first evidence of lymphocyte chemotaxis in vivo, and begin to define the cellular dynamics associated with T-cell-dependent antibody responses.
4.1. Chemotaxis of B cells to the follicle edge
Interactions between B and T cells are essential for antibody responses but the dynamics of these interactions are poorly understood. The imaging studies of Okada et al. [10] revealed that, in the absence of antigen, T and B cells appear to respect the invisible boundary at the follicle edge, with each cell type migrating randomly within its own compartment. However, upon encountering soluble antigen, B cells near the follicle edge move along relatively straight paths, directed toward the follicle edge (Fig. 5; Video 3), where they then continued to migrate more randomly without deviating far from the T-cell zone. Simultaneous imaging of antigen-engaged B cells together with control, unactivated B cells of differing antigen specificity revealed that the antigen-engaged cells are selectively depleted about 20-100 μm from the follicle boundary. This directional migration requires upregulation of the chemokine receptor CCR7 together with the presence of the chemokine CCL21, a ligand for CCR7 that is present with a concentration gradient that tapers from the T zone into the follicle. Levels of CCL21, as detected by immunofluorescence, extend about 100 μm into the follicle, consistent with the region where directed migration of B cells occurs. Chemotaxis has long been suspected to direct the traffic of lymphocytes within secondary lymphoid organs, but this is the first instance where directional migration was observed.
Fig. 5.
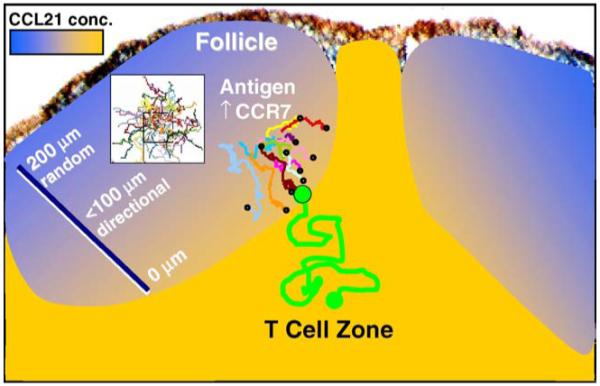
Chemotaxis of antigen-engaged B cells to the follicle edge. The diagram depicts the directional migration of activated B cells to the edge of the follicle. At distances >100 μm from the follicle edge, B cells migrate in a random walk, resulting in trails that disperse in all directions, as depicted by superimposed tracks in the white box. Closer to the edge, B cells with upregulated levels of the chemokine receptor CCR7 detect a CCL21 chemokine gradient and migrate along relatively direct paths to the edge of the follicle (colored tracks ending with circles), where they then can encounter helper T cells depicted by the green track. See also Video 3. B-cell tracks reproduced with permission from Okada et al. [10].
4.2. Motile T-B conjugate pairs
Once the B cells arrive at the follicle edge, they interact with antigen-specific T cells. In distinct contrast to T cell-DC contacts, these T-B conjugate pairs are highly motile, moving with a speed (averaging 9 μm/min) characteristic of the activated B cells [10]. B cells always lead the way in motile conjugate pairs (Fig. 6; Video 4) and drag rounded T cells along as they “waltz” together, sometimes by tethers projecting back from the B cell to the T cell. Such motile conjugates are quite stably associated. Antigen-engaged B cells remained paired with antigen-specific helper T cells for 10 to >60 min, whereas non-antigen-specific interactions lasted <10 min. B cells occasionally contacted more than one T cell, leading to serial monogamy, but T cells remained strictly monogamous. Motility and partner exchange may be vital to optimize T-B cooperation during a mixed antigen response and to colonize fresh territory for formation of germinal centers.
Fig. 6.
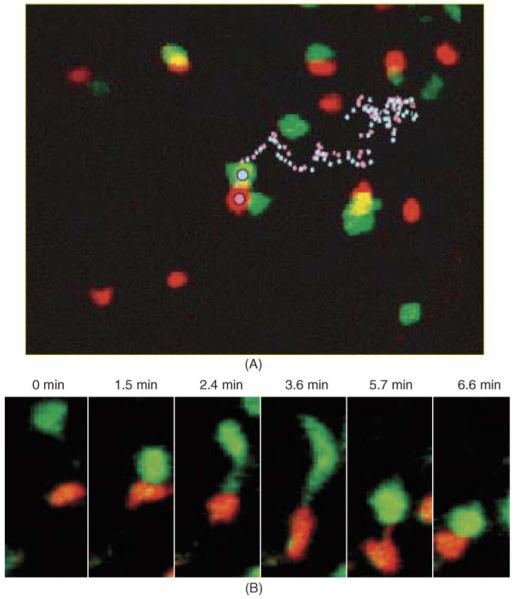
Migration of T-B conjugate pairs. HEL-specific B cells (red) and HEL-specific helper T cells (green) form conjugate pairs that migrate near the follicle edge within the lymph node. (A) B cells and T cells ~30 h after challenge with antigen in adjuvant. The tracks of a B cell (pink dotted line) and a T cell (blue dotted line) remaining bound to each other for >1 h are shown. (B) Formation of a T-B conjugate pair. Time-lapse images show the initial encounter between HEL-specific B and T cells following antigen challenge. T cells sometimes remained attached by a tether to the B cell before rounding up (3.6 min). As the conjugate pair moved, contact was sometimes maintained by a B-cell tether extending from the trailing edge of the B cell to the T cell (5.7 min). See also Video 4. Images reproduced with permission from Okada et al. [10].
5. Comparison of close encounters in the lymph node
We now have the data to compare the two of the crucial cell-cell interactions that initiate an adaptive immune response: the interactions of CD4+ T cells with DC, and with B cells. Interaction with a DC is initiated by a random encounter in the T zone near HEV and proceeds through several distinct phases to generate activated T cells. The cognate interaction between T and B cells is favored by CCR7 upregulation and chemotaxis of antigen-engaged B cells to the follicle edge. Unlike the T cell-DC interaction, this results in formation of conjugate pairs that are highly motile. These cognate T-B interactions provide “help” for B cells to differentiate into antibody-secreting plasma cells and are essential for the humoral immune response (Table 1).
Table 1.
Comparison of T-DC and T-B interactions
| T-DC | T-B | |
|---|---|---|
| Location | T zone near HEV | Follicle edge |
| Initial encounter | Random (stochastic scanning) | B-cell chemotaxis (CCR7 → CCL21) |
| Recognition and activation | Serial contacts → stable clusters → swarming, T-cell proliferation | Stable pairs, partner exchange, serial monogamy |
| Ratio | Variable, can be >10:1 | Usually 1:1 |
| Motility of conjugate | Slow | Fast (9 μm/min), B cells lead |
6. Summary
Motility of lymphocytes in vivo. Within the lymph node, T and B lymphocytes are highly motile in the diffuse cortex and in the follicles, respectively.
T cell-dendritic cell interactions. Dendritic cells (DC) labeled in vivo are slowly motile within the lymph node, but exhibit rapid changes in dendritic morphology. Contacts between T cells and DC are facilitated by robust T-cell motility in combination with rapid extension and retraction of DC dendrites. The initial recognition of antigen appears to arise through random encounters.
Dynamic stages of the immune response. During an immune response, T-cell contacts with DC change progressively from intermittent, serial engagements through formation of stable clusters to swarming and proliferation of T cells.
T cell-B cell interactions. Chemotaxis of antigen-engaged B cells, mediated by upregulation of the chemokine receptor CCR7, allows B cells to detect a gradient of CCL21 and migrate to the follicle edge where they form conjugates with helper T cells. Monogamous T-B conjugates are highly motile under conditions that lead to antibody secretion.
Functional immunoimaging. Two-photon microscopy has already opened a new window onto many cellular events that were previously hidden within the ‘black box’ of intact lymphoid tissue. Moreover, we anticipate that combination of this technique together with fluorescent probes of early activation events and gene expression, knockout animals, and pharmacological approaches will soon permit investigation of molecular signaling events in lymphoid organs.
Supplementary Material
Acknowledgements
We thank members of the Cahalan and Parker laboratories for many discussions over the past 4 years, both for suggested technical improvements and immunological applications. We are particularly pleased to acknowledge the pivotal contributions of Dr. Mark Miller, now at Washington University, to all aspects of this work. We also thank Melanie Matheu for help in preparing figures. We apologize in advance for not providing equal weight to all related studies in the field; we have emphasized our own work for didactic clarity and because of space limitations. The project on T cell-B cell interactions was an equal collaborative effort between our groups and Jason Cyster’s group at UCSF; we thank Taka Okada for synergistic experimentation and exchange of ideas. The study to compare oral tolerance and priming responses was a collaborative effort with Paul Garside’s group at Glasgow University; and we wish to thank Bernd Zinselheimer for performing many of the early experiments done at UCI. This research is supported by NIH grants GM41514 and GM48071.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.smim.2005.09.001.
References
- [1].Trautmann A, Valitutti S. The diversity of immunological synapses. Curr Opin Immunol. 2003;15:249–54. doi: 10.1016/s0952-7915(03)00040-2. [DOI] [PubMed] [Google Scholar]
- [2].Davis DM, Dustin ML. What is the importance of the immunological synapse? Trends Immunol. 2004;25:323–7. doi: 10.1016/j.it.2004.03.007. [DOI] [PubMed] [Google Scholar]
- [3].Friedl P, den Boer AT, Gunzer M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5:532–45. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
- [4].Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002;2:872–80. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci U S A. 2003;100:2604–9. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–50. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- [7].Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci U S A. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–73. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- [9].Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–56. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley S, et al. Antigen-engaged B cells undergo directional migration to the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:1047–61. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zinselmeyer BH, Dempster J, Gurney AM, Wokosin D, Miller MJ, Ho H, et al. In situ characterisation of CD4+ T cell behaviour in lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005:201. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–85. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- [13].Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–42. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- [14].Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–80. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- [15].Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–51. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- [16].Robey EA, Bousso P. Visualizing thymocyte motility using 2-photon microscopy. Immunol Rev. 2003;195:551–7. doi: 10.1034/j.1600-065x.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- [17].Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:1062–9. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wei SH, Miller MJ, Cahalan MD, Parker I. Two-photon imaging in intact lymphoid tissue. Adv Exp Med Biol. 2002;512:203–8. doi: 10.1007/978-1-4615-0757-4_26. [DOI] [PubMed] [Google Scholar]
- [19].Tutsch E, Griesemer D, Schwarz A, Stallmach A, Hoth M. Two-photon analysis of calcium signals in T lymphocytes of intact lamina propria from human intestine. Eur J Immunol. 2004;34:3477–84. doi: 10.1002/eji.200425265. [DOI] [PubMed] [Google Scholar]
- [20].Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, et al. Dynamics and function of Langerhans cells in vivo dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- [21].Nitsch R, Pohl EE, Smorodchenko A, Infante-Duarte C, Aktas O, Zipp F. Direct impact of T cells on neurons revealed by two-photon microscopy in living brain tissue. J Neurosci. 2004;24:2458–64. doi: 10.1523/JNEUROSCI.4703-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–14. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- [24].Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, et al. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–14. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Demotz S, Grey HM, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–30. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- [26].Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–6. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- [27].Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–9. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- [28].Ngo VN, Tang HL, Cyster JG. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med. 1998;188:181–91. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- [30].Mackay CR. Chemokines: immunology’s high impact factors. Nat Immunol. 2001;2:95–101. doi: 10.1038/84298. [DOI] [PubMed] [Google Scholar]
- [31].Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci U S A. 2003;100:2604–9. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cahalan MD, Parker I, Wei SH, Miller MJ. Real-time imaging of lymphocytes in vivo. Curr Opin Immunol. 2003:15. doi: 10.1016/s0952-7915(03)00079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dustin ML, de Fougerolles AR. Reprogramming T cells: the role of extracellular matrix in coordination of T cell activation and migration. Curr Opin Immunol. 2001;13:286–90. doi: 10.1016/s0952-7915(00)00217-x. [DOI] [PubMed] [Google Scholar]
- [34].Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- [35].Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–96. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- [36].McConnell HM, Watts TH, Weis RM, Brian AA. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta. 1986;864:95–106. doi: 10.1016/0304-4157(86)90016-x. [DOI] [PubMed] [Google Scholar]
- [37].Wulfing C, Sjaastad MD, Davis MM. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc Natl Acad Sci U S A. 1998;95:6302–7. doi: 10.1073/pnas.95.11.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:3909–13. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Donnadieu E, Bismuth G, Trautmann A. Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium. Curr Biol. 1994;4:584–95. doi: 10.1016/s0960-9822(00)00130-5. [DOI] [PubMed] [Google Scholar]
- [40].Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- [41].Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–32. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- [42].Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–6. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- [43].Bajenoff M, Granjeaud S, Guerder S. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J Exp Med. 2003;198:715–24. doi: 10.1084/jem.20030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–96. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- [45].Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–30. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- [46].Zinselmeyer BH, Dempster J, Gurney AM, Wokosin D, Miller M, Ho H, et al. In situ characterization of CD4+ T cell behavior in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005;201:1815–23. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.