Summary
Learning through experience underlies the ability to adapt to novel tasks and unfamiliar environments. However, learning must be regulated so that relevant aspects of the environment are selectively encoded. Acetylcholine (ACh) has been suggested to regulate learning by enhancing the responses of sensory cortical neurons to behaviorally-relevant stimuli [1]. In this study, we increased synaptic levels of ACh in the brains of healthy human subjects with the cholinesterase inhibitor donepezil (trade name: Aricept) and measured the effects of this cholinergic enhancement on visual perceptual learning. Each subject completed two five-day courses of training on a motion direction discrimination task [2], once while ingesting 5 mg of donepezil before every training session and once while placebo was administered. We found that cholinergic enhancement augmented perceptual learning for stimuli having the same direction of motion and visual field location used during training. In addition, perceptual learning under donepezil was more selective to the trained direction of motion and visual field location. These results, combined with previous studies demonstrating an increase in neuronal selectivity following cholinergic enhancement [3–5], suggest a possible mechanism by which ACh augments neural plasticity by directing activity to populations of neurons that encode behaviorally-relevant stimulus features.
Results
The neurotransmitter acetylcholine (ACh) has been proposed to regulate neural plasticity by selectively increasing the responses of neurons to behaviorally-relevant stimuli [1]. Cholinergic neurons in the basal forebrain project widely to cortex, where they release more ACh when animals are performing a task requiring sustained attention [6]. In addition, application of ACh to sensory cortex induces persistent modifications of neuronal tuning [7], and pairing of basal forebrain electrical stimulation with presentation of a sensory stimulus causes changes in cortical tuning that are similar to those observed when the animal performs a task on the presented stimulus [8]. In humans, pharmacological reduction of cholinergic transmission has been shown to prevent learning-dependent changes in fMRI responses [9]. Cholinesterase inhibitors such as donepezil (trade name: Aricept) reduce the activity of the enzyme that breaks down ACh in the synaptic cleft, thereby prolonging the effects of endogenously released ACh. Previous results suggest that cholinesterase inhibitors may benefit cognitive functions such as attention and memory [10, 11].
We examined the effects of cholinergic enhancement with donepezil on perceptual learning of a motion direction discrimination task [2]. Perceptual learning is a pervasive and stimulus-specific improvement in performance of a perceptual task with training [12]. The specificity of perceptual learning suggests possible changes in coding in neurons selectively tuned to the characteristics of the training stimuli. Indeed, physiological studies in humans [13] and other primates [14–16] have described changes in coding in visual cortical areas containing neurons exhibiting selectivity for the stimulus characteristics employed during training.
Twelve participants (seven female; mean age: 23 +/− 6; tobacco smokers were excluded from participation; all subjects had normal or corrected-to-normal vision) performed a task in which they reported whether two fields of moving dots, presented sequentially, were moving in the same direction [2] (Figure 1A; see Supplemental Experimental Procedures). The angular difference between the stimuli was adjusted according to a psychophysical staircase, converging on 70% correct performance, and a threshold was estimated from all of the trials in each staircase [17]. Two quadrants of the stimulus, located on opposite sides of the fixation point, contained 100% coherent motion, and the remaining quadrants contained 0% coherent motion (Figure 1B). Stimuli were created using the Psychophysics Toolbox [18, 19].
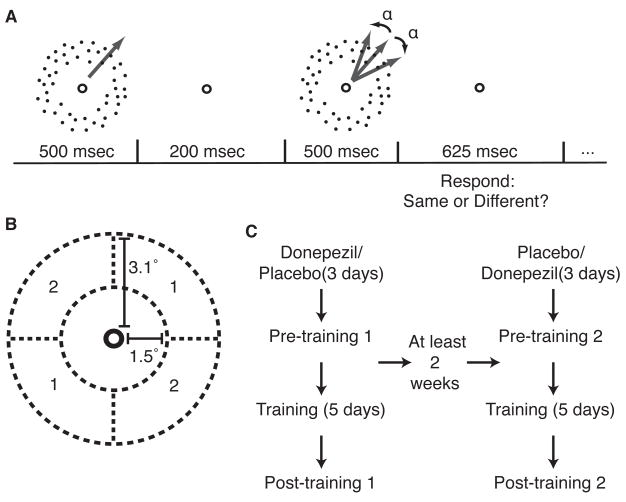
Figure 1. Experimental procedure.
a, Task description. In each trial, two fields of dots with 100% coherent motion were sequentially presented. The two fields contained either the same or slightly different directions of motion. b, Stimulus configuration. Coherent motion was presented in one of two pairs of spatial locations (1 or 2) and in the same direction of motion throughout the course of training. c, Training procedure. Subjects participated in two courses of training. Donepezil or placebo was administered beginning three days before the pre-training measurement and daily throughout training and the post-training measurement.
Each subject completed two courses of training (Figure 1C), once while ingesting a pill containing 5 mg of donepezil before every training session and once while an inactive placebo was administered. Drug administration was double-blind, and the order of drug and placebo administration was counter-balanced between subjects. Each course of training was preceded by three days of donepezil or placebo administration, bringing drug plasma levels to within the steady-state range (the half-life of donepezil in the human body is approximately 80 hours [20]), and drug/placebo administration continued daily throughout training and the post-training assessment. Before and after training, thresholds were measured for both pairs of visual field locations and for eight different directions of motion.
For each course of training, subjects performed the task for a particular stimulus with coherent motion in one direction and in one of the two possible pairs of locations (Figure 1B). Since training in this task is specific for visual field location and motion direction [21], the effects of the two courses of training were separately assessed in each subject by training under donepezil with a stimulus presented in the other visual field location and in the opposite motion direction than the stimulus used for the placebo training course. Human subjects exhibit differences in performance of this task for oblique and cardinal directions of motion [21, 22]. We therefore used only oblique directions for training. During training, participants performed 1000 trials every day. Subjects underwent five days of training, except for one subject who trained for six days in both the placebo and donepezil conditions. At least two weeks passed between the two courses of training, allowing for donepezil, if present, to be eliminated.
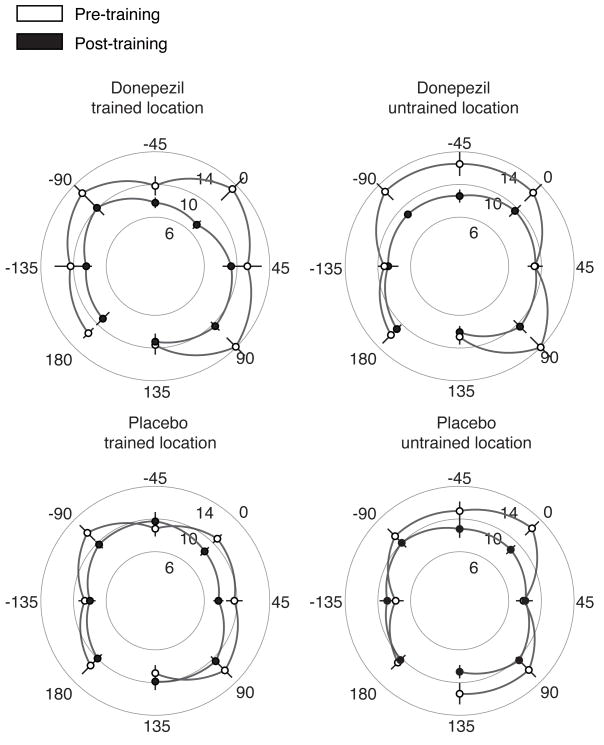
Perceptual learning resulted in an improvement in performance for the trained condition, defined as the direction of motion and visual field location used for training (Figure 2). The average decrease in angular difference threshold (Figure 1A) for the trained condition, combining placebo and donepezil training courses, was 4.2 +/− 1.2 degrees. However, the main effect of training (pre- vs. post-training thresholds, across all directions of motion, both locations, and both drug conditions (placebo and donepezil) as assessed by the significance of the training factor in the ANOVA) was not significant (F1,9=0.53, p=0.49), demonstrating the specificity of learning for the training stimulus.
Figure 2. Effects of training and cholinergic enhancement with donepezil on motion direction discrimination thresholds.
Each plot displays angular difference thresholds in degrees (Figure 1A) for different directions of motion, where zero degrees corresponds to the direction used for training. There was a significant improvement in performance for the trained condition (trained direction and visual field location), and this improvement was substantially larger under donepezil than under placebo. Error bars denote SEM. Single-subject data are presented in Supplemental Figure 1.
To further characterize the specificity of learning, we compared the improvement (change in threshold) that occurred in the trained condition (combining drug and placebo training courses), relative to the improvement that occurred in performance of the task on other stimuli. Direction specificity of perceptual learning was assessed by subtracting the improvement in performance in the untrained directions of motion (in the trained location) from the improvement in the trained direction (in the trained location). This difference in improvement was 2.7 +/− 0.9 degrees (planned comparison, t36=3.26, p<0.05), indicating that learning was specific to the trained direction of motion.
A similar measure of location selectivity was also calculated by subtracting the improvement in the untrained visual field locations (in the trained direction) from the improvement in the trained locations (in the trained direction). This difference in improvement was 1.3 +/− 0.7 degrees and was not significant (planned comparison, t36=1.53, p>0.5). The lack of location specificity of learning for the combined donepezil and placebo training courses replicates previous results in which this paradigm showed substantial transfer of learning to visual field locations immediately adjacent to the training location [21]. In addition, the spatial layout of our training stimulus, which requires simultaneous discrimination of motion direction in both visual hemifields, may have led to more spatial generalization of learning than in previous studies.
Comparison of the drug and placebo conditions revealed that administration of donepezil had an overall effect on learning, as evidenced by a significant interaction of drug and training factors in the ANOVA (F1,9=5.89, p<0.05; see Supplemental Experimental Procedures). There were significant reductions in threshold in the trained condition following perceptual learning, both when subjects were taking donepezil, with an average improvement of 6.2 +/− 2.1 deg (planned comparison, t36=6.81, p<0.05), and under placebo, 2.2 +/− 0.8 deg (planned comparison, t36=2.42, p<0.05). However, the improvement in performance in the trained condition during donepezil administration was significantly larger than the improvement under placebo (planned comparison, t36 =3.1, p<0.05).
In addition to enhancing the amount of learning in the trained condition, donepezil also increased its selectivity to this condition. Direction and location selectivity were computed separately for the placebo and donepezil training courses using the same procedure described above for combined drug and placebo data. Direction selectivity was 4.0 +/− 1.2 deg under donepezil (planned comparison, t36=3.94, p<0.05) and 1.4 +/− 1.1 deg under placebo (planned comparison, t36=1.34, p>0.05). The difference between these two values was statistically significant, indicating that, compared to placebo, donepezil increased the direction selectivity of learning (planned comparison, t36=2.82, p<0.05). Similarly, location selectivity was 3.0 +/− 1.2 deg under donepezil (planned comparison, t36=2.36, p<0.05) and −1.2 +/− 1.0 deg under placebo (planned comparison, t36=0.92, p>0.05). The increase in location specificity under donepezil (relative to placebo) was also statistically significant (planned comparison, t36 =2.68, p<0.05).
Comparisons of raw threshold values are sensitive to between-subject performance differences and to effects of the drug on overall task performance. In addition to donepezil’s enhancement of the magnitude of perceptual learning, there was an overall deleterious effect of donepezil on discrimination thresholds (F 1,9=12.76, p<0.05, combining all directions, both locations, and both pre- and post-training measurements, as assessed by the significance of the drug factor in the ANOVA), which may stem from non-specific effects of cholinergic enhancement on visual perception and/or task performance (see Discussion).
In particular, pre-training thresholds for the direction of motion and visual field location used for training were numerically higher under donepezil (13.3 +/− 2.4 deg) than under placebo (10.7 +/− 0.8 deg), raising the possibility that the drug effect on the magnitude of learning was due to this drug/placebo difference in pre-training thresholds. However, this difference in pre-training thresholds for the condition that was then used for training was not statistically significant (post-hoc t-test: t11=1.17, p>0.05). On the other hand, post-training thresholds for the trained condition were significantly lower under donepezil (7.2 +/− 0.6 deg) than under placebo (8.5 +/− 0.5 deg, post-hoc t-test: t11= 2.81, p<0.05). There were no significant effects of the drug on thresholds for any other combination of visual field location and motion direction, either before or after training. This finding provides additional evidence that donepezil enhances perceptual learning in a stimulus-specific manner, as any general effect of the drug on task performance would presumably have affected both pre- and post-training thresholds (see Supplemental Data for additional analysis of donepezil’s effect on overall thresholds and Supplemental Figure 1 for individual subject data).
To isolate donepezil’s effects on perceptual learning from its effects on overall task performance, we calculated the percent learning for each subject relative to that subject’s pre-training performance (Figure 3). Percent learning in the trained condition was greater for donepezil than for placebo (planned comparison, t36=2.5, p<0.05), further demonstrating that the beneficial effects of donepezil on learning were not due to the drug’s effects on overall performance.
Figure 3. Donepezil increases magnitude and specificity of perceptual learning.
Percent reduction in threshold following training was significantly larger under donepezil in the trained condition, and learning under donepezil was more specific to the direction of motion and visual field location used for training. Error bars denote SEM. These data are presented without normalization (i.e., raw threshold values) in Supplemental Figure 2.
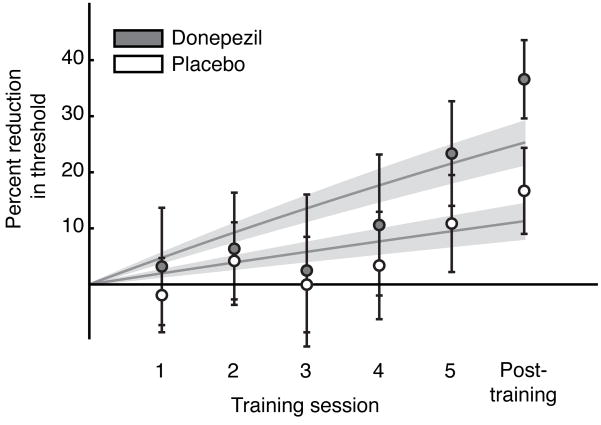
In order to determine whether the increase in the magnitude of learning under donepezil was a consequence of more rapid learning, we examined the progression of learning in the trained condition for both donepezil and placebo (Figure 4). A single-parameter model of learning was fit to the data (see Supplemental Experimental Procedures). Average learning rates (in units of % change/session) were greater for donepezil (4.9 +/− 0.8) than for placebo (2.0 +/− 0.5). Statistical significance of the effect of cholinergic enhancement on learning rate was calculated using a non-parametric permutation test (see Supplemental Experimental Procedures). This test demonstrated that learning was significantly more rapid under donepezil (p<0.05).
Figure 4. Donepezil increases rate of perceptual learning.
Percent learning in the trained condition is presented as a function of training session. Training under donepezil (filled circles) proceeded at a more rapid rate than training under placebo (empty circles). Learning rates were computed by fitting a single-parameter model of learning to the data (gray continuous lines, where the shaded area is the standard deviation derived from a jackknife estimate; see Supplemental Experimental Procedures). Error bars denote SEM.
Discussion
Cholinesterase inhibitors such as donepezil are commonly prescribed drug treatments for Alzheimer’s disease. It would therefore be beneficial to understand the specific aspects of cognition and behavioral performance that are enhanced by increases in synaptic ACh. A previous study has shown that administration of the cholinesterase inhibitor physostigmine to healthy humans enhanced the behavioral effects of visual spatial attention [11], but another study reported no effects of this drug on performance in tasks requiring visual spatial attention [23]. Also, administration of physostigmine [10], as well as donepezil [24], can improve long-term retention of memorized items, but this effect has also not always been found [25]. Our findings demonstrate the possibility of enhancing the beneficial cognitive effects of the cholinergic system, even in a young healthy population, and suggest that the cognitive improvement associated with cholinergic enhancement in Alzheimer’s disease may stem from an augmented capacity to learn new information.
Studies in animals have shown that ACh increases transmission at feedforward thalamocortical synapses relative to lateral intracortical connections [26]. ACh reduces the spatial spread of excitatory activity following electrical stimulation of rat visual cortical slices [27] and decreases the preferred stimulus length of cells in marmoset area V1 [4]. In addition, electrical stimulation of the basal forebrain results in a more reliable representation of the stimulus in visual cortical neurons [28]. In humans, donepezil reduces the spatial spread of excitatory fMRI visual responses in early visual cortex [5], consistent with a reduction in excitatory receptive field size of visual cortical neurons, and physostigmine increases the selectivity of responses in visual association cortex [3]. Our findings suggest that during perceptual learning, these increases in neural selectivity by ACh may enhance learning-dependent changes in tuning of the neurons that encode task-relevant stimuli. This is consistent with previous models of the role of the cholinergic system in learning and memory [29].
One factor that could be mediating the effects of cholinergic transmission on learning is visual attention. Attention has been found to play an important facilitatory role in some types of perceptual learning [30], and ACh modulates allocation of attention [1, 31]. In particular, when an animal is attending to a particular visual field location, visual cortical neurons with receptive fields at that location exhibit larger responses to visual stimulation. This increase in firing rate due to visual spatial attention is augmented by local administration of ACh to cortical area V1 and is attenuated following local administration of the muscarinic ACh receptor antagonist scopolamine [32]. Previous studies in humans have shown increases in the effects of sustained visual attention following pharmacological enhancement of the cholinergic system [11]. In the present study, donepezil may have facilitated processing of the training stimulus through enhanced allocation of attention to this stimulus, thereby augmenting perceptual learning. Nevertheless, we cannot rule out the possibility that donepezil’s enhancement of perceptual learning is due to a direct modulation of plasticity rather than to an effect mediated by attention.
It is important to note that perceptual learning does not always require attention to be directed to the stimulus and that learning can occur even in the absence of conscious perception of the stimulus. Watanabe et al. [33] instructed participants to perform a difficult sensory judgment in the center of the visual field while a task-irrelevant motion stimulus was presented in the peripheral visual field. Although the amount of coherent motion in the peripheral stimulus was undetectable, subjects improved in performance of a motion discrimination task for the direction of motion contained in the peripheral stimulus, and the learning was specific to that direction of motion. However, even for this kind of task-irrelevant perceptual learning, training was still not entirely subliminal. Specifically, for the peripheral subthreshold stimuli, learning occurred only when the peripheral stimulus was presented at the same time that the target appeared in central vision [34]. That is, simultaneous presentation of task-relevant information was required in order for plasticity of the neural representations of the task-irrelevant stimulus to take place.
Furthermore, another study [35] demonstrated that task-irrelevant perceptual learning depends on the relative visual field locations of the task-irrelevant and task-relevant stimuli. Task-irrelevant perceptual learning was demonstrated for stimuli that were near the task-relevant stimulus but was not observed for stimuli that were farther away (6.6 degrees of visual angle) from the attended stimulus. Acetylcholine is released in cortex when animals are performing a task requiring sustained attention [6], and a recent study showed that ACh can be released in frontal cortex in a transient and spatially-specific manner and that this transient release of ACh increases the probability of stimulus detection [36]. We hypothesize that ACh release may facilitate task-irrelevant perceptual learning when the task-irrelevant stimuli appear in temporal and spatial proximity to the allocation of spatial attention. Further research is needed to determine the role of ACh in task-irrelevant perceptual learning (see [37] for a review of perceptual learning, attention, and neuromodulatory signals).
In the present study, subjects’ overall task performance (across both trained and untrained conditions) was impaired by administration of donepezil, indicating that the presumed increase in selectivity of the neural response by ACh did not translate into an overall improvement in motion direction discrimination. However, the decrease in performance could also be the result of other effects of the drug. Donepezil was administered systemically in our study, and although this drug is relatively selective for the form of cholinesterase expressed in the central nervous system [38, 39], it may have affected non-specific task-related cognitive functions as well as cholinergic synapses regulating processes such as lens accommodation and pupil dilation [40]. These non-specific effects of the drug would have affected performance in all conditions (including both pre- and post-training measurements) and therefore would have been independent of the effect of donepezil on the magnitude and specificity of perceptual learning. Importantly, increased learning in the trained condition under donepezil was observed even when performance was normalized to each subject’s pre-training threshold (Figure 3). Thus, overall differences in performance do not account for the beneficial effects of the drug on perceptual learning (see Supplemental Data).
In conclusion, we have shown that the magnitude, direction and location specificity, and rate of perceptual learning of a visual motion direction discrimination task are greater when donepezil is administered during the training procedure. These results demonstrate the possibility of enhancing the beneficial cognitive effects of the cholinergic system, even in a young healthy population. Our finding that donepezil increases the specificity of perceptual learning suggests that ACh may augment plasticity and tuning in populations of neurons that encode task-relevant stimulus features.
Highlights.
Pharmacological enhancement of acetylcholine augments perceptual learning.
Increased acetylcholine (cholinergic enhancement) amplifies benefits of training.
Learning during cholinergic enhancement is more specific to the training stimulus.
Supplementary Material
Acknowledgments
We thank Dave Garg and Greg Lam for their help in collecting the data and Merav Ahissar, Dennis Levi, and Mark D’Esposito for comments on the manuscript. This work was supported by NIH grants R21-EY17926 and R21-EY19992 (MAS), the Hellman Family Faculty Fund (MAS), and National Research Service Award F31-AG032209 (AR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- 3.Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- 4.Roberts MJ, Zinke W, Guo K, Robertson R, McDonald JS, Thiele A. Acetylcholine dynamically controls spatial integration in marmoset primary visual cortex. J Neurophysiol. 2005;93:2062–2072. doi: 10.1152/jn.00911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver MA, Shenhav A, D’Esposito M. Cholinergic enhancement reduces spatial spread of visual responses in human early visual cortex. Neuron. 2008;60:904–914. doi: 10.1016/j.neuron.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- 7.Greuel JM, Luhmann HJ, Singer W. Pharmacological induction of use-dependent receptive field modifications in the visual cortex. Science. 1988;242:74–77. doi: 10.1126/science.2902687. [DOI] [PubMed] [Google Scholar]
- 8.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 9.Thiel CM, Friston KJ, Dolan RJ. Cholinergic modulation of experience-dependent plasticity in human auditory cortex. Neuron. 2002;35:567–574. doi: 10.1016/s0896-6273(02)00801-2. [DOI] [PubMed] [Google Scholar]
- 10.Davis KL, Mohs RC, Tinklenberg JR, Pfefferbaum A, Hollister LE, Kopell BS. Physostigmine: improvement of long-term memory processes in normal humans. Science. 1978;201:272–274. doi: 10.1126/science.351807. [DOI] [PubMed] [Google Scholar]
- 11.Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41:969–982. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- 12.Fahle M, Poggio T, editors. Perceptual Learning. Cambridge, MA: The MIT Press; 2002. [Google Scholar]
- 13.Furmanski CS, Schluppeck D, Engel SA. Learning strengthens the response of primary visual cortex to simple patterns. Curr Biol. 2004;14:573–578. doi: 10.1016/j.cub.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghose GM, Yang T, Maunsell JH. Physiological correlates of perceptual learning in monkey V1 and V2. J Neurophysiol. 2002;87:1867–1888. doi: 10.1152/jn.00690.2001. [DOI] [PubMed] [Google Scholar]
- 16.Schoups A, Vogels R, Qian N, Orban GA. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- 17.Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- 18.Pelli DG. The VideoToolbox software for visual psychophsyics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- 19.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 20.Rogers SL, Cooper NM, Sukovaty R, Pederson JE, Lee JN, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following multiple oral doses. Br J Clin Pharmacol. 1998;46(Suppl 1):7–12. doi: 10.1046/j.1365-2125.1998.0460s1007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vis Res. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- 22.Rokem A, Silver MA. A model of encoding and decoding in V1 and MT accounts for motion perception anisotropies in the human visual system. Brain Res. 2009;1299:3–16. doi: 10.1016/j.brainres.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003;20:58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- 24.Grön G, Kirstein M, Thielscher A, Riepe M, Spitzer M. Cholinergic enhancement of episodic memory in healthy young adults. Psychopharmacology (Berl) 2005;182:170–179. doi: 10.1007/s00213-005-0043-2. [DOI] [PubMed] [Google Scholar]
- 25.Nathan PJ, Baker A, Carr E, Earle J, Jones M, Nieciecki M, Hutchison C, Stough C. Cholinergic modulation of cognitive function in healthy subjects: acute effects of donepezil, a cholinesterase inhibitor. Hum Psychopharmacol. 2001;16:481–483. doi: 10.1002/hup.323. [DOI] [PubMed] [Google Scholar]
- 26.Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- 27.Kimura F, Fukuda M, Tsumoto T. Acetylcholine suppresses the spread of excitation in the visual cortex revealed by optical recording: possible differential effect depending on the source of input. Eur J Neurosci. 1999;11:3597–3609. doi: 10.1046/j.1460-9568.1999.00779.x. [DOI] [PubMed] [Google Scholar]
- 28.Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn and Mem. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 30.Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proc Natl Acad Sci USA. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rokem A, Landau AN, Garg D, Prinzmetal W, Silver MA. Cholinergic enhancement increases the effects of voluntary attention but does not affect involuntary attention. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.118. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe T, Nanez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- 34.Seitz AR, Watanabe T. Is subliminal learning really passive? Nature. 2003;422:36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- 35.Nishina S, Seitz AR, Kawato M, Watanabe T. Effect of spatial distance to the task stimulus on task-irrelevant perceptual learning of static Gabors. J Vis. 2007;7:1–10. doi: 10.1167/7.13.2. [DOI] [PubMed] [Google Scholar]
- 36.Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roelfsema PR, van Ooyen A, Watanabe T. Perceptual learning rules based on reinforcers and attention. Trends Cogn Sci. 2010;14:64–71. doi: 10.1016/j.tics.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. Inhibitory effects of donepezil hydrochloride (E2020) on cholinesterase activity in brain and peripheral tissues of young and aged rats. Eur J Pharmacol. 1999;386:7–13. doi: 10.1016/s0014-2999(99)00741-4. [DOI] [PubMed] [Google Scholar]
- 39.Rogers SL, Yamanishi Y, Yamatsu K. E2020 - The pharmacology of a piperidine cholinesterase inhibitor. In: Becker R, GE, editors. Cholinergic Basis for Alzheimer Therapy. Boston MA: Birkhäuser; 1991. [Google Scholar]
- 40.Estermann S, Daepp GC, Cattapan-Ludewig K, Berkhoff M, Frueh BE, Goldblum D. Effect of oral donepezil on intraocular pressure in normotensive Alzheimer patients. J Ocul Pharmacol Ther. 2006;22:62–67. doi: 10.1089/jop.2006.22.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.