Abstract
Study objective
To compare different oral ovulation induction agents in treating infertile women with polycystic ovary syndrome
Design
Decision-analytic model comparing three treatment strategies using probability estimates derived from literature review and sensitivity analyses performed on the baseline assumptions
Setting
Outpatient reproductive medicine and gynecology practices
Patients
Infertile women with polycystic ovary syndrome
Interventions
Metformin, clomiphene citrate, or metformin with clomiphene citrate
Main Outcome Measures
Live birth
Results
Within the baseline assumptions, combination therapy with metformin and clomiphene citrate was the preferred therapy for achieving live birth in women with polycystic ovary syndrome. Sensitivity analysis revealed the model to be robust over a wide range of probabilities.
Conclusions
Combination therapy with metformin and clomiphene citrate should be considered as first-line treatment for infertile women with polycystic ovary syndrome
Keywords: polycystic ovary syndrome, anovulation, metformin, clomiphene citrate
Introduction
Polycystic ovary syndrome (PCOS) is a common cause of infertility often amenable to treatment with oral ovulation induction agents. For many years clomiphene citrate was the first-line treatment for infertile women with PCOS. As research linking PCOS and insulin resistance emerged (1) metformin, an insulin-sensitizing agent, was advocated as an alternative to clomiphene citrate as it induced ovulation and offered additional clinical benefits for women with PCOS including improved insulin secretion and action (2).
In recent years several randomized controlled trials (RCTs) have tested the efficacy of these medications individually and in combination as first-line treatment for infertility in the setting of PCOS (3-6). Unfortunately, these studies have yielded varying results. Explanations for study discrepancies include extremes in sample size, different primary outcome measures, and different study inclusion and exclusion criteria resulting in different study populations (3, 7, 8). Recently, a meta-analysis reviewed head-to-head RCTs of clomiphene citrate, metformin or both, but this study was also unable to distinguish the best regimen (7).
Because the question of how to treat infertile women with PCOS is important and still debated (7-10), we felt it was ideally suited for further investigation using a decision-analytic model.
Materials and Methods
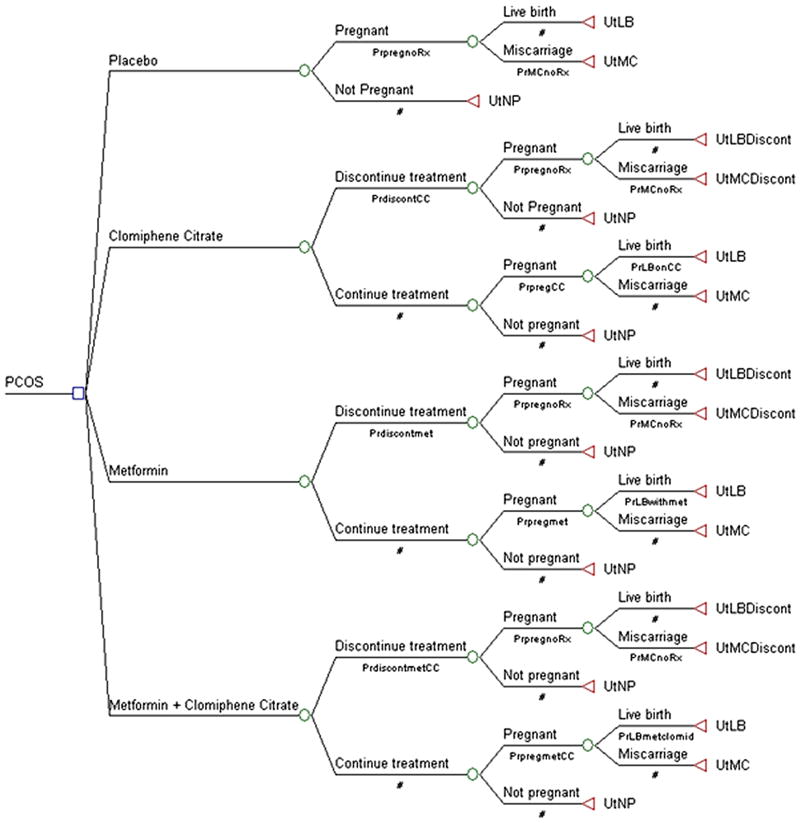
A decision-analytic model was designed comparing different oral ovulation induction agents for treatment of infertility in women with polycystic ovary syndrome. The model is depicted in figure 1 and was constructed and analyzed using TreeAge Pro 2009 Suite (Tree Age Software, Inc, Willamstown, MA). Treatment strategies included clomiphene citrate alone, metformin alone, or clomiphene citrate plus metformin in combination. Placebo was added as a fourth strategy for baseline reference. Washington University does not require institutional review board approval for decision-analytic studies.
Fig. 1.
Decision model for oral ovulation induction agents for fertility treatment in women with polycystic ovary syndrome.
The primary objective of this model was to determine the most effective oral ovulation induction agent in achieving a live birth in women with polycystic ovary syndrome. Other events relevant in the analysis included discontinuation of treatment for severe side effects, pregnancy rates, and rate of miscarriage. Probability estimates and confidence intervals for these events and our outcome of interest were derived primarily from randomized clinical trials identified through review of the literature. Estimates were calculated by totaling the number of patients responding to a given treatment and dividing it by the total treated from each study for a particular treatment group. These estimates and ranges are reported from the included studies (Table 1). Literature review included searches of the MEDLINE (January 1966-September 2009) and Cochrane Systematic Reviews databases, and review of reference lists from retrieved trials and meta-analyses (7, 11-13). Studies were excluded if they failed to report pregnancy data, if they included patients proven to be resistant to clomiphene citrate, or if they used additional agents such as hCG to induce ovulation. Utility values were based on the authors' expert opinions. Live birth was assigned a utility value of 1, no pregnancy was assigned a value of 0, and miscarriage was assigned a value of 0.3. Live birth after discontinuing medications due to an adverse event was assigned a utility of 0.8, and miscarriage after discontinuing medications due to an adverse event was assigned a utility of 0.2.
TABLE 1.
Estimated probabilities for variables in the decision tree
| Name in tree | Description | Baseline estimate | Range | Reference |
|---|---|---|---|---|
| PrdiscontCC | Probability of discontinuing treatment because of adverse reaction on CC | 0.027 | 0-0.053 | (3-6) |
| Prdiscontmet | Probability of discontinuing treatment because of an adverse reaction on metformin | 0.024 | 0-0.029 | (3, 5, 6) |
| PrdiscontmetCC | Probability of discontinuing treatment because of an adverse reaction on metformin + CC | 0.07 | 0-0.162 | (4-6) |
| PrpregCC | Probability of pregnancy on CC | 0.296 | 0.076-0.46 | (3-6, 35-39) |
| Prpregmet | Probability of pregnancy on metformin | 0.18 | 0.08-0.62 | (3, 5, 6, 40, 41) |
| PrpregmetCC | Probability of pregnancy on metformin + CC | 0.407 | 0.21-0.585 | (3-5, 38, 39) |
| PrpregnoRx | Probability of pregnancy on placebo | 0.032 | 0-0.09 | (35-37, 40, 41) |
| PrLBonCC | Probability of live birth on CC | 0.21 | 0.15-0.22 | (3-6) |
| PrLBwithmet | Probability of live birth on metformin | 0.15 | 0.07-0.52 | (3, 5, 6) |
| PrLBmetclomid | Probability of live birth on metformin + CC | 0.23 | 0.18-0.27 | (3-5) |
| PrMCnoRx | Probability of pregnancy loss on placebo | 0.246 | 0.228-0.25 | (42, 43) |
In the baseline analysis, pathway probabilities were used to calculate the optimal oral ovulation induction strategy for achieving a live birth. A theoretic cohort of 10,000 women was considered to compare each strategy. One-way sensitivity analysis was performed by varying probability estimates across plausible ranges obtained from the literature (Table 1) and by varying assigned utilities across their 95% confidence intervals (Table 2). Confidence intervals for utilities were obtained by using exact 95% confidence intervals of binomial proportions for each value as determined with STATA 11.0 (StatCorp, College Station, TX). A one-sided 97.5% confidence interval was determined for the lower-bound confidence interval for the utility value of 1.
TABLE 2.
Utility values used in decision analytic model
| Name in tree | Description | Baseline estimate | Ranges entered in sensitivity analysis* |
|---|---|---|---|
| UtLB | Utility of a live birth | 1 | 0.96-1 |
| UtMC | Utility of a miscarriage | 0.3 | 0.21-0.4 |
| UtLBDiscont | Utility of a live birth after having to discontinue treatment because of an adverse reaction | 0.8 | 0.71-0.87 |
| UtMCDiscont | Utility of a miscarriage after having to discontinue treatment because of an adverse reaction | 0.2 | 0.13-0.29 |
| UtNP | Utility of no pregnancy | 0 | 0 |
see methods text
The following assumptions were made in our analysis: 1) Efficacy of clomiphene citrate and metformin formulations and dosing was equivalent across included studies, 2) Differences in durations of included studies did not affect the analysis, 3) Totaling the number of live births and number of miscarriages equaled the number of pregnancies in each treatment arm, 4) Probability of pregnancy, miscarriage or live birth in the women who discontinued treatments because of adverse reactions would be the same as the probability of pregnancy among women treated with placebo, and 5) Probabilities for pregnancy and miscarriage in women who conceived on placebo or after discontinuing treatment was derived mainly from cohort studies as many more recent RCTs did not include placebo arms or follow outcomes in women who discontinued treatment.
Results
With our baseline assumptions, the model favored the use of combination therapy with metformin and clomiphene citrate in achieving a live birth in women with PCOS. Applying results from our baseline analysis to a hypothetical group of 10,000 PCOS patients undergoing ovulation induction, treatment with metformin alone would result in 1500 live births, 1259 more births than placebo (metformin vs. placebo, OR 7.15, 95% CI 6.2-8.23). The next best therapy would be clomiphene citrate alone resulting in 2100 live births, 1859 more births than placebo and 600 more than metformin alone (clomiphene citrate vs. metformin, OR 1.51, 95% CI 1.4-1.62). Metformin plus clomiphene citrate would result in 2300 live births, 2059 more than placebo and 200 more than clomiphene citrate alone (metformin plus clomiphene citrate vs. clomiphene citrate alone, OR 1.12, 95% CI 1.05-1.2).
We performed a one-way sensitivity analysis comparing each of the treatment modalities to evaluate the impact of varying the probabilities and utilities on the primary outcome measure. In our analysis, combination therapy performed better than metformin or clomiphene citrate alone in achieving live birth over a wide range of probabilities and utilities (Figure 2). Variables that influenced our model included probability of pregnancy on clomiphene citrate alone, metformin alone, and combination metformin and clomiphene citrate. Clomiphene citrate alone became the preferred strategy when the probability of pregnancy on combination metformin and clomiphene citrate fell to 30% from the base-case probability of 41%, or when the probability of pregnancy on clomiphene citrate rose to 40% from the base-case probability of 30%. Metformin alone became the preferred strategy when the probability of pregnancy on metformin rose to 44% from the base-case probability of 18%. Sensitivity analysis also revealed the model to be sensitive to a low probability of live birth on combination metformin and clomiphene citrate of < 5.4%, which was lower than the live birth rates with metformin and clomiphene citrate in any of the published studies encountered in our literature review. At this low probability clomiphene citrate alone became the optimal treatment strategy.
Fig. 2.
Tornado diagram summary of the 1-way sensitivity analysis for ovulation induction in women with polycystic ovary syndrome.
Discussion
The optimal first-line ovulation induction treatment for PCOS is debated. Two mainstays of therapy have included clomiphene citrate and metformin—medications with different mechanisms of action. Clomiphene citrate, a selective estrogen-receptor modulator, presumably works to induce ovulation by inhibiting negative, endogenous, estrogen-feedback on the hypothalamic-pituitary axis resulting in increased FSH secretion, follicular growth, and ovulation. Metformin, an adenosine monophosphate-kinase activator introduced to treat type 2 diabetes mellitus, may work to improve ovulation by several different mechanisms. Two proposed mechanisms include meiotic induction of immature oocytes(14), and inhibition of aromatase (15). Regardless of the mechanisms, there is evidence to support the use of clomiphene citrate or metformin either alone or in combination to treat infertile women with PCOS, however, randomized controlled trials and meta-analyses comparing these medications have failed to yield consistent results. Using results from the existing literature, our decision analysis indicates a treatment with a combination of metformin and clomiphene citrate is the optimal strategy for achieving live birth in women with PCOS.
Discrepancies in previous studies investigating this population of women could be due to several factors including differences in dosing and administration of medication, differences in study duration, differences in study location (e.g. the United States vs. Italy) or study inclusion and exclusion criteria leading to different study populations—all of these differences are likely representative of the heterogeneity of women who meet diagnostic criteria for PCOS and the heterogeneity of practice patterns among physicians who treat these women. One of the largest and most recent of these RCTs by the Reproductive Medicine Network (RMN) sponsored by the National Institutes of Health demonstrated clomiphene citrate to be superior to metformin in achieving live birth with a live-birth rate of 22.5% in the clomiphene group and 7.2% in the metformin group (3). In the combination therapy group, there was a live birth rate of 26.8%. The sample size for this study was calculated to detect a 15% difference among the therapies. This is a large difference, and the authors of this study point out, “even a 5% increase in the chances of a live birth would be important to some patients” (Myers et al, 2005(16)). On the other hand, the authors also point out that it would have required a sample size of 6,144 subjects to detect a 5% difference among groups with 80% power. A study of this size is unreasonable given that many resources have already been invested in the question of the best first-line treatment for infertile women with PCOS. Therefore, a decision analysis using our approach is the next reasonable step to investigate the question of which regimen is the optimal first-line strategy.
Since the publication of the RMN trial, many have responded with increased scrutiny of the role of metformin in treating infertility in women with PCOS (17, 18). In a recent 2010 Cochrane review by Tang et al, authors concluded “there is no evidence that metformin improves live birth rates whether it is used alone or in combination with clomiphene, or when compared with clomiphene. Therefore, the use of metformin in improving reproductive outcomes in women with PCOS appears to be limited” (13). This meta-analysis provides valuable information regarding the existing body of evidence surrounding the use of metformin in treating women with PCOS, however, the primary studies included and the analytic techniques employed in this meta-analysis were different from ours, and may be responsible in part for our disparate conclusions. First, the Cochrane review did not eliminate studies that included women previously proven to be clomiphene-resistant (19-22)—we were interested in the question of first-line treatment and therefore eliminated such studies from our analysis. Second, the Cochrane review did not eliminate studies in which additional agents such as hCG were used in conjunction with clomiphene citrate to induce ovulation—a step that adds extra cost and complexity to treatment and therefore not ideal in first-line treatment of these patients either (23). Finally, the Cochrane review piece is a collection of meta-analyses comparing each treatment strategy for individual steps of the reproductive process. Because we used decision analysis to approach the question, our study takes each step of the reproductive process into consideration when comparing the different treatment strategies. Because of these differences, we stand by our conclusion that treatment with a combination of metformin and clomiphene citrate is a reasonable first-line strategy for achieving live birth in women with PCOS.
We agree with critics of metformin that there is a need to further elucidate metformin's mechanism of action and to monitor its use in women with PCOS who are trying to conceive. However, we also believe there is a plethora of data to support continued use and research of metformin as an agent for improving ovulation in women with PCOS. In addition to improved ovulation, metformin may delay the onset of type 2 diabetes mellitus and cardiovascular risk factors in this at risk population (17), and for women who conceive it may decrease the risk of developing gestational diabetes(24). While controversial, there are also observational data suggesting metformin may decrease the risk of spontaneous pregnancy loss (25, 26) although overall differences between pregnancy rates and live birth rates did not differ in the studies incorporated into our decision analysis. Finally, data from a recent RCT of metformin versus insulin in women with gestational diabetes found metformin to be a reasonable alternative to insulin in preventing perinatal complications(27). Together these data demonstrate the potential value of metformin as a maintenance medication for women with PCOS in the pre-, peri-, and post conceptional periods as they are at high risk for developing gestational diabetes (27). While further clinical study is necessary to substantiate this proposal, there is compelling data from laboratory models suggesting a mechanism for decreased pregnancy loss and improved pregnancy outcomes with the application of metformin early in pregnancy in the setting of insulin-resistance (26).
The heterogeneity in the clinical presentation of PCOS has contributed to the difficulty of determining the etiology of anovulation in PCOS (28). Lifestyle appears to play a role as a number of women with PCOS are obese and weight loss often results in the resumption of ovulation, however, genetics may also play a role. Research investigating the interplay between environment and genetics may come in time and will be helpful in determining optimal treatments for women of different PCOS phenotype. The recent discovery that the ovulatory response varies among women with PCOS and different polymorphisms in the STK11 gene is an example of this type of research (29).
The limitations of our study include those of any decision-analysis. More specifically, the strength of our findings is dependent on the data included in our decision tree. As demonstrated in Table 1, the probabilities taken from the literature for live birth rates in each of the treatment strategies varied greatly. This may be a reflection in the heterogeneity of women who meet diagnostic criteria for PCOS, or it may be a reflection in variations in strategies for ovulation induction with the chosen regimens. While some may consider this a limitation of the study, others may consider it a strength as it may be indicative of true clinical experience. In any case, we adjusted for this in our sensitivity analysis and found our results robust over a wide range of probabilities. Combination metformin and clomiphene citrate was the preferred therapy over a wide range of probabilities. Only when the probability of pregnancy with clomiphene citrate rose above 40% or the probability of pregnancy on metformin and clomiphene fell below 30%, did clomiphene citrate alone become the preferred therapy in this model. Metformin alone became the preferred strategy when the probability of pregnancy on metformin rose above 44%.
Another limitation in this study was our choice of utility states. Thorough search of the literature for health utility states did not reveal reliable utility values for pregnancy, miscarriage, and live birth in this population. We used judgment to determine reasonable utilities for these outcomes, but our inability to find published utility values underscores the need for future health services research in reproductive medicine. Finally, we did not include probabilities for conceiving multiples in our study. This is a major limitation as the risk of conceiving multiples is significant with clomiphene citrate, however, not all of the published studies used to derive our probability estimates included data regarding multiple pregnancy rates, and delivery data was lacking from those that did. Estimating utility values for multiple gestations versus singleton gestations is also difficult as patients and clinicians may differ in their views over preferences for multiples and their perceptions of the risks involved (30-32).
Our decision analysis did not consider the use of letrozole or other aromatase inhibitors although these medications are effective for ovulation induction in women with PCOS. We are anxiously awaiting the results of the RMN trial comparing letrozole to clomiphene citrate in achieving live births in infertile women with PCOS (33) as letrozole is associated with few side effects, and it does not carry the same risk of conceiving multiples as clomiphene citrate. On the other hand, there is recent data that metformin may work to inhibit aromatase and similar to letrozole, it does not carry the risk of conceiving multiples as clomiphene citrate. Furthermore, there is experience demonstrating safe outcomes for children born to mothers who have conceived while on metformin(34). Given these data and the results of our decision analysis, we argue that it is an appropriate strategy to use of metformin with clomiphene citrate for ovulation induction as first-line therapy to treat infertility in women with polycystic ovary syndrome.
Acknowledgments
Support: This work was supported by National Institutes of Health Grant UL1RRO24992 to Washington University and National Institutes of Health Grant K12 HD063086-01 to ESJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–6. doi: 10.1210/jcem-50-1-113. [DOI] [PubMed] [Google Scholar]
- 2.Ehrmann DA, Cavaghan MK, Imperial J, Sturis J, Rosenfield RL, Polonsky KS. Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:524–30. doi: 10.1210/jcem.82.2.3722. [DOI] [PubMed] [Google Scholar]
- 3.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–66. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 4.Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ. 2006;332:1485. doi: 10.1136/bmj.38867.631551.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zain MM, Jamaluddin R, Ibrahim A, Norman RJ. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril. 2009;91:514–21. doi: 10.1016/j.fertnstert.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Palomba S, Orio F, Jr, Falbo A, Manguso F, Russo T, Cascella T, et al. Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4068–74. doi: 10.1210/jc.2005-0110. [DOI] [PubMed] [Google Scholar]
- 7.Palomba S, Pasquali R, Orio F, Jr, Nestler JE. Clomiphene citrate, metformin or both as first-step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head-to-head randomized controlled studies and meta-analysis. Clin Endocrinol (Oxf) 2009;70:311–21. doi: 10.1111/j.1365-2265.2008.03369.x. [DOI] [PubMed] [Google Scholar]
- 8.Guzick DS. Treating the polycystic ovary syndrome the old-fashioned way. N Engl J Med. 2007;356:622–4. doi: 10.1056/NEJMe068295. [DOI] [PubMed] [Google Scholar]
- 9.Nestler JE. Metformin in the treatment of infertility in polycystic ovarian syndrome: an alternative perspective. Fertil Steril. 2008;90:14–6. doi: 10.1016/j.fertnstert.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbieri RL. Clomiphene versus metformin for ovulation induction in polycystic ovary syndrome: the winner is. J Clin Endocrinol Metab. 2007;92:3399–401. doi: 10.1210/jc.2007-1393. [DOI] [PubMed] [Google Scholar]
- 11.Moll E, van der Veen F, van Wely M. The role of metformin in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2007;13:527–37. doi: 10.1093/humupd/dmm026. [DOI] [PubMed] [Google Scholar]
- 12.Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009:CD002249. doi: 10.1002/14651858.CD002249.pub4. [DOI] [PubMed] [Google Scholar]
- 13.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. :CD003053. doi: 10.1002/14651858.CD003053.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratchford AM, Chang AS, Chi MM, Sheridan R, Moley KH. Maternal diabetes adversely affects AMP-activated protein kinase activity and cellular metabolism in murine oocytes. Am J Physiol Endocrinol Metab. 2007;293:E1198–206. doi: 10.1152/ajpendo.00097.2007. [DOI] [PubMed] [Google Scholar]
- 15.Rice S, Pellatt L, Ramanathan K, Whitehead SA, Mason HD. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology. 2009;150:4794–801. doi: 10.1210/en.2009-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers ER, Silva SG, Hafley G, Kunselman AR, Nestler JE, Legro RS. Estimating live birth rates after ovulation induction in polycystic ovary syndrome: sample size calculations for the pregnancy in polycystic ovary syndrome trial. Contemp Clin Trials. 2005;26:271–80. doi: 10.1016/j.cct.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Use of insulin-sensitizing agents in the treatment of polycystic ovary syndrome. Fertil Steril. 2008;90:S69–73. doi: 10.1016/j.fertnstert.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Hwu YM, Lin SY, Huang WY, Lin MH, Lee RK. Ultra-short metformin pretreatment for clomiphene citrate-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2005;90:39–43. doi: 10.1016/j.ijgo.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Malkawi HY, Qublan HS. The effect of metformin plus clomiphene citrate on ovulation and pregnancy rates in clomiphene-resistant women with polycystic ovary syndrome. Saudi Med J. 2002;23:663–6. [PubMed] [Google Scholar]
- 21.Sturrock ND, Lannon B, Fay TN. Metformin does not enhance ovulation induction in clomiphene resistant polycystic ovary syndrome in clinical practice. Br J Clin Pharmacol. 2002;53:469–73. doi: 10.1046/j.1365-2125.2002.01575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandermolen DT, Ratts VS, Evans WS, Stovall DW, Kauma SW, Nestler JE. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril. 2001;75:310–5. doi: 10.1016/s0015-0282(00)01675-7. [DOI] [PubMed] [Google Scholar]
- 23.El-Biely MM, Habba M. The use of metformin to augment the induction of ovulation in obese infertile patients with polycsytic ovary syndrome. Middle East Fertility Society Journal. 2001;6:43–9. [Google Scholar]
- 24.Glueck CJ, Pranikoff J, Aregawi D, Wang P. Prevention of gestational diabetes by metformin plus diet in patients with polycystic ovary syndrome. Fertil Steril. 2008;89:625–34. doi: 10.1016/j.fertnstert.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:524–9. doi: 10.1210/jcem.87.2.8207. [DOI] [PubMed] [Google Scholar]
- 26.Eng GS, Sheridan RA, Wyman A, Chi MM, Bibee KP, Jungheim ES, et al. AMP kinase activation increases glucose uptake, decreases apoptosis, and improves pregnancy outcome in embryos exposed to high IGF-I concentrations. Diabetes. 2007;56:2228–34. doi: 10.2337/db07-0074. [DOI] [PubMed] [Google Scholar]
- 27.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–15. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 28.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 29.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Ovulatory response to treatment of polycystic ovary syndrome is associated with a polymorphism in the STK11 gene. J Clin Endocrinol Metab. 2008;93:792–800. doi: 10.1210/jc.2007-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan GL, Zhang SH, Dokras A, Syrop CH, Van Voorhis BJ. The desire of infertile patients for multiple births. Fertil Steril. 2004;81:500–4. doi: 10.1016/j.fertnstert.2003.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Child TJ, Henderson AM, Tan SL. The desire for multiple pregnancy in male and female infertility patients. Hum Reprod. 2004;19:558–61. doi: 10.1093/humrep/deh097. [DOI] [PubMed] [Google Scholar]
- 32.Gleicher N, Barad D. Twin pregnancy, contrary to consensus, is a desirable outcome in infertility. Fertil Steril. 2009;91:2426–31. doi: 10.1016/j.fertnstert.2008.02.160. [DOI] [PubMed] [Google Scholar]
- 33.http://www.nichd.nig.gov/research/supported/rmn.cfm. In. Vol. 2009.
- 34.Harborne L, Fleming R, Lyall H, Norman J, Sattar N. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet. 2003;361:1894–901. doi: 10.1016/S0140-6736(03)13493-9. [DOI] [PubMed] [Google Scholar]
- 35.Cudmore DW, Tupper WR. Induction of ovulation with clomiphene citrate. A double-blind study. Fertil Steril. 1966;17:363–73. doi: 10.1016/s0015-0282(16)35947-7. [DOI] [PubMed] [Google Scholar]
- 36.Garcia CR, Freeman EW, Rickels K, Wu C, Scholl G, Galle PC, et al. Behavioral and emotional factors and treatment responses in a study of anovulatory infertile women. Fertil Steril. 1985;44:478–83. doi: 10.1016/s0015-0282(16)48915-6. [DOI] [PubMed] [Google Scholar]
- 37.Johnson JE, Jr, Cohen MR, Goldfarb AF, Rakoff AE, Kistner RW, Plotz EJ, et al. The efficacy of clomiphene citrate for induction of ovulation. A controlled study. Int J Fertil. 1966;11:265–70. [PubMed] [Google Scholar]
- 38.Singh I, B MA, Hatwal A, Agarwal A. Increased pregnancy with metformin and clomiphene citrate in non-obese patients with polycystic ovary syndrome: prospective randomized study. Fertil Steril. 2001;76:S94. [Google Scholar]
- 39.Raja A, Hashmi SN, Sultana N, Rashid H. Presentation of polycystic ovary syndrome and its management with clomiphene alone and in combination with metformin. J Ayub Med Coll Abbottabad. 2005;17:50–3. [PubMed] [Google Scholar]
- 40.Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo-controlled, double-blind multicentre study. Hum Reprod. 2006;21:80–9. doi: 10.1093/humrep/dei311. [DOI] [PubMed] [Google Scholar]
- 41.Fleming R, Hopkinson ZE, Wallace AM, Greer IA, Sattar N. Ovarian function and metabolic factors in women with oligomenorrhea treated with metformin in a randomized double blind placebo-controlled trial. J Clin Endocrinol Metab. 2002;87:569–74. doi: 10.1210/jcem.87.2.8261. [DOI] [PubMed] [Google Scholar]
- 42.Koivunen R, Pouta A, Franks S, Martikainen H, Sovio U, Hartikainen AL, et al. Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Hum Reprod. 2008;23:2134–9. doi: 10.1093/humrep/den136. [DOI] [PubMed] [Google Scholar]
- 43.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–9. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]