Abstract
Galectin-1 (Gal-1), a β-galactoside–binding lectin, plays a profound role in modulating adaptive immune responses by altering the phenotype and fate of T cells. Experimental data showing recombinant Gal-1 (rGal-1) efficacy on T cell viability and cytokine production, nevertheless, is controversial due to the necessity of using stabilizing chemicals to help retain Gal-1 structure and function. To address this drawback, we developed a mouse Gal-1 human Ig chimera (Gal-1hFc) that did not need chemical stabilization for Gal-1 ligand recognition, apoptosis induction, and cytokine modulation in a variety of leukocyte models. At high concentrations, Gal-1hFc induced apoptosis in Gal-1 ligand+ Th1 and Th17 cells, leukemic cells, and granulocytes from synovial fluids of patients with rheumatoid arthritis. Importantly, at low, more physiologic concentrations, Gal-1hFc retained its homodimeric form without losing functionality. Not only did Gal-1hFc–binding trigger IL-10 and Th2 cytokine expression in activated T cells, but members of the CD28 family and several other immunomodulatory molecules were upregulated. In a mouse model of contact hypersensitivity, we found that a non-Fc receptor-binding isoform of Gal-1hFc, Gal-1hFc2, alleviated T cell-dependent inflammation by increasing IL-4+, IL-10+, TGF-β +, and CD25high/FoxP3+ T cells, and by decreasing IFN-γ + and IL-17+ T cells. Moreover, in human skin-resident T cell cultures, Gal-1hFc diminished IL-17+ T cells and increased IL-4+ and IL-10+ T cells. Gal-1hFc will not only be a useful new tool for investigating the role of Gal-1 ligands in leukocyte death and cytokine stimulation, but for studying how Gal-1–Gal-1 ligand binding shapes the intensity of immune responses.
Over the last 20 years, the study of carbohydrate-binding proteins (lectins) and their significance in immunology has intensified. Interestingly, the role of lectin-mediated control mechanisms in immunity has more recently been expanded to include a class of lectins called galectins. Galectins belong to a novel family of carbohydrate-binding proteins with affinity for β-galactosides. Galectin-1 (Gal-1), a 14.3-kDa protein bearing a single carbohydrate recognition domain, has been widely studied in the context of both adaptive and innate immune activities (1–4).
Gal-1 is functionally active in nature as a homodimer, recognizing glycoconjugates exposing polyvalent chains of N-acetyllactosamine type 1 (Galβ1,3GlcNAc) or type 2 (Galβ1,4GlcNAc) disaccharides (5). Gal-1 lines the surfaces of epithelial, stromal, and hematopoietic cells, and is a component of extracellular matrix and basement membrane (6). It mediates homotypic and heterotypic cell adhesion, migration, and growth-regulatory activities (7). In immunity, Gal-1 helps preserve the fetomaternal tolerance (8), promote negative selection of T cells in the thymus (9), establish cancer “immune privilege” sites (10), and induce proapoptotic (or apoptotic) activity in activated leukocytes (11–14). These events appear to be regulated by Gal-1-death–sparing effect on Th2 cells (3, 4, 15), production of IL-10 in regulatory T cells (Tregs) (1, 16–18), and exposure of phosphatidylserine (PS) on Gal-1 ligand-positive leukocytes (1, 4, 19). However, precise mechanisms by which Gal-1 induces selective and dramatic immunoregulatory activities in T cells are still under debate, largely because of controversial data on use of recombinant forms of Gal-1 in immune cell bioassays (12–14).
Because Gal-1 does not require any posttranslational modification, recombinant human Gal-1 (rGal-1) purified from transformed Escherichia coli using lactosyl Sepharose columns has been described (20, 21). Nevertheless, both native Gal-1 and rGal-1 preparations are susceptible to oxidative inactivation; and when concentrations decline to <7 μM, dimeric forms of Gal-1 spontaneously dissociate into monomers with limited binding capacity and functional activity (20). Conversely, Gal-1 can aberrantly oligomerize through disulfide bonding, which also limits its function (22). Reducing agents, such as DTT and 2-ME, are routinely added to confer resistance to oxidative inactivation to avert structural decomposition and functional inactivity. In fact, nearly all mechanistic data on T cell death induction are produced by using these agents, which do sensitize cells to undergo Gal-1–mediated apoptosis (13). Recent reports described increased stability of iodoacetamide-alkylated rGal-1. However, although this preparation promotes PS exposure on human leukocytes in the absence of reducing agents, it does not cause morphologic changes in the nucleus or mitochondria, challenging the paradigm of Gal-1–mediated apoptosis on T cells (13, 14).
Considering the controversy surrounding Gal-1 stabilization approaches, how and to what extent Gal-1 regulates the immune system and induces effector leukocyte death at physiologic concentrations is unsettled (1, 4, 19). To help clarify mechanistic insights of Gal-1–Gal-1 ligand immunoregulatory activity, we produced and characterized a genetically engineered chimeric protein consisting of mouse Gal-1 fused to the Fc region of human IgG1 (Gal-1hFc). Because Gal-1 dimerization was facilitated and sustained through Ig homodimerization, Gal-1hFc was stable and functional at low micromolar concentrations, bound characteristic N-acetyllactosamine moieties, bound leukocyte Gal-1 ligands in flow cytometric and immunohistochemical assays, recognized CD43 in Western blots of Th1 cell lysates, and triggered apoptosis in leukemic cells and in activated mouse and human Th1 and Th17 cell subsets, whereas augmenting levels of IL-4, IL-10, IL-13, CTLA-4, and programmed death (PD)-1 production in T cells. Moreover, analysis of activated T cell supernatants harvested from Gal-1hFc incubations on cytokine arrays showed that a number of anti-inflammatory molecules, including IL-1R antagonist (IL-1ra), IL-4, IL-10, IL-13, soluble ICAM-1 (sICAM-1), and tissue inhibitor of metalloproteinase (TIMP-1), were induced. Binding characterization of Gal-1hFc mutants containing substitutions in the AA-45 and/or AA-69 positions, validated the necessity of these residues for optimal functional activity. To examine Gal-1hFc effects in other effector leukocyte models, we found that granulocytes in inflamed joints of patients with rheumatoid arthritis (RA) were susceptible to Gal-1hFc–mediated cell death. In mice treatment with a non-Fc receptor-binding isoform of Gal-1hFc, Gal-1hFc2, suppressed hapten mediated contact hypersensitivity, by increasing IL-4+, IL-10+, TGF-β+, and CD25high/FoxP3+ T cells and reducing IFN-γ– and IL-17–producing T cells. Importantly, Gal-1hFc and Gal-1hFc2 elicited its T cell skewing, cell death, and cytokine modulation without the use of chemical stabilization approaches. These data support the use of Gal-1hFc and its mutants as new glycobiological tools for dissecting the roles of Gal-1 ligands on various immune processes.
Materials and Methods
Cell lines, Abs, and chemicals
J558L, HL-60, Wehi-3, and PC-3 cells all from American Type Culture Collection (ATCC, Manassas, VA) were maintained in RPMI 1640/10% FBS/1% penicillin/streptomycin (Invitrogen, Carlsbad, CA). Abs included anti-mouse Gal-1 (N16), anti-mouse β-actin and anti-human CD43 mAb (843C; Santa Cruz Biotechnology, Santa Cruz, CA); alkaline phosphatase (AP)-conjugated anti-human Fc and anti-goat IgG (Southern Biotech, Birmingham, AL); allophycocyanin-conjugated anti-human Fc (Jackson Immuno-Research, West Grove, PA); anti-mouse CD43 mAb (1B11), biotin-conjugated anti-mouse CTLA-4 mAb, FITC-labeled anti-mouse CD4, -CD25, and -ICOS mAbs and FITC-Annexin V; anti-human CD19 and -CD177 mAbs; PE–anti-FoxP3, anti-mouse IL-4, –IFN-γ, –IL-10, –IL-13, –IL-17, and allophycocyanin PD-1 mAbs, anti-human IL-13, and propidium iodide (PI; Biolegend, San Diego, CA). Goat IgG, mouse IgG, PE–anti-human CD3; allophycocyanin–anti-human CD3, –IL-10, and -FoxP3 mAbs; PerCP-conjugated anti-human CD8 mAb; PE–anti-human CD25, –TNF-α, and –IL-4 mAbs; FITC-labeled anti-human CD4, CD15, IFN-γ, and CD69 mAbs, and Alexa Fluor 647-conjugated anti-human IL-17 mAb (BD Biosciences, San Diego, CA); PE-labeled anti-human TGF-β and allophycocyanin-conjugated anti-mouse TGF-β mAb (R&D Systems, Minneapolis, MN). Mouse cytokine array and recombinant mouse Gal-1 were purchased from R&D Systems. TRIzol, diethyl pyrocarbonate-treated water, Moloney Murine Leukemia Virus Reverse Transcriptase kit, Platinum PCR SuperMix High Fidelity, DTT, FBS, Zeocin, OPTI-MEM, Lipofectamine 2000, protein-G agarose, Gene-Tailor Site-Directed Mutagenesis kit were from Invitrogen.
Production and purification of Gal-1hFc and its mutants
Mouse Gal-1 was PCR-cloned from C57BL/6 mouse splenocyte cDNA using the following primers: forward 5′-CGACCTCGAGGCCACCCGTCTCTCGGGTGGAGTC-3′, reverse 5′-CCGAGATCTCTCAAAGGCCACGCACTTAATCTT-3′. The PCR product and pFUSE-hIgG1-Fc1 plasmid encoding the Zeocin resistance gene (InvivoGen, San Diego, CA) were digested with XhoI and BglII (New England BioLabs, Ipswich, MA), PCR product was ligated in-frame using the Rapid DNA Ligation Kit (Roche Applied Science, Indianapolis, IN), and resultant plasmid was transformed into DH5α competent cells (Invitrogen) selected on Zeocin plates (InvivoGen). Mouse Gal-1 insert was confirmed by restriction enzyme digestion and DNA sequencing at the Brigham and Women’s Hospital High Throughput Sequencing Facility. J558L murine plasmacytoma cells were transfected using Lipofectamine 2000 and selected with Zeocin (400 μg/ml) for 10 d. Gal-1hFc expression was then examined by RT-PCR and by immunoblotting for Gal-1 and hFc in cell lysates.
Transfected cells were grown in RPMI 1640/10% FBS until a concentration of 3 × 106 cells/ml was achieved. Cells were then washed with cold PBS and resuspended in OPTI-MEM medium supplemented with 1% penicillin/streptomycin and 1% sodium pyruvate for 48 h. Supernatants were then collected and passed twice through a previously equilibrated protein G agarose column (pH 6). After washing with PBS, hFc chimeras were eluted with 8 ml elution buffer (0.1 M glycine pH 2.4) and neutralized with 1 M Tris (pH 9.4). Eluates were concentrated using 10-kDa centrifugal filter tubes (Millipore, Bedford, MA) and dialyzed against PBS.
A mutated Gal-1hFc construct, wherein tryptophan-69 was substituted for a glycine (W69G Gal-1hFc or mGal-1hFc), was produced using the PCR-site directed mutagenesis Gene Tailor System with the following primers: forward 5′-ACCAAGGAAGATGGGACCGGGGGAACCGAACAC-3′, reverse 5′-GGTCCCATCTTCCTTGGTGTTACACACAAT-3′. DH5α-T1r competent cells were transformed with the methylated Gal-1hFc PCR product; plasmid DNA was purified using a Plasmid mini kit and further validated by DNA sequencing. Using the mGal-1hFc plasmid DNA as a template, a double-mutant (W69G H45L Gal-1hFc or dmGal-1hFc) construct, wherein histidine-45 was substituted for a leucine, was produced as described earlier with the following primers: forward 5′-ACAACCTGTGCCTACTCTTCAATCCTCGCT-3′, reverse 5′-GTAGGCACAGGTTGTTGCTGTCTCTTCCCA-3′.
For in vivo studies, we fused mouse Gal-1 cDNA in-frame to the pFUSE-hIgG1e3-Fc2 plasmid (InvivoGen) to produce Gal-1hFc2. This vector carries specific mutations in the Fc domain that prevents Fc receptor-binding, and complement and Ab-mediated cytotoxicity. In brief, we subcloned Gal-1 from the Gal-1hFc vector with the following primers: forward 5′-ATTCGATATCTATGGCCTGTGGTCTGGTCGCCA-3′, reverse 5′-CCGAGATCTCTCAAAGGCCACGCACTTAATCTT-3′, then digested PCR product with EcoRV and BglII, dephosphorylated with shrimp AP, gel purified, and ligated in-frame to EcoRV/BglII digested pFUSE-hIgG1e3-Fc2 vector.
Characterizing carbohydrate-binding specificity of Gal-1hFc
Carbohydrate-binding profiles of Gal-1hFc were performed by the Core H of Consortium for Functional Glycomics using a printed glycan microarray of 442 elements as previously described (23). In brief, Gal-1hFc (200 μg/ml) or control hFc (200 μg/ml) in binding buffer (1% BSA, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.05% Tween 20, and 20 mM Tris-HCL; pH 7.4) was incubated for 1 h at room temperature (RT) on the printed glycan array (Version 4.0) followed by a 1-h incubation with allophycocyanin-conjugated goat F(ab′)2 anti-human Fc (5 μg/ml). After washing to remove excess reagents, the slides were dried and scanned at an excitation wavelength of 633 nm to detect the allophycocyanin fluorophore. The array is composed of glycans printed in replicates of 6, and relative fluorescence was reported as the average of 4 after removal of the highest and lowest point from each set of six replicates. Mean fluorescence intensities of Gal-1hFc binding for each glycan tested were normalized by dividing Gal-1hFc–binding intensities by hFc-binding intensities and graphically represented as mean fold difference. Glycan-binding intensities with standard deviations greater than relative fluorescence unit were not considered. Raw data can be accessed online at: http://www.functionalglycomics.org/glycomics/HServlet?operation=view&sideMenu=no&psId=primscreen_2707.
Gal-1hFc–binding assays
Cells (1 × 106) previously incubated were incubated at 4°C for 45 min with Gal-1hFc or mutants (all at 20 μg/ml) in the presence or absence of 50 mM lactose or sucrose. Then cells were incubated for 30 min at 4°C with allophycocyanin-conjugated goat F(ab′)2 anti-human Fc, and analyzed by flow cytometry in a FACSCanto (BD Biosciences) and then using FlowJo 8.1 (Tree Star, Ashland, OR).
Polarization of mouse Th cells
Naive CD4+ T cells (CD62L+/CD44low) were isolated from 6-wk-old C57BL/6 mouse spleens by immunomagnetic bead-positive selection (Miltenyi Biotec, Auburn, CA) and polarized into Th1, Th2, Th17 subsets, as previously described (4). CD43-deficient C57BL/6 mice were generously provided by Dr. Hermann Ziltener (University of British Columbia, Vancouver, British Columbia, Canada). In brief, cells were resuspended (1.5 × 106 cells/ml) and stimulated for 48 h with plate-bound anti-CD3 mAb (5 μg/ml) and soluble anti-CD28 mAb (1 μg/ml). For Th1 polarization, 10 ng/ml IL-12 and 40 U/ml anti–IL-4 mAb were added; for Th2 polarization, 20 ng/ml IL-4, 2 μg/ml anti–IL-12 mAb, and 25 U/ml IL-2 were added; for Th17 polarization, 20 ng/ml IL-6, 10 ng/ml IL-23, 5 ng/ml TGF-β1, 10 μg/ml anti–IFN-γ mAb, 10 μg/ml anti–IL-4 mAb, and 10 μg/ml anti–IL-2 mAb were added. Th1 and Th2 cells were expanded with RPMI-1640 supplemented with 25 U/ml IL-2, and Th17 cells were expanded with 10 ng/ml IL-23 for 4 additional days. All cytokines and Abs were purchased from BD Biosciences.
Western blotting and immunoprecipitation
Cell lysates from naive mouse CD4+ T cells, ex vivo-polarized Th1 cells, ex vivo-polarized Th1 cells from CD43−/− mice or KG1a cells, and anti-CD43 mAb (843C) or isotype control (mouse IgG) immunoprecipitates were separated by reducing 4–20% SDS-PAGE gradient gels and transferred to immunoblot membrane (Bio-Rad, Hercules, CA). After a 1-h blocking step with 100% FBS at RT, membranes were incubated overnight with 10 μg/ml Gal-1hFc in TBS at 4°C, washed three times, incubated 30 min with AP–anti-human Fc, extensive washing and development with Western Blue (Promega, Madison, WI). Alternatively, blots containing mouse T cell lysates were probed with anti-CD43 (1B11) or with anti–β-actin mAbs and then with respective AP-secondary Ab and developed with Western Blue. For CD43 immunoprecipitation, 300 μg precleared KG1a lysate was incubated with 2 μg anti-human CD43 (843C) Ab or isotype control Ab and 30 μl protein-G agarose for 2 h on a rotator at 4°C, as previously described (24).
In vivo T cell activation and induction of hapten-dependent contact hypersensitivity
Shaved abdomens of 6-wk-old C57BL/6 mice were painted on days 0 and 1 with 25 μl 0.5% 2,4-dinitro-1-fluorobenzene (DNFB) or with 1% oxazolone (Sigma) in a 4:1 acetone/olive oil vehicle. To assess T cell activation during the afferent phase, we harvested draining inguinal lymph nodes (LNs) on day 3, minced with frosted slides, and cells were stained with Gal-1hFc (± 50 mM lactose) and Abs to CD4 and CD69, and analyzed by flow cytometry. In vivo treatments with Gal-1hFc2, a Gal-1hFc mutant that does not bind Fc receptor, though binds equally well to Gal-1 ligands as Gal-1hFc, were administered i.p. at 2.3 mg/kg on days 2, 4, and 5. Mice were alternatively treated with human Fc at an identical dose, which served as a treatment control. Hapten-induced inflammation was then induced on day 5 by challenging mice with 10 μl 0.25% DNFB or 0.5% oxazolone on both sides of the right ear, and as a negative inflammatory control, mice received 10 μl vehicle alone on both sides of the left ear. Ear swelling responses were determined by calculating the difference in ear thickness between days 5 and 6. To assess T cell cytokine levels, we harvested Ag-draining inguinal LNs and minced with frosted slides; then lymphocytes were activated with PMA/ionomycin in the presence of brefeldin A for 6 h, stained for surface markers or intracellular cytokines, and analyzed by flow cytometry. After a 24-h challenge, ears were harvested, fixed in 10% formalin, embedded in paraffin, sectioned, and stained with H&E.
Mouse T cell cytokine analysis
Naive CD4+ T cells (CD62L+/CD44low) isolated from 6-wk-old C57/BL6 mouse spleens by immunomagnetic bead technology (Miltenyi Biotec) were stimulated with plate-bound anti-mouse CD3 and soluble anti-mouse CD28 for 48 h. Then, after 24-h incubation with Gal-1hFc or its controls, supernatants were collected in triplicate and analyzed for cytokine levels by using a mouse cytokine array (R&D Systems). Blots were processed as indicated by the manufacturer and developed by chemiluminescence. Signal intensities were analyzed by optic densitometry (OD), using ImageJ software. OD values were normalized to control hFc-treated group. In parallel, cells were stimulated with PMA/ionomycin and brefeldin A for 6 h to analyze intracellular cytokine levels by flow cytometry.
Cell death assays
Synovial fluid cells, HL-60 cells, or polarized mouse Th cell subsets (1.5 × 106 cells/ml) were incubated with Gal-1hFc at 10 or 100 μg/ml (±50 mM lactose) or with molecular controls for 12 or 24 h in RPMI 1640/10% FBS/1% penicillin/streptomycin. Apoptotic cells were identified by staining with FITC-conjugated Annexin V and PI. Cell viability was also determined by trypan blue dye exclusion and forward/side scatter plots.
Real-time RT-PCR
Activated mouse Th cells were treated for 8 h with 0.25 μM Gal-1hFc or relevant controls. Total RNA extraction and cDNA conversion were performed as previously described (24). SYBR green PCR was performed using the following primers: GATA-3, forward 5′-GGTTGCCAAGCCTTATCGGA-3′, reverse 5′-ACCTGCTCCACTGCCTTGCT-3′; IL-10, forward 5′-CTAACCATAAAATGAATGGGCAG-3′, reverse 5′-CTCTCCTTGCTGCTGCCGACAG-3′; T-bet, forward 5′-GTTCCCATTCCTGTCCTT-3′, reverse 5′-CCTTGTTGTTGGTGAGCTT-3′; and β-actin, forward: 5′-CATCGTACTCCTGCTTGCTG-3′, reverse 5′-AGCGCAAGTACTCTGTGTGG-3′. Samples were analyzed in triplicate and normalized to β-actin expression.
Human skin-explant T cell cultures
Normal healthy skin discarded from cosmetic surgeries was cultured on cell foam three-dimensional growth matrices in the presence of IL-2 and IL-15 for 21 d, as previously described (25). T cells were harvested from the matrices, washed with cold PBS, and cultured for 24 h in the presence of Gal-1hFc (±50 mM lactose) or its molecular controls. Cells were stimulated with PMA/ionomycin and brefeldin A for 6 h before performing surface marker/cytokine/FoxP3 staining and FACS analysis.
Human synovial fluid and leukocyte analysis
Human knee synovial fluids were obtained as discarded material from patients with RA undergoing diagnostic or therapeutic arthrocentesis. RA was diagnosed by an American Board of Internal Medicine certified rheumatologist and/or by review of laboratory, radiologic, and clinic notes and by applying American College of Rheumatology classification criteria (26). All studies received Institutional Review Board approval. For FACS analyses and functional studies of cells contained in freshly collected RA synovial fluids, cells were washed in cold PBS, stained with trypan blue for cell death exclusion, adjusted to 1 × 106 cells/100 μl FACS buffer, incubated for 20 min in FcγR-binding block, and then subsequently stained for surface markers and analyzed by flow cytometry.
Immunohistochemistry
For Gal-1hFc immunohistochemical analysis, we used Gal-1 ligand+ Wehi-3 and HL-60 cells fixed in 10% formalin and embedded in paraffin as models. Four-micrometer sections were deparaffinized, rehydrated, and then subjected to Ag retrieval in 1 mM EDTA (pH 8.0) in a cloaking chamber for 2 min. Sections were then blocked in hydrogen peroxide and 10% FBS, and incubated with 10 μg/ml Gal-1hFc, dmGal-1hFc, or hFc for 1 h at RT. After washing, sections were incubated with HRP-rabbit-anti-hIgG (1:2000; Abcam) for 30 min at RT. Staining was performed using standardized development times and substrate, diaminobenzidine. All sections were counterstained in hematoxylin.
Statistical analyses
Statistical significance was ascertained between groups using a paired t test.
Results
Gal-1hFc functions as an authentic Gal-1 ligand probe
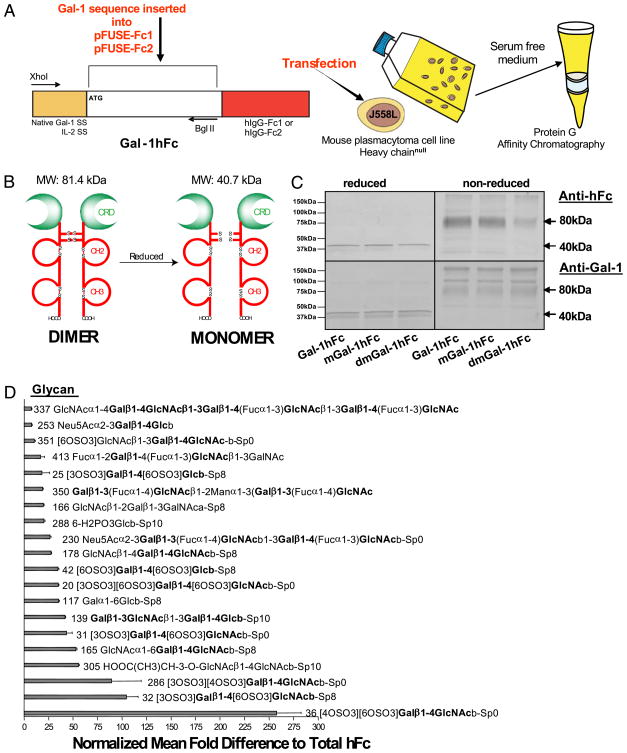
Using chemical stabilization to help promote Gal-1 dimers from a monomer/dimer/oligomer equilibrium is critical in Gal-1 research. Inclusion of DTT in rGal-1 preparations could potentially bias cell death-related conclusions and even underestimate Gal-1’s effects on cytokine modulation. To circumvent this pitfall, we engineered a chimeric protein, wherein mouse Gal-1 cDNA was linked in-frame to the Fc portion of human IgG1 using the pFUSE-hIgG1-Fc1 expression system (Gal-1hFc; Fig. 1A). Moreover, to control for carbohydrate-binding activity of Gal-1hFc, we created genetic mutants in which a key tryptophan residue for carbohydrate recognition via van der Waals interactions in the 69th amino acid position was substituted for a glycine (mGal-1hFc), and a histidine also important for carbohydrate-binding through the formation of hydrogen bonds in the 45th position was substituted for a leucine (dmGal-1hFc; Supplemental Fig. 1). Because J558L murine plasmacytoma cells are capable of producing greater amounts of ectopic fusion protein compared with CHO or HEK293 cells (27), J558L cells were transfected, drug-selected, subcloned, and assayed for Gal-1hFc expression by RT-PCR and Western blotting. Stable clones secreted a Gal-1hFc and mutant protein, which was isolated by protein G affinity chromatography and resolved at the predicted sizes of 40.7 and 81.4 kDa, under reducing and nonreducing conditions, respectively (Fig. 1A–C). To characterize specificity of glycan recognition, we assayed Gal-1hFc binding on printed glycan arrays containing 442 immobilized glycans with the assistance of Core H and the Consortium for Functional Glycomics. Because of the relative carbohydrate-binding properties of human IgG (28), we normalized Gal-1hFc binding to that of total human Fc. As expected, Gal-1hFc exhibited highest affinity for structures bearing type 1 (Galβ1,3) or type 2 (Galβ1,4) N-acetyllactosamine moieties (Fig. 1D). Interestingly, Gal-1hFc also bound sulfated lactosamine structures similar to a human Gal-1-Ig chimeric molecule previously reported (23) and bound glycans containing α2,3 sialylated N-acetyllactosamine units as reported for a covalently linked dimeric Gal-1 preparation (29). Gal-1hFc also did not bind α2,6 sialylated structures, similar to rGal-1 forms tested and reported by Core H of the Consortium for Functional Glycomics.
FIGURE 1.
Construction and purification of Gal-1hFc and its mutants. A, Mouse Gal-1 cDNA containing native signal sequence (SS) or IL-2-SS was ligated in-frame into commercially available vector encoding the Fc region of IgG1 (pFUSE-Fc1) or the non-Fc receptor-binding mutant (pFUSE-Fc2), respectively. Purified plasmid DNA was transfected into J558L mouse plasmacytoma cells, drug selected and grown in serum-free medium. Gal-1hFc was purified by protein-G affinity chromatography. B, Schematic representation of Gal-1hFc in its reduced and nonreduced forms. C, Purified Gal-1hFc and its mutants were analyzed by SDS-PAGE and Western blotting with anti-human Fc or anti-mouse Gal-1 mAbs. D, Gal-1hFc or hFc was incubated on a covalent printed glycan array (version 4.0) developed by Core H investigators of the Consortium for Functional Glycomics. Mean fluorescence intensities of Gal-1hFc binding were normalized by dividing Gal-1hFc fluorescence intensities by control hFc-binding intensities and graphed as mean fold difference. The top 20 normalized glycans are listed.
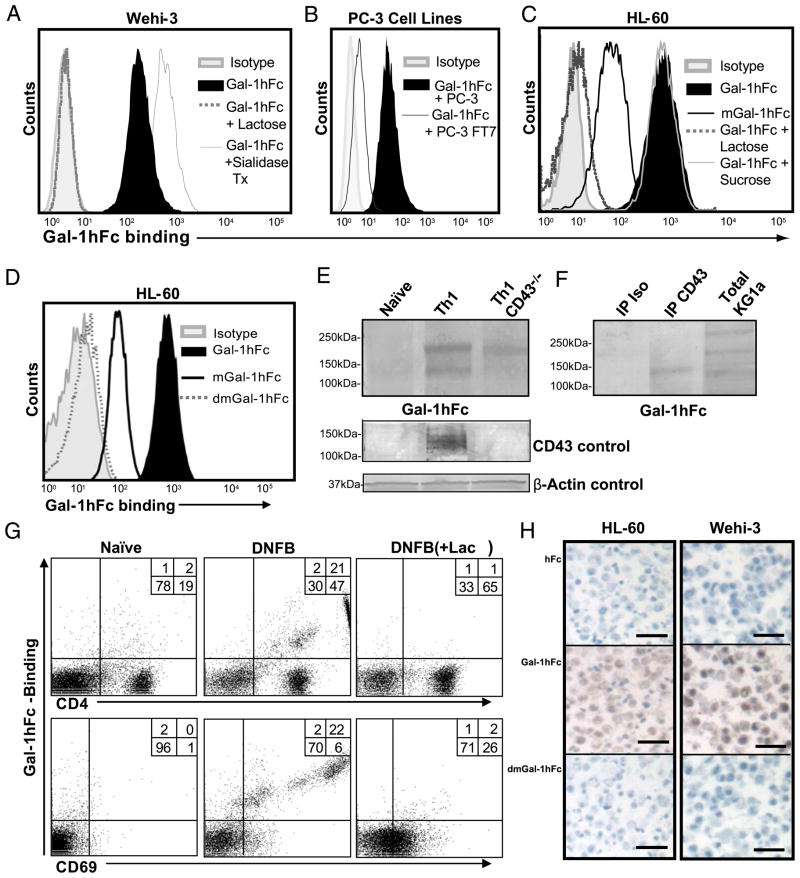
To further validate Gal-1hFc–binding function, we performed binding assays using mouse and human hematopoietic cells and solid tumor cell lines. Gal-1hFc–bound mouse WEHI-3 and human HL-60 leukemic cell lines and binding were inhibited by 50 mM lactose, but not sucrose (Fig. 2A, 2C). Importantly, removal of terminal sialic acid residues by neuraminidase treatment enhanced WEHI-3 binding, whereas binding to human PC-3 prostate cancer cells overexpressing α1,3 fucosyltransferase 7 (30) was diminished compared with parental cells, validating published data on Gal-1 glycan-binding properties (29) (Fig. 2B). Binding of mGal-1hFc to HL-60 cells was diminished by ~50% (Fig. 2D) (31, 32), whereas dmGal-1hFc binding was completely abolished (Fig. 2D).
FIGURE 2.
Carbohydrate-binding activity of Gal-1hFc and its mutants to hematopoietic and nonhematopoietic cells by flow cytometry, Western blotting, and immunohistochemistry. A, Gal-1hFc binding to Wehi-3 cells or cells treated with Vibrio cholerae sialidase (0.2 U/ml) for 30 min at 37°C was assessed in the presence or absence of 50 mM lactose. B, Gal-1hFc binding was assayed on PC-3 cells and on PC-3 α1,3 fucosyltransferase 7 (FT7) transfectants (24). C and D, Gal-1hFc, mGal-1hFc, and dmGal-1hFc binding to HL-60 cells was assayed in the presence or absence of 50 mM lactose or sucrose. E, Lysates (30 μg/lane) from naive Th cells or polarized Th1 cells isolated from wt or CD43−/− mice were subjected to reducing 4–20% SDS-PAGE gels, blotted with Gal-1hFc, anti-CD43 mAb (1B11), or anti–β-actin mAb and then with respective AP-secondary Ab. F, KG1a cell lysate (30 μg/lane) and anti-CD43 and isotype control immunoprecipitates from KG1a cells were separated by 4–20% reducing SDS-PAGE gradient gels and then blotted with Gal-1hFc and AP–anti-hFc. G, Lymphocytes from LNs draining DNFB-sensitized or naive skin were analyzed by flow cytometry with Gal-1hFc, and anti-CD4 and -CD69 mAbs. Lactose (+ lac) was added to assay and washing buffers to control for carbohydrate-mediated binding. H, Sections of paraffin-embedded, formalin-fixed HL-60 or Wehi-3 cells were immunostained with 10 μg/ml Gal-1hFc or controls (hFc and dmGal-1hFc). Scale bars, 20 μm. Original magnification ×20. All data are representative of at least three experiments.
Although CD43 is a well-recognized Gal-1 ligand on human leukemic cell lines and human PBMCs (11, 33), it has yet to be validated on mouse Th cells. Using Gal-1hFc as a Gal-1 ligand probe, we incubated Western blots of whole-cell lysates from naive CD4+ T cells and ex vivo-polarized Th1 cells from wild type (wt) or CD43-deficient mice with 10 μg/ml Gal-1hFc. Notably, whereas AP–anti-hFc alone or dmGal-1hFc control incubations did not show any staining activity (data not shown), Gal-1hFc recognized two prominent bands at ~200 and ~140 kDa in wt Th1 cell lysates (Fig. 2E). However, Gal-1hFc binding to the 140-kDa band in CD43−/− Th1 cell lysate or in naive Th lysate was not observed, indicating that CD43 is a putative Gal-1 ligand on Th1 cells (Fig. 2E). To confirm the absence of CD43 in CD43−/− Th1 cell lysate or in naive Th lysate, we blotted the activated form of CD43 containing core-2 O-glycans with anti-CD43 mAb 1B11 and found that core 2 O-glycan–bearing CD43 ranging from 120–140 kDa was expressed only in wt Th1 cell lysate (Fig. 2E). To validate reactivity of CD43 to Gal-1hFc, we immunoprecipitated a heavily glycosylated glycoform of CD43 from human leukemic KG1a cells and then probed Western blots of anti-CD43 or isotype control immunoprecipitates with Gal-1hFc and found that immunoprecipitated CD43 was reactive to Gal-1hFc (Fig. 2F).
To substantiate poor Gal-1hFc–binding activity to naive CD4+ T cells and robust binding to activated Th cells, we used an in vivo T cell activation protocol (34). Accordingly, we sensitized abdominal mouse skin with 0.5% DNFB or vehicle control, harvested skin-draining inguinal LNs after 3 d, stained lymphocytes with Gal-1hFc (±50 mM lactose), anti-CD4 mAb, and anti-early activation marker CD69 mAb, and analyzed by flow cytometry. Results showed that CD69+ and CD4+ cells from LNs draining DNFB-sensitized skin bound Gal-1hFc in a β-galactoside–dependent manner, whereas cells from LNs draining naive skin did not bind Gal-1hFc (Fig. 2G).
To further demonstrate Gal-1hFc binding to Gal-1 ligands, we performed immunohistochemical studies using Gal-1hFc or controls, hFc, and dmGal-1hFc to stain sections of formalin-fixed, paraffin-embedded Gal-1 ligand+ HL-60 and Wehi-3 cells. At 10 μg/ml Gal-hFc, we observed robust cellular staining, whereas incubations with 10 μg/ml hFc or dmGal-1 did not show any staining activity (Fig. 2H).
Gal-1hFc induces cell death of human HL-60 leukemic cells
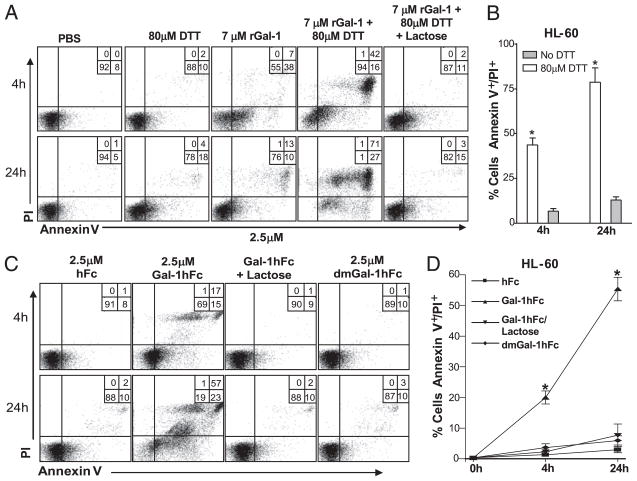
It is widely accepted that exogenous rGal-1 induces apoptosis on activated T cells and several hematopoietic cell lines after a short 4-h exposure (11). However, more recently, this paradigm has been challenged, arguing that DTT, a reducing agent commonly added to culture conditions to prevent rGal-1 oxidative inactivation during cell death assays, is itself able to induce apoptosis. Moreover, studies show that, although monomeric Gal-1 is unable to induce apoptosis, dimeric Gal-1 can induce transient PS exposure in leukocytes that is reversible on Gal-1 removal or oxidative inactivation of the carbohydrate-recognition domain after a short 4-h incubation (13, 35). Importantly, data suggest that activated human leukocytes are resistant to Gal-1–mediated PS exposure when no DTT is present, and that PS exposure does not lead to changes in the integrity of the plasma membrane, mitochondrial potential, or nuclei, a scenario referred to as preaparesis (14). To help clarify these conflicting data, we compared Gal-1hFc with rGal-1 efficacy on cell death induction using human HL-60 leukemic cells. Incubations were performed with 7 μM rGal-1 in the presence or absence of DTT, or with 2.5 μM Gal-1hFc for a period of 4 or 24 h. At 4 h, rGal-1 caused marked PS exposure, as evidenced by Annexin V positivity (or PS exposure), though PI staining was minimally increased (Fig. 3A, 3B). In contrast, inclusion of 80 μM DTT enhanced apoptotic potential, as PI positivity accompanied PS exposure (Fig. 3A, 3B). At 24 h, rGal-1 and DTT caused most HL-60 cells to exhibit Annexin V and PI positivity, whereas rGal-1 incubation alone did not further enhance, or even sustain, its proapoptotic potential. In contrast, Gal-1hFc alone induced both PS exposure and PI uptake in as early as 4 h and was sustained and enhanced over a 24-h period in the absence of DTT (Fig. 3C, 3D). These effects were averted by addition of lactose or by using dmGal-1hFc. These results suggested that Gal-1hFc can engage cell surface ligands and trigger irreversible cell death.
FIGURE 3.
Comparative analysis of apoptosis induction in promyeloleukemic HL-60 cells using rGal-1 and Gal-1hFc. A, HL-60 cells were incubated with rGal-1 in the presence or absence of DTT and/or 50 mM lactose. Cell death analysis (Annexin V staining/PI uptake) was evaluated by flow cytometry at 4 and 24 h after incubation. B, Graphical representation of data from three different experiments depicting mean Annexin V+ staining/PI+ uptake values (±SD). *Statistically significant difference compared with no DTT control, p ≤ 0.01. C, HL-60 cells were incubated with Gal-1hFc or dmGal-1hFc in the presence or absence of 50 mM lactose. D, Graphical representation of data from three different experiments depicting mean Annexin V+ staining/PI+ uptake values (±SD) at 4 and 24 h after incubation. *Statistically significant difference compared with hFc control, p ≤ 0.01.
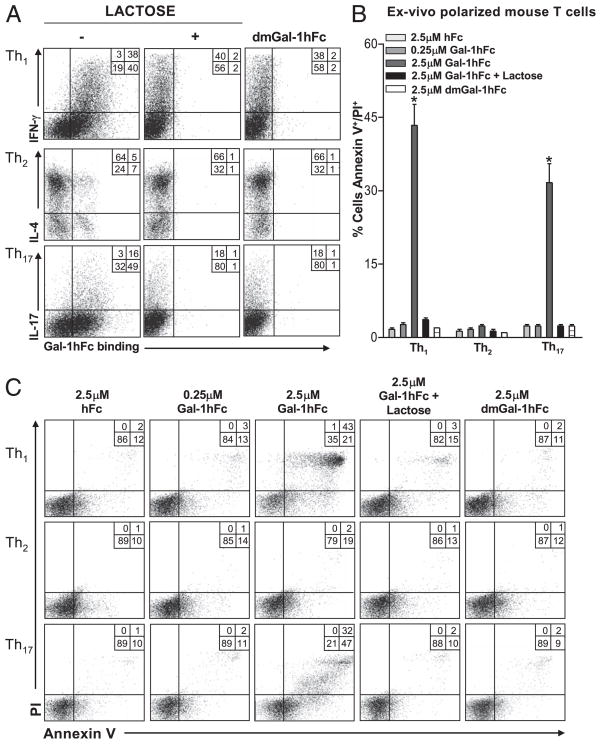
Th1 and Th17 cells are susceptible to Gal-1hFc–mediated apoptosis
Proinflammatory Th1 and Th17 cell subsets share common glycan motifs, such as abundant asialocore-1 O-glycans, together with small amounts of sialic acid α2,6-galactose residues on their surfaces (4). To determine whether Gal-1hFc can bind these Th subsets, we polarized mouse Th1, Th2, and Th17 subsets ex vivo, confirmed their identity by intracellular cytokine staining, and assayed for Gal-1hFc–binding activity. IFN-γ+ Th1 and IL-17+ Th17 cells expressed high levels of Gal-1 ligands as determined by Gal-1hFc binding, whereas only a small percentage of IL-4+ Th2 cells (5%) bound Gal-1hFc as previously described (Fig. 4A) (3). Furthermore, compared with hFc, incubating Gal-1hFc with Th1 and Th17 cell cultures for 24 h decreased their viability as observed by analysis of Annexin V staining and PI uptake in a dose-dependent manner (Fig. 4B, 4C). To the contrary, Th2 or Th1/Th17 cells incubated with dmGal-1hFc or Gal-1hFc with lactose were largely resistant to cell death (Fig. 4B, 4C). These cell death-inducing results with Gal-1-hFc on Th1 and Th17 cells, and not on Th2 cells, parallel effects caused by rGal-1 (4).
FIGURE 4.
Galectin-1 Gal-1hFc induces apoptosis in Th1 and Th17 cell subsets. A, Gal-1hFc binding was assessed on mouse ex vivo polarized Th cell subsets, and Th cell phenotype was assayed by intracellular cytokine staining for IL-4, IFN-γ, and IL-17. dmGal-1hFc and Gal-1hFc binding was performed in the presence or absence of lactose. B, Graphical representations of data from three experiments showing apoptosis (Annexin V+ and PI+) in Th1, Th2, and Th17 cell subsets after a 24-h treatment with 2.5 μM Gal-1hFc in the presence or absence of 50 mM lactose or dmGal-1hFc. *Statistically significant difference compared with hFc control, p ≤ 0.01. C, Representative FACS plots of Gal-1hFc–mediated apoptosis (Annexin V+ and PI+) on Th cell subsets are shown.
Gal-1hFc induces T cell immunomodulatory molecules and alters T cell differentiation
Typically, when investigating proapoptotic effects, high concentrations of Gal-1 (>7 μM) with DTT are needed to ensure dimerization and a functional lectin domain. Although DTT helps prevent intramolecular disulfide bond formation and retain carbohydrate-binding activity, they may favor monomer formation, which are significantly less avid than native homodimers. This biochemical relationship could, therefore, undermine Gal-1’s effects on other non–death-related pathways at lower, more physiologic concentrations. Some insights on Gal-1–related cytokine modulation have been gleaned from studies of the pathophysiology of Hodgkin’s lymphoma, and from using mouse models of autoimmune diseases wherein rGal-1 treatment upregulates Th2 cytokines and IL-10 (15, 16). Similarly, studies using a leucine zipper-based Gal-1 homodimer showed 100-fold more secreted IL-10 than rGal-1 when incubated with human PBMCs (17, 36).
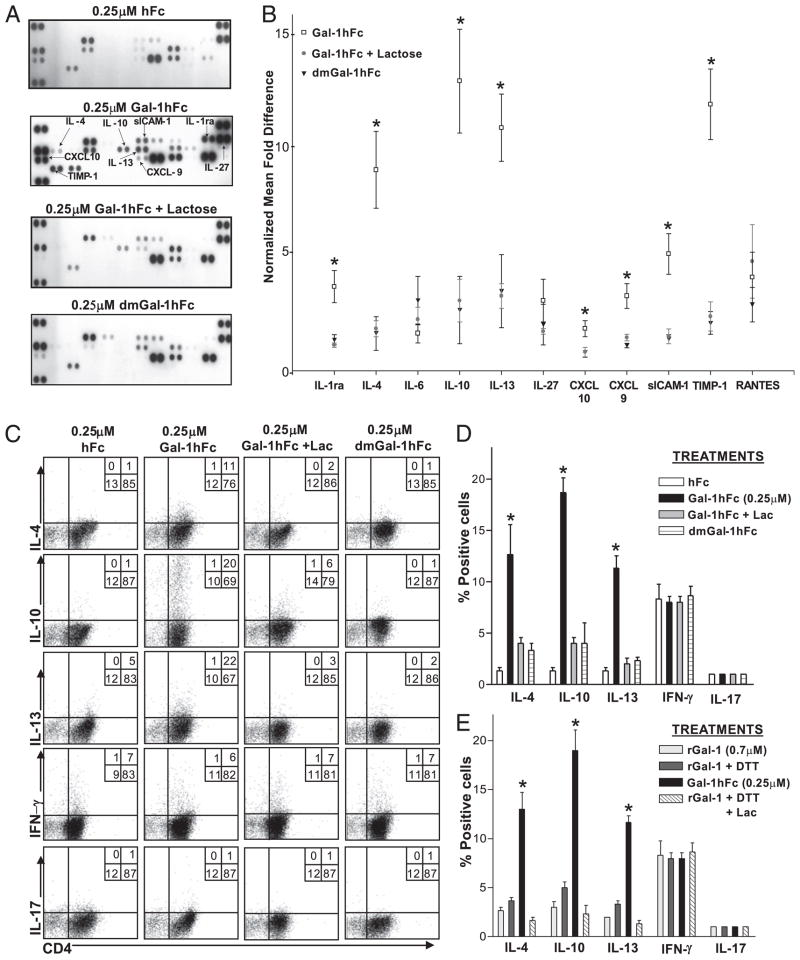
To expand on Gal-1–induced upregulation of IL-10, we investigated Gal-1hFc effects on a number of cytokines and immunomodulatory molecules in mouse and human T cells. At 10-fold less Gal-1hFc (0.25 μM) used in death assays, activated mouse Th cells still avidly bound Gal-1hFc, and naive T cells did not (Supplemental Fig. 2A). Using this concentration of Gal-1hFc, we activated sorted CD62L+/CD44low naive CD4+ T cells (Supplemental Fig. 2B) with anti-CD3/CD28 mAb and incubated them for 24 h with Gal-1hFc. Lectin-binding controls, dmGal-1hFc, Gal-1hFc with lactose, or hFc at equal concentrations were also used in this assay. Supernatants were collected and examined for the expression of 40 different cytokines and other immunologic molecules by immunoblotting (Supplemental Fig. 2C). Blots were scanned by OD, and signal levels were normalized to hFc staining levels. Results showed that Gal-1hFc triggered production/secretion of a number of immunoregulatory molecules, including IL-10, IL-1ra, sICAM-1, Th2 cytokines IL-4 and IL-13, chemokines CXCL-10 and RANTES, and anti-invasion molecule TIMP-1 in a Gal-1–dependent manner (Fig. 5A, 5B). Similarly, validation by intracellular cytokine analysis showed that Gal-1hFc enhanced expression of IL-4, IL-10, and IL-13 with little effect on IFN-γ and IL-17 production (Fig. 5C, 5D). Experiments were performed in parallel using (0.7 μM) rGal-1 with or without DTT or lactose and showed that rGal-1 did not significantly upregulate the expression of similar cytokines compared with Gal-1hFc (p < 0.01; Fig. 5E, Supplemental Fig. 2D).
FIGURE 5.
Gal-1hFc stimulates the secretion of immunoregulatory molecules and alters Th cell differentiation. A, Naive Th cells were isolated by immunomagnetic beads from mouse spleens and activated for 48 h with anti-CD3/CD28, and further incubated for an additional 24 h with Gal-1hFc (±50 mM lactose), control hFc, or dmGal-1hFc. Supernatants were collected and analyzed for expression of 40 cytokines with a mouse cytokine panel array kit and quantified by OD, and mean densities were normalized to hFc-treated group. The complete list of cytokines and their spatial arrangement in the array are shown in Supplemental Fig. S2C. B, Graphic representation of data from three experiments is shown as normalized mean fold difference. *Statistically significant difference compared with lactose control, p ≤ 0.01. C, Activated Th0 cells incubated with Gal-1hFc (0.25 μM) or controls for 24 h were stimulated with PMA/ionomycin in the presence of brefeldin A for 6 h and then stained with anti–IL-4, –IL-10, –IL-13, –IFN-γ, and –IL-17 mAbs, and analyzed by flow cytometry. D, Graphic representations of data from three experiments are shown. *Statistically significant difference compared with hFc control, p ≤ 0.01. E, Activated Th0 cells were incubated with 0.7 μM rGal-1 with or without 80 μM DTT (± lactose [Lac]) for 24 h, stimulated with PMA/ionomycin in the presence of brefeldin A for 6 h, and then stained with anti–IL-4, –IL-10, –IL-13, –IFN-γ, and –IL-17 mAbs, and analyzed by flow cytometry. Data from three independent experiments are shown. *Statistically significant difference compared with rGal-1, p ≤ 0.01. Representative FACS plots are shown in Supplemental Fig. 2D.
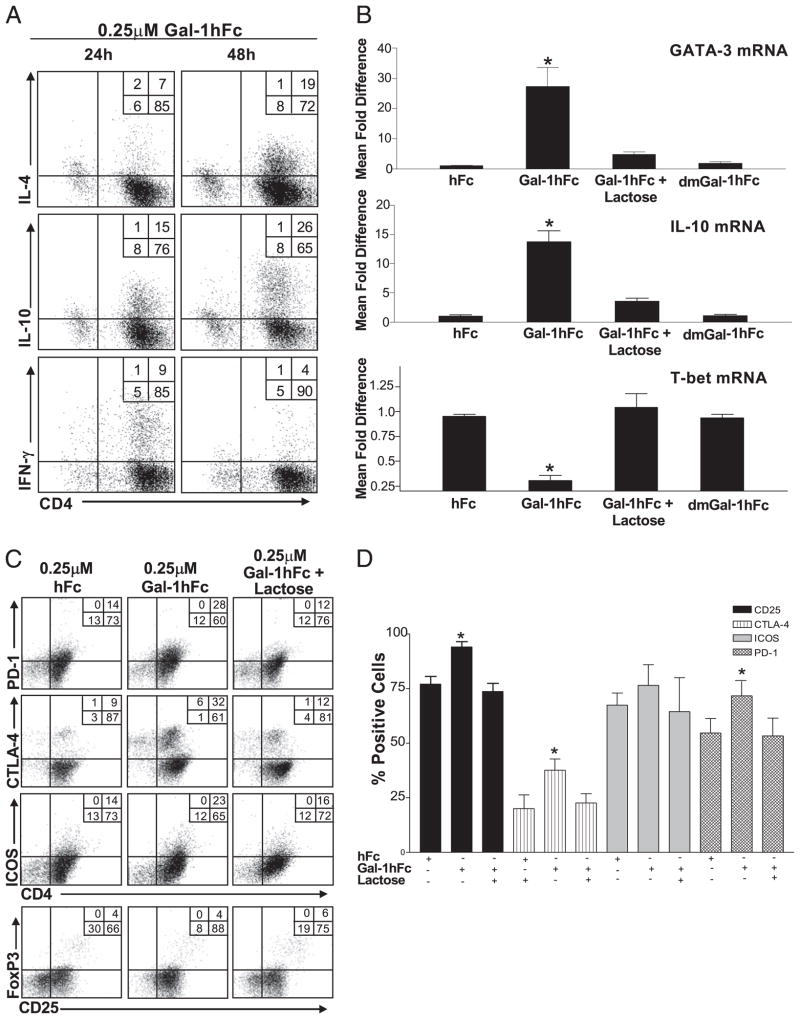
To determine whether Gal-1hFc’s effect on T cell polarization was maintained longer than 24 h, we assayed IL-4, IL-10, and IFN-γ production from Gal-1hFc–treated Th0 cells after 24 and 48 h. Indeed, levels of IL-4+ and IL-10+ cells were nearly doubled, whereas the number of IFN-γ–producing cells was decreased (Fig. 6A). Likewise, mRNA levels of IL-4 transcription activator, GATA-3, of IL-10 and of transcriptional activator in IFN-γ+ T cells, T-bet, changed accordingly after Gal-1hFc treatment (Fig. 6B), confirming that cytokines were synthesized de novo, and that Gal-1hFc can help polarize Th cells. Importantly, at this concentration of Gal-1hFc, expression differences were not attributed to selective apoptosis on Th1 cells in which Gal-1hFc was used at a 10-fold higher concentration (Fig. 4C).
FIGURE 6.
Gal-1hFc alters Th cell differentiation, cytokine production, and expression of regulatory surface molecules. A, Naive mouse Th cells activated with anti-CD3/28 were treated with 0.25 μM Gal-1hFc; and IL-4, IL-10, and IFN-γ for 24 or 48 h were assessed by intracellular cytokine FACS staining. B, Transcriptional activity of GATA-3, IL-10, and T-bet mRNA was analyzed 8 h after incubation with Gal-1hFc by quantitative RT-PCR. Data are expressed as relative mRNA levels normalized to hFc treatment. *Statistically significant difference compared with hFc control, p ≤ 0.01. C, Naive mouse Th cells activated with anti-CD3/28 were incubated for 24 h with 0.25 μM hFc or Gal-1hFc (±50 mM lactose), or stained with anti–CTLA-4, –PD-1, -ICOS, and -CD25 mAbs, and analyzed by flow cytometry. D, Graphic representation of data from three independent experiments. *Statistically significant difference compared with hFc control, p ≤ 0.01.
Gal-1hFc induces overexpression of CTLA-4, PD-1, and CD25 in Th cells
Earlier studies describe a parallel increase in Gal-1 expression and PD-1 and its ligand during the peak and recovery phases of experimental induced encephalomyelitis (1). Similarly, other studies indicate a possible synergistic and coordinated effect between molecules that turn off T cell effector functions and Gal-1 (37). To explore a possible role of Gal-1 in expression of immunoregulatory members of the CD28 family, we analyzed the expression of surface CTLA-4, PD-1, and ICOS in ex vivo-activated mouse T cell cultures after a 24-h incubation with 0.25 μM Gal-1hFc. Interestingly, Gal-1hFc upregulated the expression of CTLA-4 and PD-1 in a carbohydrate-dependent manner, as evidenced by its suppression after inclusion of 50 mM lactose (Fig. 6C, 6D). Expression of ICOS after Gal-1hFc treatment remained unaltered. Although CTLA-4 and PD-1 are associated with the phenotype and function of Tregs (38–40), we found that the levels of CD25high/FoxP3+ cells were relatively unchanged (Fig. 6C, 6E). However, most Gal-1hFc–treated cells showed high levels of CD25 compared with cells from control treatments (Fig. 6D).
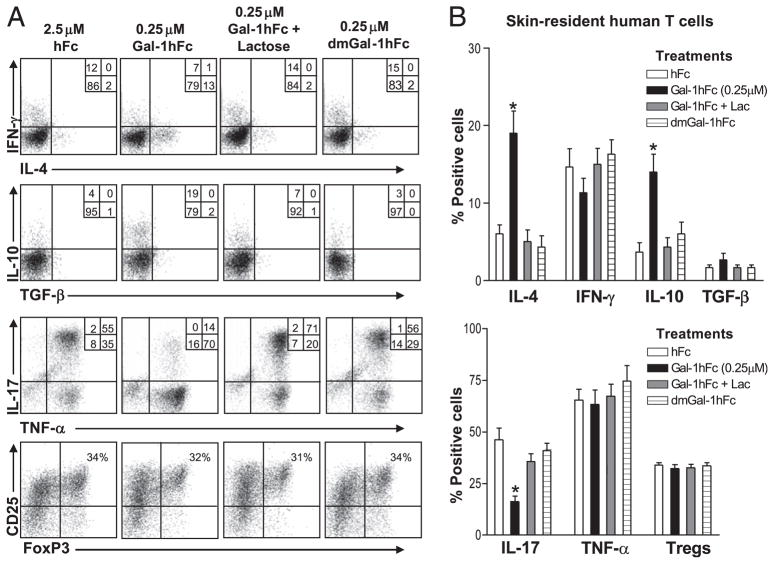
Skin-resident human T cell cytokine profile is modified by Gal-1hFc
To further explore Gal-1hFc–mediated IL-10 secretion and its potential of skewing T cell differentiation toward a Th2 profile, we studied Gal-1hFc effects on human skin-resident memory T cells. Using human skin-explant cultures of skin-resident memory T cells (CD45RO+, CCR7−, cutaneous lymphocyte Ag [CLA+]) (25), we incubated Gal-1hFc for 24 h and analyzed cytokine levels by intracellular staining. This cell model characteristically produces high numbers of TNF-α+/IL-17+ T cells (~70%) and a relatively high presence of CD25high/FoxP3+ cells (25, 41). Similar to mouse T cell data, when incubated with low concentrations of Gal-1hFc, a greater percentage of human skin-resident memory T cells expressed IL-4 and IL-10 (Fig. 7A). In contrast, a dramatically lower number of IL-17–producing T cells was observed, whereas TNF-α or IFN-γ levels were largely unaffected (Fig. 7A). The number of CD25high/FoxP3+ Tregs did not appear to be altered (Fig. 7A). Statistical analysis of data sets from six different donors showed the significance of Gal-1hFc induction of IL-4+ and IL-10+ T cells (p < 0.01) and downregulation of IL-17+ T cells (p < 0.01), indicating that Gal-1hFc can markedly affect cytokine production in human T cells (Fig. 7B).
FIGURE 7.
Gal-1hFc modulates effector molecules in skin-resident human T cells. A, Human skin-resident effector memory T cells were incubated in the presence of Gal-1hFc or molecular controls for 24 h and stained with anti-CD25, -FoxP3, –IFN-γ, –IL-4, –IL-10, –TGF-β, –IL-17, and –TNF-α mAbs, and analyzed by flow cytometry. T cells were first gated on CD3+ and CD8− cells. B, Graphic representations of data taken from six independent donors. *Statistically significant difference compared with hFc control, p ≤ 0.01.
Granulocytes infiltrating synovial fluid from patients with RA are susceptible to Gal-1hFc–mediated cell death
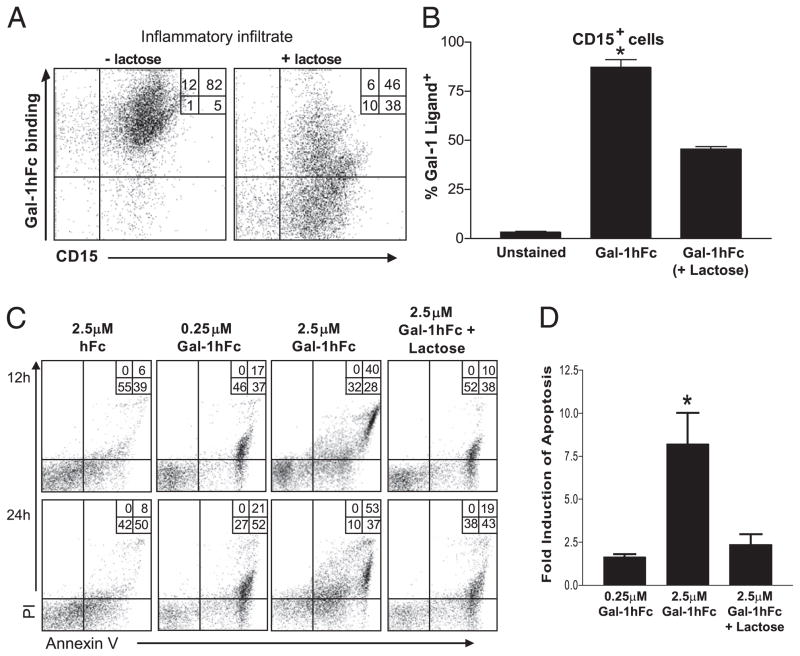
Because Gal-1 is a putative molecular regulator controlling the proliferation and viability of effector leukocytes, multiple researchers have tried to translate in vitro-generated data to experimental animal models of inflammatory diseases. Prior data using mouse models of inflammation shows that rGal-1 can suppress experimental type 1 diabetes, autoimmune encephalomyelitis, uveitis, Con A-induced hepatitis, and graft-versus-host disease (4, 16, 42–44). Of note, although T cells play an important role in the development of these disorders, relatively little is known about the anti-inflammatory properties of Gal-1 on other key cellular constituents, such as B cells and granulocytes. RA is an autoimmune disease that includes many different effector cell types, including B cells, neutrophils, monocytes/macrophages, and mast cells, which play key roles in both induction and maintenance of disease. To investigate Gal-1 efficacy as a putative anti-inflammatory agent in patients with RA, we assayed apoptotic induction properties of Gal-1hFc on freshly isolated leukocytes infiltrating synovial fluids of RA patients and incubated Gal-1hFc or control molecules. Infiltrates were freshly obtained and typically characterized by a moderate number of CD19+ B cells (12–20%) and abundant numbers of granulocytes (70–80%) as identified by surface expression of CD15 and neutrophils as identified by CD177 (Supplemental Fig. 3). We first assessed whether Gal-1hFc could bind inflamed leukocytic infiltrates. The majority of inflammatory infiltrates (>80%) bound Gal-1hFc in a lactose-dependent manner and also expressed granulocytic marker, CD15 (Fig. 8A, 8B). For cell death analysis, leukocytic infiltrates were incubated for 12 or 24 h with Gal-1hFc or controls and assayed for trypan blue staining, Annexin V positivity, and PI uptake by flow cytometric analysis. Results showed that, compared with hFc incubations, a large percentage of cells incubated with Gal-1hFc underwent apoptosis as determined by Annexin V/PI positivity, and this apoptosis was inhibited by inclusion of lactose (Fig. 8C, 8D).
FIGURE 8.
Gal-1hFc induces apoptosis of granulocytic infiltrates from synovial fluid of patients with RA. A, Inflammatory cells from synovial fluid of patients with RA were stained with Gal-1hFc and anti-CD15 mAb (±50 mM lactose), and analyzed by flow cytometry. B, Data from four independent donors are shown. C, CD15+ cells from synovial fluid samples were incubated with 0.25 or 2.5 μM Gal-1hFc (±lactose). Annexin V+ and PI+ cells were evaluated after 12 and 24 h after incubation. D, Graphed data are from a set of four independent donors. *Statistically significant difference compared with lactose control, p ≤ 0.01.
Gal-1hFc2 triggers immunomodulatory molecule production and helps alleviate hapten-mediated contact hypersensitivity
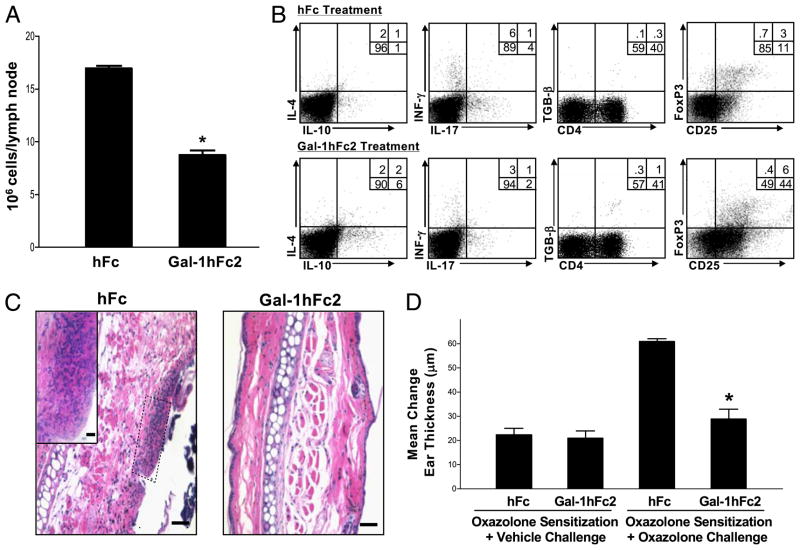
As shown in Fig. 2G, Gal-1hFc can bind activation-induced Gal-1 ligands on activated mouse T cells in LNs draining DNFB-sensitized skin. To study effects of Gal-1hFc on T cell development in vivo, we first developed a Gal-1hFc variant, Gal-1hFc2, which contains 3a mutated Fc region that prevents binding to Fc receptors and minimizes potential complement and Ab-dependent cytotoxicity, as previously reported (45, 46). Gal-1hFc2 retained identical morphologic and binding activity as Gal-1hFc (data not shown). To investigate Gal-1hFc2 anti-inflammatory efficacy, we used a model of hapten-mediated contact hypersensitivity, which consisted of oxazolone-sensitization on the abdomen on days 0 and 1, and oxazolone (or vehicle alone) challenge on the ear on day 5. Gal-1hFc2 or hFc control (both at 2.3 mg/kg mouse) was administered to mice on days 2, 4, and 5. On day 5, baseline ear thickness measurements were calculated on the right ear or vehicle alone on the left ear. After 24 h, to assess inflammation, ear thickness was remeasured and a mean change in ear thickness was computed. In addition, ear skin was analyzed for leukocytic infiltrate, and lymphocytes were harvested from oxazolone-draining LNs, enumerated, and then analyzed for cytokine expression. Gal-1hFc2–treated mice exhibited a significantly lower level of lymphocytes in draining LNs compared with hFc-treated mice (p <0.01; Fig. 9A). Of note, nondraining LNs had similar cell numbers in both groups (data not shown). Flow cytometric analysis showed that lymphocytes from Gal-1hFc2–treated mice exhibited increased levels of IL-4+, IL-10+, and TGF-β+ cells, and lower levels of IFN-γ+ and IL-17+ cells (Fig. 9B). Of note, the percentage of CD25high/FoxP3+ cells was increased twofold in Gal-1hFc2–treated mice compared with the hFc-treatment control. Together with induction of T cell immunoregulatory cytokines, mononuclear and granulocytic infiltrates in inflamed skin were markedly decreased (Fig. 9C), and change in ear thickness was significantly abrogated in Gal-1hFc2–treated mice compared with that in vehicle-alone–challenged ears (Fig. 9D).
FIGURE 9.
Galectin-1 human Ig chimera 2 (Gal-1hFc2) modulates cytokine production in T cells draining Ag-sensitized skin and alleviates Ag-dependent inflammation. A, Inguinal LNs draining oxazolone-sensitized skin from mice treated with hFc or Gal-1hFc2 (2.3 mg/kg) were harvested on day 6 after sensitization, minced, and analyzed for cellularity by trypan blue exclusion. Data are expressed as mean 106 cells/LN (±SD). *Statistically significant difference compared with hFc control, p ≤ 0.01. B, Lymphocytes from inguinal LNs were then restimulated for 6 h with PMA/ionomycin/brefeldin A and analyzed for IL-4, IL-10, IL-17, IFN-γ, TGF-β, FoxP3, and CD25 expression by flow cytometry. C, Ears from mice treated with hFc or Gal-1hFc2 were fixed in 10% formalin and stained with H&E. Scale bars, 20 μM. Original magnification ×10; inset, original magnification ×40. D, Change in ear thickness was ascertained 24 h after vehicle alone or oxazolone challenge. All experiments were repeated three times and consisted of three mice per group. *Statistically significant difference compared with hFc-treated mice receiving oxazolone sensitization and challenge, p ≤ 0.01.
Discussion
Gal-1 is a β-galactoside–binding S-type lectin that contains numerous cysteine residues capable of forming intramolecular and intermolecular disulfide bonds (22). This has necessitated the use of reducing agents, alkylation, or fixation approaches to preserve the functionality of the carbohydrate-recognition domain (21, 35, 47, 48). Reducing agents can directly induce cell death and also promote the formation of less active Gal-1 monomers (29), whereas alkylation with iodoacetamide induces leukocyte PS exposure that does not progress to full apoptosis, calling into question its ability to cause death (5, 13). It has also been difficult to study other immune-modifying properties of Gal-1 in vitro because these studies require the use of lower, nonproapoptotic concentrations of Gal-1 that favor the formation of less active and avid monomers.
To help circumvent monomer/dimer equilibrium problems and help clarify Gal-1 effects on leukocyte cell death, cytokine modulation, and T cell subset skewing, we created a mouse Gal-1–human IgG1 Fc1 chimeric protein, Gal-1hFc. We also synthesized genetic mutants mGal-1hFc and dmGal-1hFc, whose lectin domains contained amino acid substitutions at positions 45 and/or 69, to help control for the carbohydrate-binding function of Gal-1hFc (48). Gal-1hFc persisted as a predominant homodimer even after 24 h at 37°C and exhibited similar lectin properties as rGal-1 preparations. Importantly, probing Gal-1 ligands by flow cytometry, Western blotting or immunohistochemistry, triggering leukocytic apoptosis, and modulating cytokine secretion/production and T cell differentiation with Gal-1hFc were highly effective and not confounded by inclusion of reducing agents.
In cell-binding assays, Gal-1hFc exhibited characteristic Gal-1 ligand-binding activity, which was influenced by the presence of terminal α2,6 sialylation and α1,3 fucosylation. Gal-1hFc bound hallmark lactosamine-bearing glycans (18, 23) and recognized Gal-1 ligands on mouse and human hematopoietic cells, as well as on human nonhematopoietic cancer cells. Interestingly, we demonstrated that Gal-1 ligands were absent on naive LNs, though were highly expressed on activated CD4+ T cells in LNs draining Ag-sensitized skin. Moreover, Gal-1 ligands on mouse Th1 cells and on hematopoietic cell lines were detected with Gal-1hFc using Western blotting and immunohistochemical approaches, features not readily shown for other Gal-1 formulations. In fact, Western blotting experiments using Gal-1hFc and wt or CD43−/− Th1 cell lysates indicated that CD43 is a major Gal-1 ligand on Th1 cells. Collectively, Gal-1hFc and its mutants can be used in a number of leukocyte models and biochemical methodologies to analyze Gal-1 ligand expression and identity.
In cell death assays, compared with rGal-1 efficacy, 2.5 μM Gal-1hFc triggered apoptosis on Th1 and Th17 subsets, as evidenced by trypan blue exclusion, Annexin V staining, and PI uptake without using reducing agents or alkylation methods. These effects were not discernible on Th2 cells and were markedly diminished by adding lactose or by using nonbinding mutant forms of Gal-1hFc. Because of the death-inducing properties of Gal-1hFc, we believe that the authenticity of known or unknown molecular mediators of Gal-1–mediated cell death pathways can be determined.
Expanding on its T cell death-inducing properties, we investigated Gal-1hFc’s effects on leukocytic infiltrates present in inflammatory synovial fluids from patients with RA. We found that most of these inflammatory leukocytes (94%) expressed Gal-1 ligands and granulocytic marker, CD15, and were susceptible to Gal-1hFc–mediated cell death. Notwithstanding Gal-1’s well-chronicled effects in dampening T cell-mediated autoimmune responses and transplant rejection (1, 16, 43, 44), we demonstrate that granulocytes also express Gal-1 ligands, and that engagement of these moieties triggers cell death. These results provide excitement for further investigation using Gal-1hFc as a potential anti-inflammatory therapeutic to eliminate Gal-1 ligand+/CD15+ granulocytic infiltrates that predominate in swollen joints.
At a 10-fold lower concentration (0.25 μM), we studied more subtle properties of Gal-1 binding that could result in the induction/suppression of cytokines, as well as expansion/retraction of specific Th cell subsets, independent of apoptosis. In mouse Th0 cell cultures, we found that Gal-1hFc and not rGal-1 significantly increased the production of IL-4, IL-10, and IL-13. These studies were conducted using sorted naive CD4+ T cells from C57/BL-6 mice, a mouse strain innately prone to develop a Th1 phenotype (49). This Gal-1 Th2 skewing phenomenon, previously demonstrated in mouse models of autoimmunity and of cancer immune evasion (15, 16), has been, until now, difficult to replicate in vitro. Indeed, we heretofore showed that Gal-1 can directly skew Th cell subset differentiation of Th0 cells by upregulating IL-4 (GATA-3) and IL-10 expression at the mRNA and protein level without imparting cell death-inducing properties. Interestingly, although IFN-γ (T-bet) mRNA levels were depressed as early as 8 h after Gal-1hFc treatment in mouse Th0 cultures, a decrease at the protein level was still evident 40 h later. As a comparator, we also studied Gal-1hFc’s effect on cytokine production in human Th cells isolated from human skin (25, 41). These skin-resident Th cells, which contained a heterogeneous population rich in Th1, Th17, and FoxP3+ Tregs, were profoundly influenced by Gal-1hFc, as evidenced by significant increases in IL-4 and IL-10 production. Conversely, marked reductions in IL-17 production, although maintaining Treg numbers, suggested that Gal-1hFc can bias human skin T cells toward Th2 and tolerogenic (IL-10high) responses. These results indicate that Gal-1hFc may be useful in treating Th1-and Th17-mediated T cell inflammatory diseases of the skin, including psoriasis and cutaneous graft-versus-host disease.
To determine whether Gal-1hFc can trigger the formation/secretion of currently unappreciated immunoregulatory molecules, analysis of supernatants from Gal-1hFc–treated activated T cells showed that a number of molecules are secreted in a β-galactoside–dependent manner, including the IL-1ra and sICAM-1. Of interest, these two factors could synergize with increased IL-10 to augment anti-inflammatory properties of Gal-1. The significance of Gal-1hFc–mediated upregulation of TIMP-1, CXCL-9, and CXCL-10 is not clear and is currently under investigation. These studies do confirm, however, that Gal-1hFc directly enhances the production of Th2 cytokines and IL-10, making it a useful reagent for identifying immunoregulatory molecules elaborated by Gal-1 engagement and to study Gal-1 ligands necessary for conferring immunoregulatory activity.
Studies have recently suggested a potential relation between Gal-1 and other immunoregulatory molecules (1, 37). Our data highlight a potential role for Gal-1 in the upregulation of CD28 family surface molecules that are known to modulate T cell–mediated immune responses. Gal-1hFc enhanced the production of CTLA-4 and PD-1, two molecules that inhibit T cell stimulation and diminish inflammatory responses. In addition, surface expression of the IL-2R (CD25) is enhanced both in vitro and in vivo after Gal-1hFc treatment. Lastly, we demonstrate a direct influence of Gal-1 on the production of IL-10 from mouse and human CD4+ T cells. Importantly, these studies were conducted in the absence of dendritic cells, assuming that dendritic cells have previously been shown to produce IL-27 on Gal-1 engagement, which, in turn, acts on T cells to upregulate IL-10 levels (1). Although Gal-1–mediated upregulation of IL-10 production was previously described in human PBMCs using stable Gal-1 homodimers (17, 36), these studies are complicated by unknown percentages of baseline naive cells and by using 2 μM dimeric Gal-1 in the presence of 1.2 mM DTT for IL-10 induction analysis, which, at the same concentrations, causes cell death in 70% of MOLT4 T cells (17, 36). Our results showed that analyses of Gal-1–mediated cytokine expression and cell death can be attained at low (0.25 μM) and high (2.5 μM) levels of Gal-1hFc, respectively. To this end, our data suggested that Gal-1 can directly induce de novo synthesis of IL-10 from naive CD4+ T cells without inducing apoptosis of proinflammatory Th subsets or enhancing secretion of preformed cytokines from memory T cells.
Using an in vivo cutaneous model of T cell-dependent inflammation, we validated in vitro data on Gal-1hFc’s role in regulating T cell function by showing that Ag-dependent T cell activation was blunted, T cell subsets expressing IFN-γ and IL-17 were lowered, and T cell subsets expressing IL-4, IL-10, TGF-β, and FoxP3 were elevated in mice treated with a non-Fc receptor-binding variant, Gal-1hFc2. These T cell subset skewing effects prevented the expansion of activated proinflammatory T cells in Ag-draining LNs and attenuated leukocytic infiltration in Ag-challenged skin. These results provide rationale for using Gal-1hFc2 to help create an immunoregulatory environment and alter the intensity of Th1- or Th17-dependent inflammatory processes, such as psoriasis and autoimmunity.
Collectively, we show that Gal-1hFc and its mutants are powerful new tools for investigating Gal-1–Gal-1 ligand-mediated activities in the context of cellular immunity and inflammation. Because Gal-1hFc exhibits stable binding properties without ancillary stabilization procedures, we believe that a more accurate depiction of Gal-1–Gal-1 ligand binding mechanisms controlling leukocyte cell death and/or cytokine stimulation and T cell differentiation will result from future analyses. Use of Gal-1hFc will allow clarification of the immunoregulatory properties of Gal-1 not only on effector T cells, but on other leukocytes that express Gal-1 ligands. Importantly, compared with other enforced dimeric forms of Gal-1, such as the leucine zipper model, the Fc or Fc2 domain of Gal-1hFc/Gal-1hFc2 not only provides a versatile molecular probe for laboratory research, but often enhances potency, efficacy, and serum t1/2 in vivo (50–52), raising the prospect of using Gal-1hFc2 as an anti-inflammatory therapy.
Supplementary Material
Acknowledgments
We thank Dr. Vijay Kuchroo (Brigham and Women’s Hospital) and Dr. David Smith (Emory University School of Medicine) for helpful discussions.
This work was supported by American Cancer Society Research Scholar Award 06-024-01-CSM (to C.J.D.), National Institutes of Health/National Cancer Institute Grant RO1 CA118124 (to C.J.D.), National Institutes of Health/National Center for Complementary and Alternative Medicine Grant RO1 AT004268 (to C.J.D.), National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant RO1 AR056720 (to R.A.C.), a Clinical Investigator award from the Damon Runyon Cancer Research Foundation (to R.A.C.), and a New Investigator award from the Scleroderma Foundation (to R.A.C.).
Abbreviations used in this paper
- AP
alkaline phosphatase
- DNFB
2,4-dinitro-1-fluorobenzene
- Gal-1
galectin-1
- hFc
human Ig chimera
- IL-1ra
IL-1R antagonist
- Lac
lactose
- LN
lymph node
- OD
optic densitometry
- PD
programmed death
- PI
propidium iodide
- PS
phosphatidylserine
- RA
rheumatoid arthritis
- rGal-1
recombinant human galectin-1
- RT
room temperature
- sICAM-1
soluble ICAM-1
- SS
signal sequence
- TIMP-1
tissue inhibitor of metalloproteinase
- Treg
regulatory T cell
- wt
wild type
Footnotes
The online version of this article contains supplemental material.
Disclosures
C.J.D., F.C.-L., and S.R.B. are coinventors on a pending patent application that covers the subject matter of the manuscript.
References
- 1.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009;10:981–991. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 2.Garín MI, Chu CC, Golshayan D, Cernuda-Morollón E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+ CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 3.Motran CC, Molinder KM, Liu SD, Poirier F, Miceli MC. Galectin-1 functions as a Th2 cytokine that selectively induces Th1 apoptosis and promotes Th2 function. Eur J Immunol. 2008;38:3015–3027. doi: 10.1002/eji.200838295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, Rabinovich GA. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 5.Karmakar S, Stowell SR, Cummings RD, McEver RP. Galectin-1 signaling in leukocytes requires expression of complex-type N-glycans. Glycobiology. 2008;18:770–778. doi: 10.1093/glycob/cwn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Brûle FA, Fernandez PL, Buicu C, Liu FT, Jackers P, Lambotte R, Castronovo V. Differential expression of galectin-1 and galectin-3 during first trimester human embryogenesis. Dev Dyn. 1997;209:399–405. doi: 10.1002/(SICI)1097-0177(199708)209:4<399::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.He J, Baum LG. Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol. 2006;417:247–256. doi: 10.1016/S0076-6879(06)17017-2. [DOI] [PubMed] [Google Scholar]
- 8.Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 9.Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med. 1997;185:1851–1858. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ, Hong SC, Choi SK, Ha WS, Kim JW, et al. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int J Cancer. 2007;120:2331–2338. doi: 10.1002/ijc.22434. [DOI] [PubMed] [Google Scholar]
- 11.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 12.Dias-Baruffi M, Zhu H, Cho M, Karmakar S, McEver RP, Cummings RD. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J Biol Chem. 2003;278:41282–41293. doi: 10.1074/jbc.M306624200. [DOI] [PubMed] [Google Scholar]
- 13.Stowell SR, Karmakar S, Arthur CM, Ju T, Rodrigues LC, Riul TB, Dias-Baruffi M, Miner J, McEver RP, Cummings RD. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol Biol Cell. 2009;20:1408–1418. doi: 10.1091/mbc.E08-07-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA, Shipp MA. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci USA. 2007;104:13134–13139. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perone MJ, Bertera S, Shufesky WJ, Divito SJ, Montecalvo A, Mathers AR, Larregina AT, Pang M, Seth N, Wucherpfennig KW, et al. Suppression of autoimmune diabetes by soluble galectin-1. J Immunol. 2009;182:2641–2653. doi: 10.4049/jimmunol.0800839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Leij J, van den Berg A, Blokzijl T, Harms G, van Goor H, Zwiers P, van Weeghel R, Poppema S, Visser L. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J Pathol. 2004;204:511–518. doi: 10.1002/path.1671. [DOI] [PubMed] [Google Scholar]
- 18.Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 19.Baum LG, Seilhamer JJ, Pang M, Levine WB, Beynon D, Berliner JA. Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconj J. 1995;12:63–68. doi: 10.1007/BF00731870. [DOI] [PubMed] [Google Scholar]
- 20.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem. 1995;270:5207–5212. doi: 10.1074/jbc.270.10.5207. [DOI] [PubMed] [Google Scholar]
- 21.Pace KE, Hahn HP, Baum LG. Preparation of recombinant human galectin-1 and use in T-cell death assays. Methods Enzymol. 2003;363:499–518. doi: 10.1016/S0076-6879(03)01075-9. [DOI] [PubMed] [Google Scholar]
- 22.Nishi N, Abe A, Iwaki J, Yoshida H, Itoh A, Shoji H, Kamitori S, Hirabayashi J, Nakamura T. Functional and structural bases of a cysteineless mutant as a long-lasting substitute for galectin-1. Glycobiology. 2008;18:1065–1073. doi: 10.1093/glycob/cwn089. [DOI] [PubMed] [Google Scholar]
- 23.Tsai CM, Chiu YK, Hsu TL, Lin IY, Hsieh SL, Lin KI. Galectin-1 promotes immunoglobulin production during plasma cell differentiation. J Immunol. 2008;181:4570–4579. doi: 10.4049/jimmunol.181.7.4570. [DOI] [PubMed] [Google Scholar]
- 24.Barthel SR, Wiese GK, Cho J, Opperman MJ, Hays DL, Siddiqui J, Pienta KJ, Furie B, Dimitroff CJ. Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci USA. 2009;106:19491–19496. doi: 10.1073/pnas.0906074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, Kupper TS. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 26.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 27.Howard MR, Lodge AP, Reed JE, McNamee CJ, Moss DJ. High-level expression of recombinant Fc chimeric proteins in suspension cultures of stably transfected J558L cells. Biotechniques. 2002;32:1282–1286. 1288. doi: 10.2144/02326st03. [DOI] [PubMed] [Google Scholar]
- 28.von Gunten S, Smith DF, Cummings RD, Riedel S, Miescher S, Schaub A, Hamilton RG, Bochner BS. Intravenous immunoglobulin contains a broad repertoire of anticarbohydrate antibodies that is not restricted to the IgG2 subclass. J Allergy Clin Immunol. 2009;123:1268–1276. e15. doi: 10.1016/j.jaci.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leppänen A, Stowell S, Blixt O, Cummings RD. Dimeric galectin-1 binds with high affinity to alpha2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J Biol Chem. 2005;280:5549–5562. doi: 10.1074/jbc.M412019200. [DOI] [PubMed] [Google Scholar]
- 30.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, Urashima T, Oka T, Futai M, Muller WE, et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 31.Levi G, V, Teichberg I. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981;256:5735–5740. [PubMed] [Google Scholar]
- 32.Hirabayashi J, Kasai K. Effect of amino acid substitution by sited-directed mutagenesis on the carbohydrate recognition and stability of human 14-kDa beta-galactoside-binding lectin. J Biol Chem. 1991;266:23648–23653. [PubMed] [Google Scholar]
- 33.Pace KE, Lee C, Stewart PL, Baum LG. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- 34.Gainers ME, Descheny L, Barthel SR, Liu L, Wurbel MA, Dimitroff CJ. Skin-homing receptors on effector leukocytes are differentially sensitive to glycometabolic antagonism in allergic contact dermatitis. J Immunol. 2007;179:8509–8518. doi: 10.4049/jimmunol.179.12.8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stowell SR, Cho M, Feasley CL, Arthur CM, Song X, Colucci JK, Karmakar S, Mehta P, Dias-Baruffi M, McEver RP, Cummings RD. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J Biol Chem. 2009;284:4989–4999. doi: 10.1074/jbc.M808925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Leij J, van den Berg A, Harms G, Eschbach H, Vos H, Zwiers P, van Weeghel R, Groen H, Poppema S, Visser L. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol Immunol. 2007;44:506–513. doi: 10.1016/j.molimm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Di Lella S, Ma L, Ricci JC, Rabinovich GA, Asher SA, Alvarez RM. Critical role of the solvent environment in galectin-1 binding to the disaccharide lactose. Biochemistry. 2009;48:786–791. doi: 10.1021/bi801855g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 39.Chai JG, Tsang JY, Lechler R, Simpson E, Dyson J, Scott D. CD4+CD25+ T cells as immunoregulatory T cells in vitro. Eur J Immunol. 2002;32:2365–2375. doi: 10.1002/1521-4141(200208)32:8<2365::AID-IMMU2365>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, Vatan L, Finlayson E, Huang E, Simeone D, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 41.Clark RA, Kupper TS. IL-15 and dermal fibroblasts induce proliferation of natural regulatory T cells isolated from human skin. Blood. 2007;109:194–202. doi: 10.1182/blood-2006-02-002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, Hirabayashi J, Rizzo LV, Rabinovich GA. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176:6323–6332. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 43.Santucci L, Fiorucci S, Cammilleri F, Servillo G, Federici B, Morelli A. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology. 2000;31:399–406. doi: 10.1002/hep.510310220. [DOI] [PubMed] [Google Scholar]
- 44.Baum LG, Blackall DP, Arias-Magallano S, Nanigian D, Uh SY, Browne JM, Hoffmann D, Emmanouilides CE, Territo MC, Baldwin GC. Amelioration of graft versus host disease by galectin-1. Clin Immunol. 2003;109:295–307. doi: 10.1016/j.clim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Armour KL, Clark MR, Hadley AG, Williamson LM. Recombinant human IgG molecules lacking Fcgamma receptor I binding and monocyte triggering activities. Eur J Immunol. 1999;29:2613–2624. doi: 10.1002/(SICI)1521-4141(199908)29:08<2613::AID-IMMU2613>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 46.Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 47.Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. J Biol Chem. 1995;270:5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- 48.López-Lucendo MF, Solís D, André S, Hirabayashi J, Kasai K, Kaltner H, Gabius HJ, Romero A. Growth-regulatory human galectin-1: crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J Mol Biol. 2004;343:957–970. doi: 10.1016/j.jmb.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 49.Adkins B, Bu Y, Cepero E, Perez R. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J Immunol. 2000;164:2347–2353. doi: 10.4049/jimmunol.164.5.2347. [DOI] [PubMed] [Google Scholar]
- 50.Landolfi NF. A chimeric IL-2/Ig molecule possesses the functional activity of both proteins. J Immunol. 1991;146:915–919. [PubMed] [Google Scholar]
- 51.Mohler KM, Torrance DS, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 52.Way JC, Lauder S, Brunkhorst B, Kong SM, Qi A, Webster G, Campbell I, McKenzie S, Lan Y, Marelli B, et al. Improvement of Fc-erythropoietin structure and pharmacokinetics by modification at a disulfide bond. Protein Eng Des Sel. 2005;18:111–118. doi: 10.1093/protein/gzi021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.