Abstract
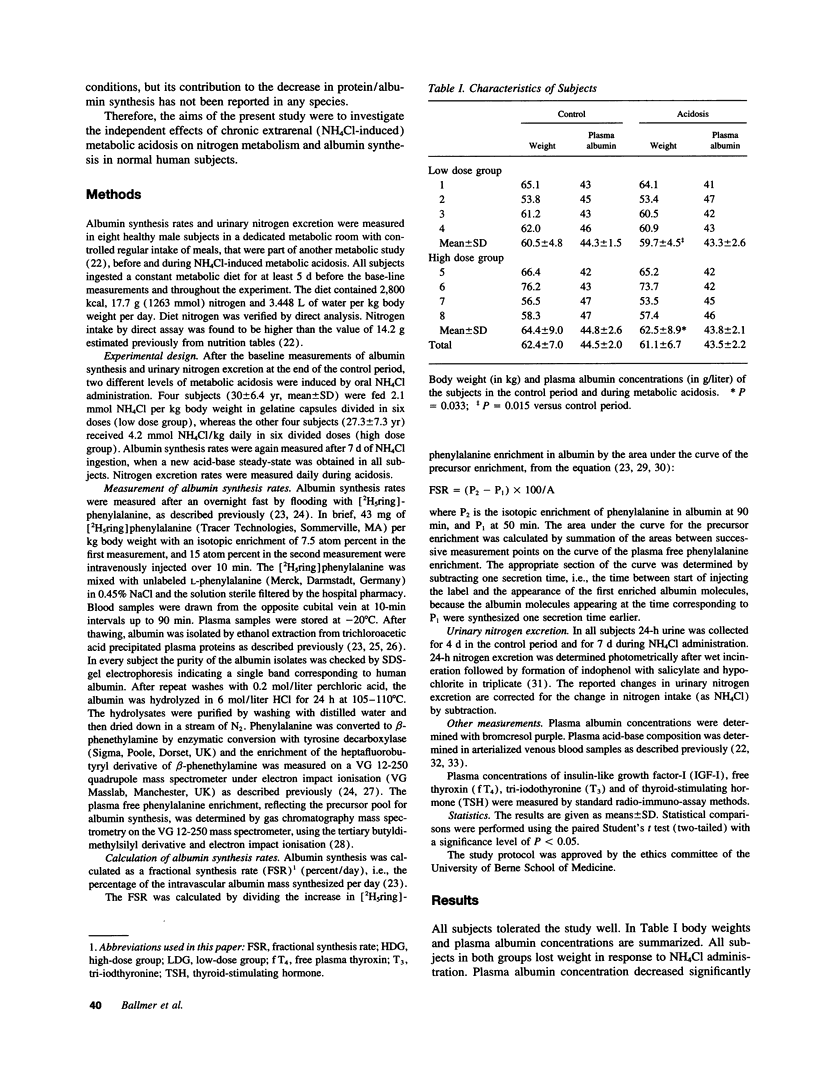
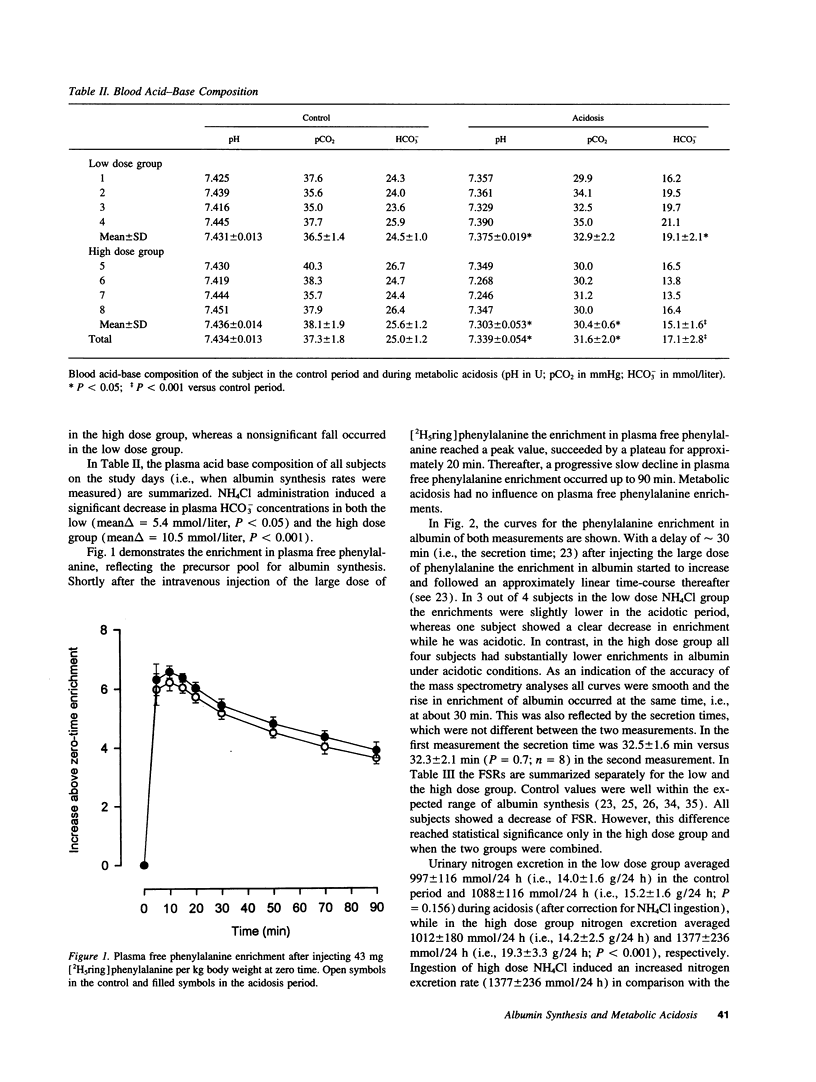
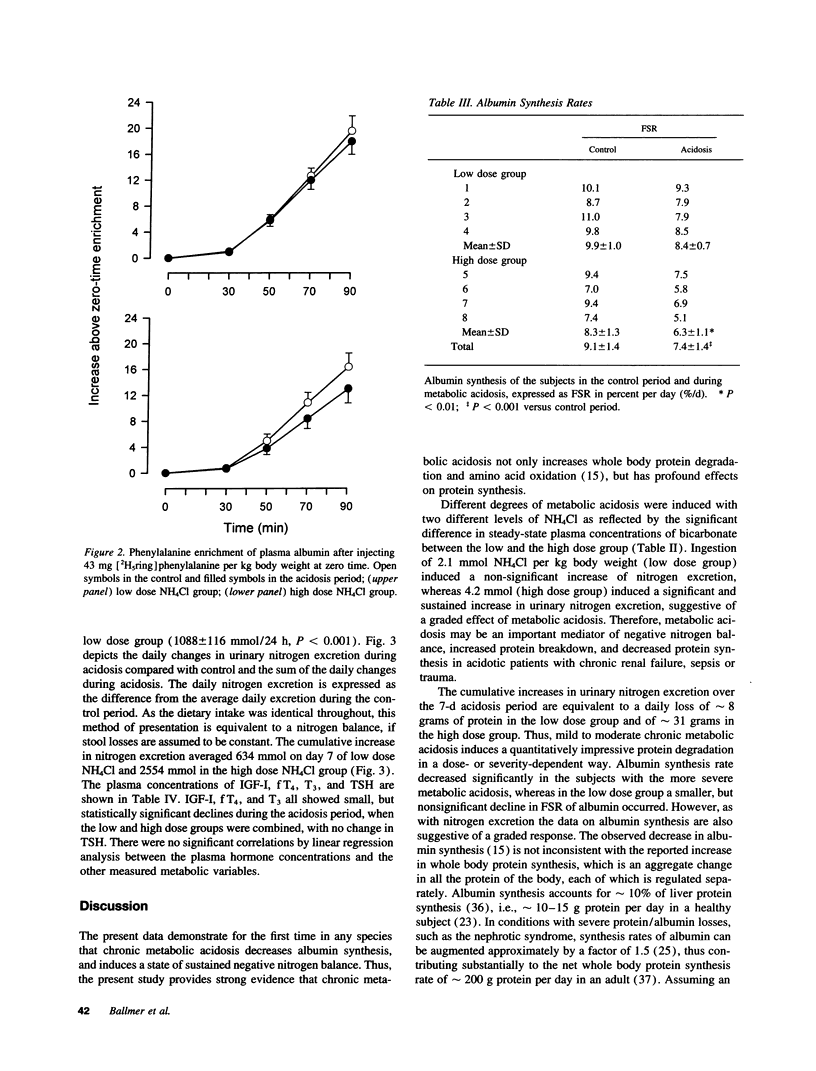
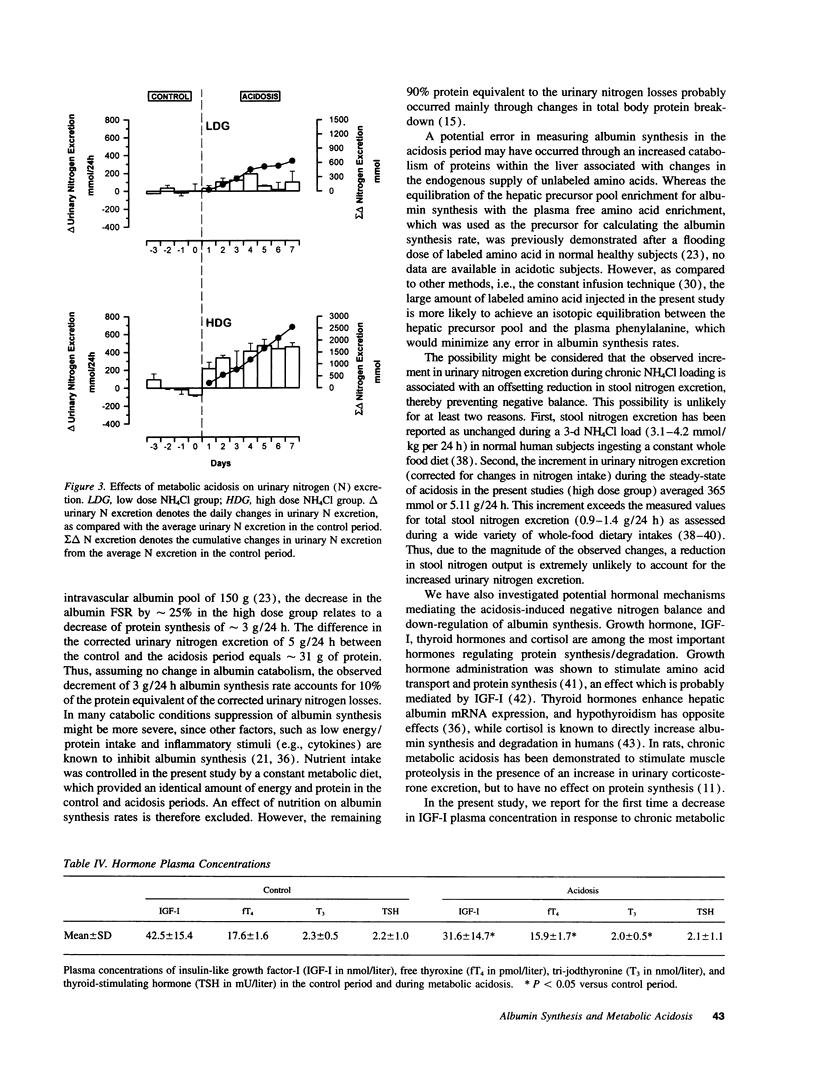
Chronic metabolic acidosis has been previously shown to stimulate protein degradation. To evaluate the effects of chronic metabolic acidosis on nitrogen balance and protein synthesis we measured albumin synthesis rates and urinary nitrogen excretion in eight male subjects on a constant metabolic diet before and during two different degrees of chronic metabolic acidosis (NH4Cl 2.1 mmol/kg body weight, low dose group, and 4.2 mmol/kg body weight, high dose group, orally for 7 d). Albumin synthesis rates were measured by intravenous injection of [2H5ring]phenylalanine (43 mg/kg body weight, 7.5 atom percent and 15 atom percent, respectively) after an overnight fast. In the low dose group, fractional synthesis rates of albumin decreased from 9.9 +/- 1.0% per day in the control period to 8.4 +/- 0.7 (n.s.) in the acidosis period, and from 8.3 +/- 1.3% per day to 6.3 +/- 1.1 (P < 0.001) in the high dose group. Urinary nitrogen excretion increased significantly in the acidosis period (sigma delta 634 mmol in the low dose group, 2,554 mmol in the high dose group). Plasma concentrations of insulin-like growth factor-I, free thyroxine and tri-iodothyronine were significantly lower during acidosis. In conclusion, chronic metabolic acidosis causes negative nitrogen balance and decreases albumin synthesis in humans. The effect on albumin synthesis may be mediated, at least in part, by a suppression of insulin-like growth factor-I, free thyroxine and tri-iodothyronine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballmer P. E., McNurlan M. A., Milne E., Heys S. D., Buchan V., Calder A. G., Garlick P. J. Measurement of albumin synthesis in humans: a new approach employing stable isotopes. Am J Physiol. 1990 Dec;259(6 Pt 1):E797–E803. doi: 10.1152/ajpendo.1990.259.6.E797. [DOI] [PubMed] [Google Scholar]
- Ballmer P. E., Walshe D., McNurlan M. A., Watson H., Brunt P. W., Garlick P. J. Albumin synthesis rates in cirrhosis: correlation with Child-Turcotte classification. Hepatology. 1993 Aug;18(2):292–297. [PubMed] [Google Scholar]
- Ballmer P. E., Weber B. K., Roy-Chaudhury P., McNurlan M. A., Watson H., Power D. A., Garlick P. J. Elevation of albumin synthesis rates in nephrotic patients measured with [1-13C]leucine. Kidney Int. 1992 Jan;41(1):132–138. doi: 10.1038/ki.1992.17. [DOI] [PubMed] [Google Scholar]
- Cabello G., Wrutniak C. Influence of experimental acidosis on the concentrations of thyreostimulin (TSH) and iodothyronines (total T4, free T4, T3) in the plasma of the newborn lamb. Reprod Nutr Dev. 1989;29(4):509–515. doi: 10.1051/rnd:19890412. [DOI] [PubMed] [Google Scholar]
- Calder A. G., Anderson S. E., Grant I., McNurlan M. A., Garlick P. J. The determination of low d5-phenylalanine enrichment (0.002-0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992 Jul;6(7):421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- Calder A. G., Smith A. Stable isotope ratio analysis of leucine and ketoisocaproic acid in blood plasma by gas chromatography/mass spectrometry. Use of tertiary butyldimethylsilyl derivatives. Rapid Commun Mass Spectrom. 1988 Jan;2(1):14–16. doi: 10.1002/rcm.1290020105. [DOI] [PubMed] [Google Scholar]
- Challa A., Chan W., Krieg R. J., Jr, Thabet M. A., Liu F., Hintz R. L., Chan J. C. Effect of metabolic acidosis on the expression of insulin-like growth factor and growth hormone receptor. Kidney Int. 1993 Dec;44(6):1224–1227. doi: 10.1038/ki.1993.372. [DOI] [PubMed] [Google Scholar]
- Clark H. E., Howe J. M., Magee J. L., Malzer J. L. Nitrogen balances of adult human subjects who consumed four levels of nitrogen from a combination of rice, milk and wheat. J Nutr. 1972 Dec;102(12):1647–1654. doi: 10.1093/jn/102.12.1647. [DOI] [PubMed] [Google Scholar]
- Clark H. E., Moon W. H., Malzer J. L., Pang R. L. Nitrogen retention of young men who consumed between sixteen and eight grams of nitrogen from a combination of rice, wheat, chicken and milk. Am J Clin Nutr. 1974 Oct;27(10):1059–1064. doi: 10.1093/ajcn/27.8.1059. [DOI] [PubMed] [Google Scholar]
- De Feo P., Horber F. F., Haymond M. W. Meal stimulation of albumin synthesis: a significant contributor to whole body protein synthesis in humans. Am J Physiol. 1992 Oct;263(4 Pt 1):E794–E799. doi: 10.1152/ajpendo.1992.263.4.E794. [DOI] [PubMed] [Google Scholar]
- England B. K., Chastain J. L., Mitch W. E. Abnormalities in protein synthesis and degradation induced by extracellular pH in BC3H1 myocytes. Am J Physiol. 1991 Feb;260(2 Pt 1):C277–C282. doi: 10.1152/ajpcell.1991.260.2.C277. [DOI] [PubMed] [Google Scholar]
- Forster H. V., Dempsey J. A., Thomson J., Vidruk E., DoPico G. A. Estimation of arterial PO2, PCO2, pH, and lactate from arterialized venous blood. J Appl Physiol. 1972 Jan;32(1):134–137. doi: 10.1152/jappl.1972.32.1.134. [DOI] [PubMed] [Google Scholar]
- Fryburg D. A., Gelfand R. A., Barrett E. J. Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol. 1991 Mar;260(3 Pt 1):E499–E504. doi: 10.1152/ajpendo.1991.260.3.E499. [DOI] [PubMed] [Google Scholar]
- Fuller S. J., Gaitanaki C. J., Sugden P. H. Effects of increasing extracellular pH on protein synthesis and protein degradation in the perfused working rat heart. Biochem J. 1989 Apr 1;259(1):173–179. doi: 10.1042/bj2590173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J. Skeletal muscle protein and amino acid metabolism in experimental chronic uremia in the rat: accelerated alanine and glutamine formation and release. J Clin Invest. 1978 Sep;62(3):623–632. doi: 10.1172/JCI109169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto G., Russo R., Sala M. R., Ancarani P., Robaudo C., Sofia A., Deferrari G., Tizianello A. Muscle protein turnover and amino acid metabolism in patients with chronic renal failure. Miner Electrolyte Metab. 1992;18(2-5):217–221. [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Ballmer P. E. Influence of dietary protein intake on whole-body protein turnover in humans. Diabetes Care. 1991 Dec;14(12):1189–1198. doi: 10.2337/diacare.14.12.1189. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Ryan G., Miller S. B. Expression of insulin-like growth factor in adult and embryonic kidney. Miner Electrolyte Metab. 1992;18(2-5):253–255. [PubMed] [Google Scholar]
- Hannaford M. C., Leiter L. A., Josse R. G., Goldstein M. B., Marliss E. B., Halperin M. L. Protein wasting due to acidosis of prolonged fasting. Am J Physiol. 1982 Sep;243(3):E251–E256. doi: 10.1152/ajpendo.1982.243.3.E251. [DOI] [PubMed] [Google Scholar]
- Krapf R., Beeler I., Hertner D., Hulter H. N. Chronic respiratory alkalosis. The effect of sustained hyperventilation on renal regulation of acid-base equilibrium. N Engl J Med. 1991 May 16;324(20):1394–1401. doi: 10.1056/NEJM199105163242003. [DOI] [PubMed] [Google Scholar]
- Krapf R., Vetsch R., Vetsch W., Hulter H. N. Chronic metabolic acidosis increases the serum concentration of 1,25-dihydroxyvitamin D in humans by stimulating its production rate. Critical role of acidosis-induced renal hypophosphatemia. J Clin Invest. 1992 Dec;90(6):2456–2463. doi: 10.1172/JCI116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber K. Photometric determination of nitrogen. Wet incineration followed by formation of indophenol blue with salicylate/hypochlorite. Clin Chim Acta. 1976 Feb 16;67(1):107–110. doi: 10.1016/0009-8981(76)90224-2. [DOI] [PubMed] [Google Scholar]
- Li J. B., Wassner S. J. Protein synthesis and degradation in skeletal muscle of chronically uremic rats. Kidney Int. 1986 Jun;29(6):1136–1143. doi: 10.1038/ki.1986.119. [DOI] [PubMed] [Google Scholar]
- MCFARLANE A. S. MEASUREMENT OF SYNTHESIS RATES OF LIVER-PRODUCED PLASMA PROTEINS. Biochem J. 1963 Nov;89:277–290. doi: 10.1042/bj0890277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. C., Kelly R. A., Mitch W. E. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J Clin Invest. 1987 Apr;79(4):1099–1103. doi: 10.1172/JCI112924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. C., Kelly R. A., Mitch W. E. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986 Feb;77(2):614–621. doi: 10.1172/JCI112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May R. C., Masud T., Logue B., Bailey J., England B. Chronic metabolic acidosis accelerates whole body proteolysis and oxidation in awake rats. Kidney Int. 1992 Jun;41(6):1535–1542. doi: 10.1038/ki.1992.223. [DOI] [PubMed] [Google Scholar]
- McSherry E., Morris R. C., Jr Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J Clin Invest. 1978 Feb;61(2):509–527. doi: 10.1172/JCI108962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch W. E., May R. C., Maroni B. J. Review: mechanisms for abnormal protein metabolism in uremia. J Am Coll Nutr. 1989 Aug;8(4):305–309. doi: 10.1080/07315724.1989.10720306. [DOI] [PubMed] [Google Scholar]
- Moshage H. J., Kleter B. E., van Pelt J. F., Roelofs H. M., Kleuskens J. A., Yap S. H. Fibrinogen and albumin synthesis are regulated at the transcriptional level during the acute phase response. Biochim Biophys Acta. 1988 Sep 7;950(3):450–454. doi: 10.1016/0167-4781(88)90144-3. [DOI] [PubMed] [Google Scholar]
- Nash M. A., Torrado A. D., Greifer I., Spitzer A., Edelmann C. M., Jr Renal tubular acidosis in infants and children. Clinical course, response to treatment, and prognosis. J Pediatr. 1972 May;80(5):738–748. doi: 10.1016/s0022-3476(72)80124-0. [DOI] [PubMed] [Google Scholar]
- Papadoyannakis N. J., Stefanidis C. J., McGeown M. The effect of the correction of metabolic acidosis on nitrogen and potassium balance of patients with chronic renal failure. Am J Clin Nutr. 1984 Sep;40(3):623–627. doi: 10.1093/ajcn/40.3.623. [DOI] [PubMed] [Google Scholar]
- Preedy V. R., Garlick P. J. The influence of restraint and infusion on rates of muscle protein synthesis in the rat. Effect of altered respiratory function. Biochem J. 1988 Apr 15;251(2):577–580. doi: 10.1042/bj2510577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaich D., Channon S. M., Scrimgeour C. M., Goodship T. H. Ammonium chloride-induced acidosis increases protein breakdown and amino acid oxidation in humans. Am J Physiol. 1992 Oct;263(4 Pt 1):E735–E739. doi: 10.1152/ajpendo.1992.263.4.E735. [DOI] [PubMed] [Google Scholar]
- Rodriguez N. R., Miles J. M., Schwenk W. F., Haymond M. W. Effects of acute metabolic acidosis and alkalosis on leucine metabolism in conscious dogs. Diabetes. 1989 Jul;38(7):847–853. doi: 10.2337/diab.38.7.847. [DOI] [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Schreiber S. S. Serum albumin. Hepatology. 1988 Mar-Apr;8(2):385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ W. B., JENSON R. L., RELMAN A. S. The disposition of acid administered to sodium-depleted subjects: the renal response and the role of the whole body buffers. J Clin Invest. 1954 Apr;33(4):587–597. doi: 10.1172/JCI102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambelan M., Sebastian A., Katuna B. A., Arteaga E. Adrenocortical hormone secretory response to chronic NH4Cl-induced metabolic acidosis. Am J Physiol. 1987 Apr;252(4 Pt 1):E454–E460. doi: 10.1152/ajpendo.1987.252.4.E454. [DOI] [PubMed] [Google Scholar]
- Schwander J. C., Hauri C., Zapf J., Froesch E. R. Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology. 1983 Jul;113(1):297–305. doi: 10.1210/endo-113-1-297. [DOI] [PubMed] [Google Scholar]
- Straumann E., Keller U., Küry D., Bloesch D., Thélin A., Arnaud M. J., Stauffacher W. Effect of acute acidosis and alkalosis on leucine kinetics in man. Clin Physiol. 1992 Jan;12(1):39–51. doi: 10.1111/j.1475-097x.1992.tb00292.x. [DOI] [PubMed] [Google Scholar]
- WILKINSON P., MENDENHALL C. L. SERUM ALBUMIN TURNOVER IN NORMAL SUBJECTS AND PATIENTS WITH CIRRHOSIS MEASURED BY 131I-LABELLED HUMAN ALBUMIN. Clin Sci. 1963 Oct;25:281–292. [PubMed] [Google Scholar]
- Williams B., Layward E., Walls J. Skeletal muscle degradation and nitrogen wasting in rats with chronic metabolic acidosis. Clin Sci (Lond) 1991 May;80(5):457–462. doi: 10.1042/cs0800457. [DOI] [PubMed] [Google Scholar]
- Yudkoff M., Nissim I., McNellis W., Polin R. Albumin synthesis in premature infants: determination of turnover with [15N]glycine. Pediatr Res. 1987 Jan;21(1):49–53. doi: 10.1203/00006450-198701000-00012. [DOI] [PubMed] [Google Scholar]


