Abstract
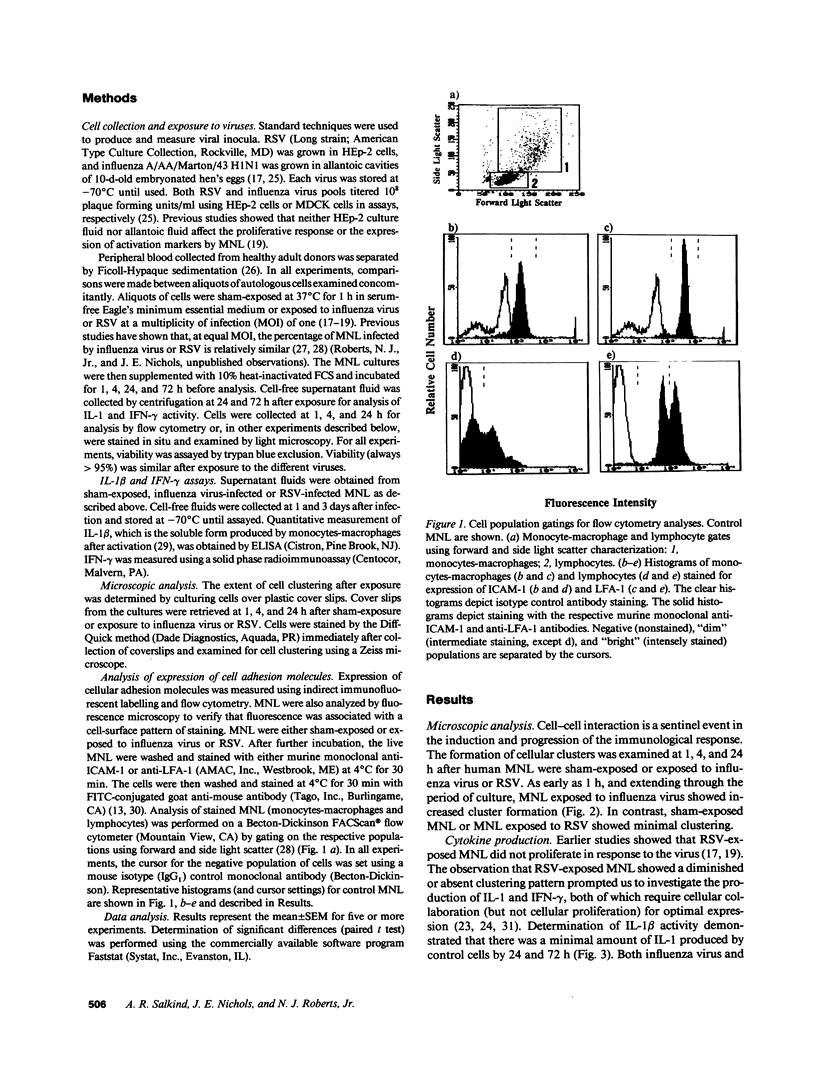
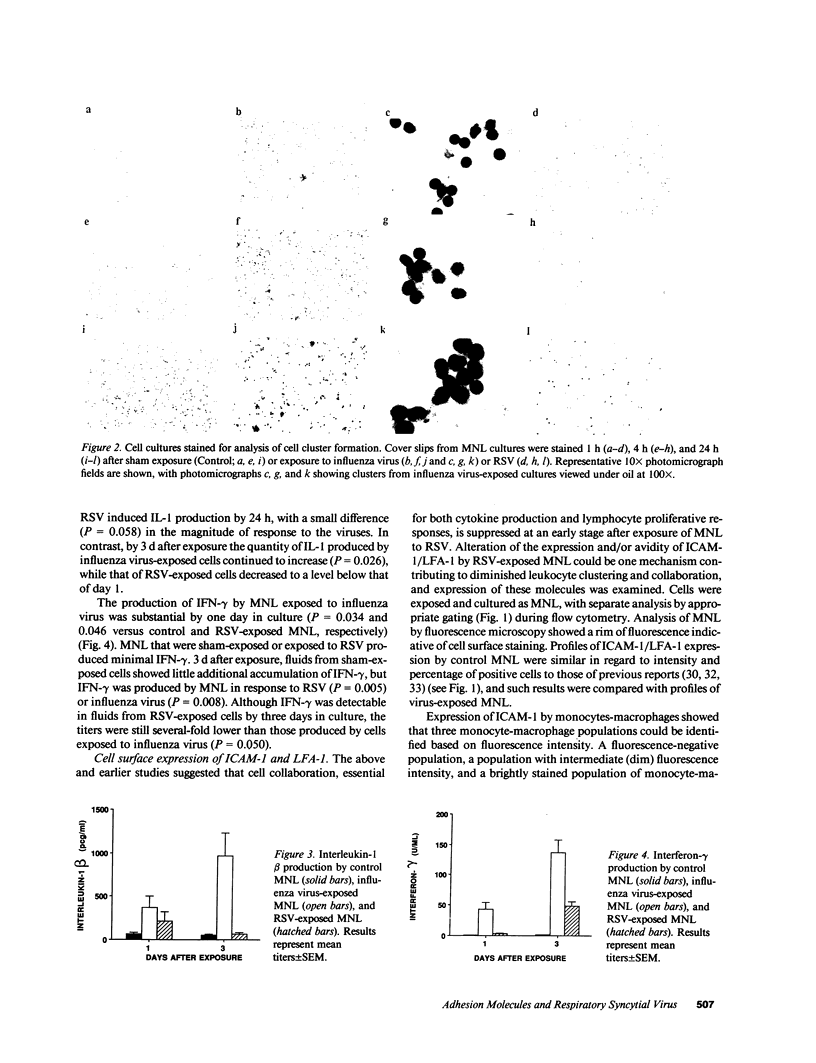
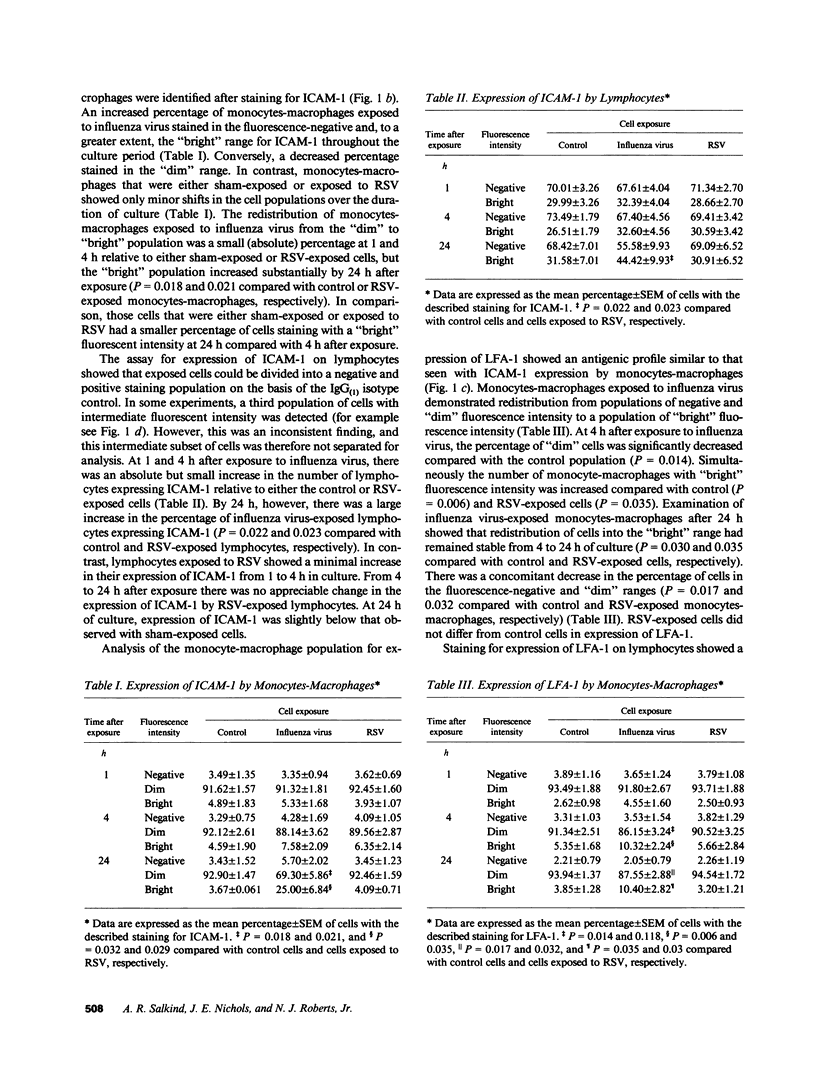
Human mononuclear leukocytes (MNL) exposed to respiratory syncytial virus (RSV) produce net IL-1 inhibitor bioactivity with the anticipated consequences of cell cycle arrest, suppressed virus-specific proliferation, and reduced expression of activation markers. These studies were undertaken to investigate effects of exposure and resultant net IL-1 inhibitor activity on the expression of the intercellular adhesion molecule-1 (ICAM-1), and its ligand the lymphocyte function-associated antigen (LFA-1). MNL collected at 1, 4, and 24 h after exposure to influenza virus (which induces net IL-1 bioactivity) showed enhanced expression of ICAM-1 and LFA-1 relative to sham-exposed MNL and exhibited cell clustering. In contrast, exposure to RSV was associated with suppressed expression of both ICAM-1 and LFA-1 and with minimal detectable cell clustering throughout the culture period. Influenza virus-exposed MNL produced significantly more IL-1 and IFN-gamma (which require cell-cell collaboration for optimal production) than did RSV-exposed MNL. These data raise the possibility that exposure of MNL to RSV fails to elicit or blocks the early events necessary for cellular collaboration, contributing to early suppression of the clonal expansion of RSV-specific lymphocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Warner S. J., Webb A. C., Cannon J. G., Bernheim H. A., McAdam K. J., Rosenwasser L. J., LoPreste G., Mucci S. F., Dinarello C. A. Studies on the molecular nature of human interleukin 1. J Immunol. 1987 Mar 1;138(5):1447–1456. [PubMed] [Google Scholar]
- Beem M. Repeated infections with respiratory syncytial virus. J Immunol. 1967 Jun;98(6):1115–1122. [PubMed] [Google Scholar]
- Braendstrup O., Werdelin O., Shevach E. M., Rosenthal A. S. Macrophage-lymphocyte clusters in the immune response to soluble protein antigen in vitro. VII. Genetically restricted and nonrestricted physical interactions. J Immunol. 1979 Apr;122(4):1608–1613. [PubMed] [Google Scholar]
- Brownson J. M., Mahy B. W., Hazleman B. L. Interaction of influenza A virus with human peripheral blood lymphocytes. Infect Immun. 1979 Aug;25(2):749–756. doi: 10.1128/iai.25.2.749-756.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle A. M., Hogg N. Human memory T cells express intercellular adhesion molecule-1 which can be increased by interleukin 2 and interferon-gamma. Eur J Immunol. 1990 Feb;20(2):337–341. doi: 10.1002/eji.1830200216. [DOI] [PubMed] [Google Scholar]
- Chonmaitree T., Roberts N. J., Jr, Douglas R. G., Jr, Hall C. B., Simons R. L. Interferon production by human mononuclear leukocytes: differences between respiratory syncytial virus and influenza viruses. Infect Immun. 1981 Apr;32(1):300–303. doi: 10.1128/iai.32.1.300-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier C., Haeffner-Cavaillon N., Weiss L., Fischer E., Kazatchkine M. D. Induction of cell-associated interleukin 1 through stimulation of the adhesion-promoting proteins LFA-1 (CD11a/CD18) and CR3 (CD11b/CD18) of human monocytes. Eur J Immunol. 1990 May;20(5):999–1005. doi: 10.1002/eji.1830200508. [DOI] [PubMed] [Google Scholar]
- Desroches C. V., Andréoni C., Rigal D. Differential expression of the LFA-1 molecule on the human peripheral blood mononuclear cell subpopulations. Immunol Lett. 1990 Mar-Apr;24(1):13–20. doi: 10.1016/0165-2478(90)90030-t. [DOI] [PubMed] [Google Scholar]
- Domurat F., Roberts N. J., Jr, Walsh E. E., Dagan R. Respiratory syncytial virus infection of human mononuclear leukocytes in vitro and in vivo. J Infect Dis. 1985 Nov;152(5):895–902. doi: 10.1093/infdis/152.5.895. [DOI] [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. PPD-stimulated interferon: in vitro macrophage-lymphocyte interaction in the production of a mediator of cellular immunity. Cell Immunol. 1971 Dec;2(6):602–613. doi: 10.1016/0008-8749(71)90008-6. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. The interaction of human macrophages and lymphocytes in the phytohemagglutinin-stimulated production of interferon. J Clin Invest. 1971 Apr;50(4):744–753. doi: 10.1172/JCI106545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON K. M., CHANOCK R. M., RIFKIND D., KRAVETZ H. M., KNIGHT V. Respiratory syncytial virus. IV. Correlation of virus shedding, serologic response, and illness in adult volunteers. JAMA. 1961 May 27;176:663–667. [PubMed] [Google Scholar]
- KRAVETZ H. M., KNIGHT V., CHANOCK R. M., MORRIS J. A., JOHNSON K. M., RIFKIND D., UTZ J. P. Respiratory syncytial virus. III. Production of illness and clinical observations in adult volunteers. JAMA. 1961 May 27;176:657–663. [PubMed] [Google Scholar]
- Kawakami K., Yamamoto Y., Kakimoto K., Onoue K. Requirement for delivery of signals by physical interaction and soluble factors from accessory cells in the induction of receptor-mediated T cell proliferation. Effectiveness of IFN-gamma modulation of accessory cells for physical interaction with T cells. J Immunol. 1989 Mar 15;142(6):1818–1825. [PubMed] [Google Scholar]
- Kirchner H., Zawatzky R., Engler H., Schirrmacher V., Becker H., von Wussow P. Production of interferon in the murine mixed lymphocyte culture. II. Interferon production is a T cell-dependent function, independent of proliferation. Eur J Immunol. 1979 Oct;9(10):824–826. doi: 10.1002/eji.1830091015. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Rosenthal A. S. Macrophage-lymphocyte interaction. II. Antigen-mediated physical interactions between immune guinea pig lymph node lymphocytes and syngeneic macrophages. J Exp Med. 1975 Jan 1;141(1):138–154. doi: 10.1084/jem.141.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Shaw S. The CD2-LFA-3 and LFA-1-ICAM pathways: relevance to T-cell recognition. Immunol Today. 1989 Dec;10(12):417–422. doi: 10.1016/0167-5699(89)90039-X. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- McCarthy D. O., Domurat F. M., Nichols J. E., Roberts N. J., Jr Interleukin-1 inhibitor production by human mononuclear leukocytes and leukocyte subpopulations exposed to respiratory syncytial virus: analysis and comparison with the response to influenza virus. J Leukoc Biol. 1989 Sep;46(3):189–198. doi: 10.1002/jlb.46.3.189. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Schwarting R., Springer T. A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986 Nov 1;137(9):2891–2900. [PubMed] [Google Scholar]
- Mincheff M. S., Meryman H. T. Costimulatory signals necessary for induction of T cell proliferation. Transplantation. 1990 Apr;49(4):768–772. doi: 10.1097/00007890-199004000-00023. [DOI] [PubMed] [Google Scholar]
- Mock D. J., Domurat F., Roberts N. J., Jr, Walsh E. E., Licht M. R., Keng P. Macrophages are required for influenza virus infection of human lymphocytes. J Clin Invest. 1987 Feb;79(2):620–624. doi: 10.1172/JCI112856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Horan P. K. Expression of viral antigens after infection of human lymphocytes, monocytes, and macrophages with influenza virus. J Infect Dis. 1985 Feb;151(2):308–313. doi: 10.1093/infdis/151.2.308. [DOI] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Nichols J. E. Regulation of lymphocyte proliferation after influenza virus infection of human mononuclear leukocytes. J Med Virol. 1989 Mar;27(3):179–187. doi: 10.1002/jmv.1890270302. [DOI] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Prill A. H., Mann T. N. Interleukin 1 and interleukin 1 inhibitor production by human macrophages exposed to influenza virus or respiratory syncytial virus. Respiratory syncytial virus is a potent inducer of inhibitor activity. J Exp Med. 1986 Mar 1;163(3):511–519. doi: 10.1084/jem.163.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Salkind A. R., McCarthy D. O., Nichols J. E., Domurat F. M., Walsh E. E., Roberts N. J., Jr Interleukin-1-inhibitor activity induced by respiratory syncytial virus: abrogation of virus-specific and alternate human lymphocyte proliferative responses. J Infect Dis. 1991 Jan;163(1):71–77. doi: 10.1093/infdis/163.1.71. [DOI] [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Seeger R. C., Oppenheim J. J. Synergistic interaction of macrophages and lymphocytes in antigen-induced transformation of lymphocytes. J Exp Med. 1970 Jul 1;132(1):44–65. doi: 10.1084/jem.132.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacholtz M. C., Patel S. S., Lipsky P. E. Leukocyte function-associated antigen 1 is an activation molecule for human T cells. J Exp Med. 1989 Aug 1;170(2):431–448. doi: 10.1084/jem.170.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. T cell induction of membrane IL 1 on macrophages. J Immunol. 1986 Dec 15;137(12):3868–3873. [PubMed] [Google Scholar]
- Werdelin O., Braendstrup O., Pedersen E. Macrophage-lymphocyte clusters in the immune response to soluble protein antigen in vitro. J Exp Med. 1974 Nov 1;140(5):1245–1259. doi: 10.1084/jem.140.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Ransil B. J., Shapiro H. M., Strom T. B. Accessory cell requirement for activation antigen expression and cell cycle progression by human T lymphocytes. J Immunol. 1984 Dec;133(6):2986–2995. [PubMed] [Google Scholar]



