Abstract
Vascular endothelial growth factor (VEGF)-A mRNA was previously identified as one of the significantly upregulated transcripts in spinal cord injured tissue from adult rats that developed allodynia. To characterize the role of VEGF-A in the development of pain in spinal cord injury (SCI), we analyzed mechanical allodynia in SCI rats that were treated with either vehicle, VEGF-A isoform 165 (VEGF165), or neutralizing VEGF165-specific antibody. We have observed that exogenous administration of VEGF165 increased both the number of SCI rats that develop persistent mechanical allodynia, and the level of hypersensitivity to mechanical stimuli. Our analysis identified excessive and aberrant growth of myelinated axons in dorsal horns and dorsal columns of chronically injured spinal cords as possible mechanisms for both SCI pain and VEGF165-induced amplification of SCI pain, suggesting that elevated endogenous VEGF165 may have a role in the development of allodynia after SCI. However, the neutralizing VEGF165 antibody showed no effect on allodynia or axonal sprouting after SCI. It is possible that another endogenous VEGF isoform activates the same signaling pathway as the exogenously-administered 165 isoform and contributes to SCI pain. Our transcriptional analysis revealed that endogenous VEGF188 is likely to be the isoform involved in the development of allodynia after SCI. To the best of our knowledge, this is the first study to suggest a possible link between VEGF, nonspecific sprouting of myelinated axons, and mechanical allodynia following SCI.
Key words: microarray analysis, myelinated axon sprouting, neuropathic pain, rat spinal cord injury, vascular endothelial growth factor
Introduction
Although published studies have reported a widely divergent incidence of chronic pain among individuals with traumatic spinal cord injury (SCI), ranging from 26–96% (Dijkers et al., 2009), chronic pain is nevertheless a devastating and debilitating condition, and current treatments are largely ineffective in relieving it (Finnerup et al., 2001; Hulsebosch, 2005; Siddall et al., 2003; Yezierski, 1996).
Chronic pain results from persistent sensitization within the pain transmission pathways and thus may develop peripherally, at the level of spinal cord, or in supraspinal centers (Bolay and Moskowitz, 2002). Almost all of the studies investigating sensitization underlying SCI-induced pain have focused on cellular and molecular changes within the injured spinal cords. It appears that a plethora of different mechanisms triggered by SCI (Hulsebosch, 2005; Hulsebosch et al., 2009) underlie persistent alterations in neuronal excitability (Hains et al., 2003; Ji et al., 2003; Waxman and Hains, 2006), and contribute to the potentiation of nociceptive signals. For example, SCI induces upregulation and redistribution of sodium channels (Hains and Waxman, 2007), upregulation of vanilloid (DomBourian et al., 2006) or glutamate receptors (Mills and Hulsebosch, 2002; Mills et al., 2001), and triggers pathological astrocytic and microglial activation (Hulsebosch et al., 2009) that underlie the development of pain.
Maladaptive alterations and sensitization of nociceptive signaling in injured cords are also driven by a more complex remodeling of pain-processing circuits within the dorsal horns. It has been shown that this functional reorganization within the dorsal horns results from excessive and nonspecific sprouting of primary afferent sensory fibers, and loss of supraspinal innervations/neurons after injury (Hoschouer et al., 2009; Kalous et al., 2007). It was also shown that contusion SCI induces increased abundance and extensive spreading of primary afferents, and that a reduction in the abundance of sensory axons is associated with decreased pain after SCI (Nesic et al., 2008).
Vascular endothelial growth factor (VEGF) is among the significantly upregulated genes found in SCI rats with allodynia (Nesic et al., 2005). Despite the well-established role of VEGF in axonal growth (Zachary, 2005), there are no reports linking VEGF, excessive sprouting, and neuropathic pain in SCI. Therefore the goal of this study was to directly test whether exogenous administration of VEGF165 enhances development of the neuropathic pain-like behavior in SCI rats, and whether treatment with neutralizing VEGF antibody affects mechanical allodynia in rats with SCI. The results of our study suggest a novel finding that VEGF165 treatment increases the incidence of mechanical allodynia in injured rats that is associated with excessive axonal sprouting of myelinated axons in the dorsal horns/columns of SCI rats. To the best of our knowledge, this study is the first to suggest a possible link between VEGF, nonspecific axonal sprouting in both dorsal horns and dorsal columns, and mechanical allodynia after SCI.
Methods
Rat spinal cord injury
The protocol used in this study was reviewed and approved by the institutional animal welfare committee. The guidelines provided in the National Institutes of Health Guide for the Care and Use of Laboratory Animals were strictly followed.
Spinal cord injuries were produced as previously described (Herrera et al., 2009). Briefly, rats (male Sprague-Dawley rats, weight 300–350 g) were anesthetized with 4% isoflurane, and maintained under anesthesia with a mixture of 2% isoflurane, air, and oxygen, administered through a Harvard rodent ventilator (Model 683; Harvard Apparatus, Holliston, MA), during the entire procedure. A laminectomy was performed at the seventh thoracic vertebra (T7), and a 150-kDyn force with a 1 sec dwell time was delivered to the exposed cord using an Infinite Horizon Impactor (Precision Systems and Instrumentation, Lexington, KY), to produce a moderate level of injury. The sham animals underwent the same surgical procedure, but received only a laminectomy.
The animals were allowed to recover in warmed cages and received subcutaneous injections of enrofloxacin (2.5 mg/kg; Bayer Healthcare LLC Animal Division, Shawnee Mission, KS) twice a day for 3–5 days, and buprenorphine (0.01 mg/kg; Hospira, Inc., Lake Forest, IL) twice a day for 5 days. The animals were also administered subcutaneous injections of saline twice daily for 5 days. The animals' bladders were manually expressed twice daily until the return of spontaneous urination. The animals had free access to food and water.
VEGF165 and anti-VEGF treatments
The injured rats were randomly assigned to one of three groups: group 1 (n = 12) received a single 1.5-μL injection of recombinant VEGF165 (4 μg/mL, #293-VE; R&D Systems, Minneapolis, MN); group 2 (n = 11) received 1.5 μL of anti-VEGF (4 μg/mL, #AF564; R&D Systems); and group 3 (n = 13) received saline immediately after injury. The sham controls in group 4 received laminectomy only (n = 9). The injections were delivered at a depth of 1.2 mm below the surface directly into the contusion site at a rate of 0.5 μL/min through a glass pulled needle driven by a picospritzer (Parker Hannifin Corporation, Fairfield, NJ). We used the same concentration of VEGF165 that was employed in a previous study (Widenfalk et al., 2003), while the concentration of anti-VEGF165 was based on the study done by Fischer and associates (Fischer et al., 1999). Our previous study confirmed that this antibody recognizes the endogenous rat VEGF164 isoform (Hererra et al., 2009; rat VEGF isoforms are one amino acid shorter than human isoforms). Animals were sacrificed 8 weeks after SCI for immunocytochemical or biochemical analyses. For immunocytochemical analysis we used six animals per group.
Immunocytochemistry and image acquisition
The animals were transcardially perfused with 0.1 M phosphate-buffered saline (PBS), followed by 4% paraformaldehyde, on post-injury day 56. The spinal cords were then removed, post-fixed overnight in 4% paraformaldehyde, and then immersed in 30% sucrose-phosphate-buffered saline (0.1 M PBS) for 2–3 days at 4°C. The spinal cords were coronally sectioned at 35 μm using a cryostat (model CM1800; Leica, Bannockburn, IL), and stored at −20°C in tissue-storing medium.
Tissue sections (n = 8/animal) from rostral and caudal areas spanning a 1-cm section of cord that included the injury epicenter were examined. We assessed the abundance of myelinated axons using the SMI-31 antibody, which recognizes phosphorylated neurofilament (1:1000, SMI-31R; Covance, Emeryville, CA), and non-myelinated primary afferents by using calcitonin gene-related peptide (CGRP, 1:1000, Abcam, Cambridge, U.K.). The specificity of the primary antibodies was checked using negative controls, which showed no non-specific labeling. Alexa-Fluor dye-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) were used at a dilution of 1:500 in 0.1 M PBS. The tissue sections were viewed and captured using a Spot Flex digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) attached to a Leica RX1500 upright microscope. The operator acquiring the images was blinded to group assignment. We measured the relative fluorescence intensities of SMI-31- and CGRP-labeled axons in the dorsal columns and dorsal horns, as previously described (Herrera et al., 2008). Briefly, the sections were imaged and a region of interest was drawn. The fluorescence intensities were quantified using ImagePro Plus software (Media Cybernetics, Inc., Silver Spring, MD). The relative threshold levels were determined from control sections, and then applied to the treatment groups. The levels were then compiled in a Microsoft Excel spreadsheet and statistically analyzed, as described below.
DNA microarray analysis
A detailed description of this procedure can be found in Nesic et al., 2005. Total RNA was prepared from frozen spinal cord segments using TRI-Reagent (Molecular Research Center, Cincinnati, OH). Spinal segments (five segments rostral and five segments caudal from the site of injury) were homogenized in TRI-Reagent, and total RNA was extracted in chloroform, ethanol precipitated, and stored at −80°C. Total RNA was assayed for integrity on 1% denaturing agarose gels. Approximately 15 μg of total RNA was used for each target. The rat RNA microarray from Affymetrix (Santa Clara, CA) was used in all hybridizations. The results were analyzed with Affymetrix GeneChip Analysis Suite 5.0 software. Genes with significantly changed expression levels in any group being compared were identified using the statistical analysis of microarrays (SAM), a robust statistical method devised specifically for the analysis of microarray data (Tusher et al., 2001). Only those mRNA values with a fold change that was higher than 1.5-fold for upregulated genes, and lower than 0.66 for downregulated genes, were used for SAM analysis. This pre-filtering procedure decreased the number of genes that were being analyzed, and reduced the number of false-positives from the SAM analysis.
Behavioral tests
Each animal was evaluated on days 3, 7, 14, 28, 42, and 56 post-SCI using the open field locomotion scale (Basso, Beattie, and Bresnahan scale; BBB scale) for testing hindlimb function (Basso et al., 1995). Once the animals were able to maintain a weight-supported stance, the von Frey filament test for assessing sensory function (Christensen and Hulsebosch, 1997; Peng et al., 2006) was performed in addition to the BBB scale.
Mechanical allodynia is one of the typical symptoms of neuropathic pain, characterized by hypersensitivity to innocuous mechanical stimuli (Woolf and Mannion, 1999). To test sensitivity to mechanical stimuli, the rats were placed in clear acrylic glass cubicles on an elevated metal mesh floor and allowed to acclimate to the new environment for 15 min. Following the acclimation period, the forepaw-withdrawal response to mechanical stimuli was recorded using an electronic Von Frey anesthesiometer (IITC Life Science, Inc., Woodland Hills, CA). The von Frey filament was applied to the plantar surface of each paw, and the minimum pressure required to elicit a response was automatically recorded (Peng et al., 2006). Five scores for each paw were recorded; the lowest and highest values were discarded and the middle three scores were used for analysis. To determine whether animals were experiencing pain at a given time point, the average of the three pressure values recorded for each paw were compared to the baseline scores, which were recorded prior to injury.
Novel analysis of the allodynia in SCI rats
For testing the effect of VEGF165 on allodynic behavior in SCI rats, we assessed: (1) the difference in pain levels (e.g., decreases in mechanical thresholds) between vehicle- and VEGF165-treated SCI rats, and (2) the incidence of allodynic behavior among SCI rats (the number of SCI rats developing allodynia in those two groups of SCI rats).
To establish the difference in pain levels between the two SCI groups, we first normalized the post-SCI mechanical thresholds (grams of force measured with the von Frey filaments) to baseline (pre-SCI) threshold values (set to 100%; Nesic et al., 2005, 2008), because of the considerable inter-individual variability in the mechanical thresholds of the forelimbs among the uninjured rats (ranging from 2.5–20 g). Therefore, the pain level in an SCI group was calculated as the mean percent change in mechanical thresholds compared to the pre-SCI baseline (Fig. 1).
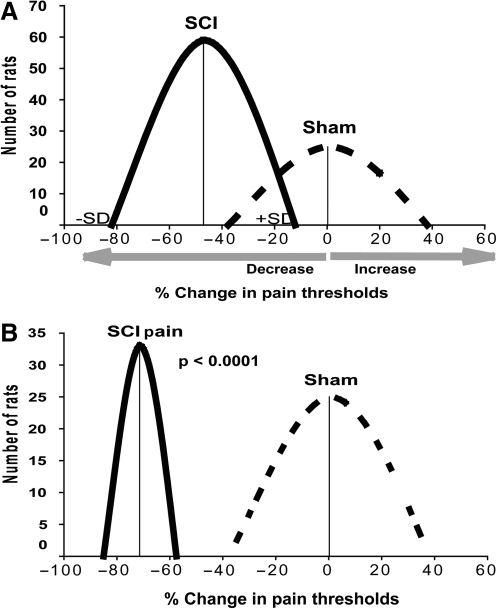
FIG. 1.
Categorization of uninjured and injured rats based on the percentage of mechanical threshold changes seen in the forelimbs after spinal cord injury (SCI), or sham treatment, versus pre-SCI baseline values, using K-means clustering. The y axis shows the number of rats analyzed, and the x axis the percentage change in forelimb mechanical thresholds at 4 weeks compared to baseline values (before sham treatment or SCI, set to 100%). (A) Sham rats (n = 25) showed a mean decrease of −0.23 ± 37.8%, while contused rats (SCI; n = 59) showed a mean decrease of −47 ± 34.93%. (B) After introducing a 40% criterion, a group of SCI rats (33 out of 59) showed significantly increased sensitivity to mechanical stimuli compared to sham rats.
Not all SCI patients develop neuropathic pain. Similarly, not all moderately contused rats developed allodynia, although the reported incidence varies from 20% (Nesic, et al., 2005), to 50% (Drew et al., 2004), to 100% (Tan et al., 2008). To determine the number of SCI rats that developed allodynia after SCI, we first established a cutoff criterion for discriminating the mechanical sensitivity of SCI rats that displayed painful behavior from spontaneous fluctuations in mechanical sensitivity among uninjured rats.
Ideally, the mechanical threshold in an uninjured rat should remain the same regardless of the time it is measured. However, mechanical thresholds measured in a group of sham rats (n = 9) at four time points after laminectomy (0, 2, 4, 6, and 8 weeks), showed that the mechanical threshold in the same sham rat could fluctuate around baseline value by as much as 40%. In a group of uninjured rats (n = 25, collected over 1 year), mechanical thresholds were measured at baseline and at 4 weeks after laminectomy. As shown in Figure 1A, sham animals showed marked differences in mechanical sensitivity between baseline levels and those seen at 4 weeks post-laminectomy. This likely was the result of spontaneous fluctuations in the mechanical sensitivity of the forelimbs of uninjured rats, given that laminectomy alone has not been reported to generate neuropathic pain. Furthermore, similar fluctuations were also observed in naive rats (data not shown). Although the average percent change in mechanical thresholds of the 25 sham rats was close to zero (Fig. 1), the standard deviation (SD) showed a large variation in mechanical threshold changes, ranging from a ∼35% decrease to a ∼35% increase.
The change seen in mechanical thresholds in SCI rats at 4 weeks post-SCI (n = 59, data collected over 1 year; allodynia was measured only at 4 weeks post-SCI) showed that the average percent change was nearly a 50% decrease (e.g., sensitivity to mechanical stimuli was increased by 50% after SCI). However, a large range of inter-individual variability, similar to that seen in the sham rats (±30%), resulted in an overlap of the threshold decreases between injured and sham rats. This result clearly indicates that not all of the decreases seen in mechanical thresholds among SCI rats reflected pain-like behavior. The K-means clustering method demonstrated that all SCI rats that showed decreases in pain thresholds greater than 40% at 4 weeks post-SCI could be categorized as SCI rats manifesting pain-like behavior (Fig. 1B). After introduction of the 40%-decrease criterion, the separation between threshold fluctuations among sham rats and pain-like behavior among SCI rats became clear (Fig. 1B). This analysis suggested that 44% of all moderately contused rats (33 versus 59; compare the number of rats shown on the y axis in Fig. 1A and B) could be considered to be SCI rats that developed pain 4 weeks after SCI.
However, neuropathic pain after SCI is defined not as a transient, but as a chronic condition that lasts for years if not for life in some SCI patients (Baastrup and Finnerup, 2008). Therefore the mechanical thresholds should be persistently lower in SCI rats that develop allodynia. This must be confirmed at different time points during the chronic phase of injury.
We suggest that the analysis of allodynia after SCI should include in each experiment and for each injury level (severe, moderate, or mild) the following: (1) the determination of the cutoff criterion using the K-means clustering method (e.g., the percentage of the decrease in mechanical thresholds that discriminates the normal variable mechanical sensitivity of SCI versus sham-treated rats); and (2) several measurements of mechanical thresholds during the chronic post-SCI phase to confirm the persistent nature of allodynia in SCI rats. Such stringent criteria would likely reduce the number of rats that would be considered to be manifesting chronic allodynia (Figs. 1 and 2), and the discrepancies seen among different studies, thus allowing the use of a more reliable model for studying neuropathic pain after SCI.
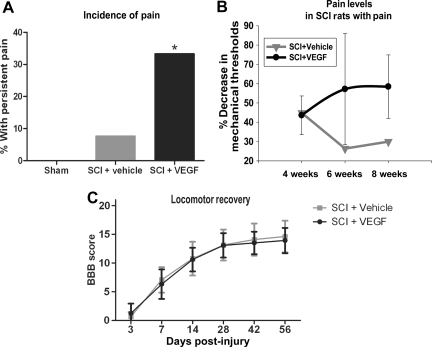
FIG. 2.
(A) Analysis of mechanical allodynia, as described in Figure 1 and in the methods section. (A) Incidence of pain. Animals that had increased sensitivity in both forelimbs at all time points tested were considered to demonstrate persistent pain. None of the sham animals had persistent pain, while 8% of vehicle-treated spinal cord injury (SCI) rats and 34% of VEGF165-treated animals had persistent pain. The chi square test showed a statistically significant difference between VEGF165-treated and vehicle-treated SCI rats (*p < 0.05). (B) Pain levels. Shown are the percentage decreases in mechanical thresholds seen at 4, 6, and 8 weeks post-SCI in rats that developed persistent allodynia. Although the percentage decrease is represented by negative numbers in Figure 1, here we present it as a positive number (on the y axis), so a higher percentage indicates increased sensitivity (e.g., lower thresholds to mechanical stimuli or increased pain levels in post-SCI rats compared to pre-injury baseline levels). (C) Basso, Beattie, and Bresnahan (BBB) scale scores (mean ± standard deviation) of all SCI rats used in this study showed no effect of VEGF165 administration on motor recovery after SCI (VEGF, vascular endothelial growth factor).
In our experiments rats that showed decreased thresholds in both forelimbs (1) by ≥ 40% at 4 weeks after SCI, and (2) in which they remained decreased at 6 and 8 weeks after SCI, were considered as manifesting chronic allodynia.
Statistical analysis
All statistical tests were evaluated at an alpha level of 0.05, two-tailed. We used parametric methods (t-test) for our analyses. However, if the assumptions for these tests were not met, we proceeded with non-parametric analyses (Mann-Whitney U test). Likewise, we used non-parametric methods to check all parametric test results as a safeguard. If the results were not consistent, we reported the results from the non-parametric tests. The K-means clustering was performed using SPSS software (SPSS Inc., Chicago, IL). To analyze the incidence of chronic pain-like behavior among SCI rats we used the X2 test. The decision process of determining if pain was present was based on the significance level (p < 0.05) obtained from the X2 test.
Results
VEGF and SCI pain
Using the approach described above, we found that none of the sham rats developed allodynia, while ∼8% of vehicle-treated SCI rats developed persistent allodynia (Fig. 2A). In contrast to vehicle-treated SCI rats, a significantly higher number of VEGF165-treated SCI rats (∼34%; p < 0.05) developed chronic allodynia. VEGF165 treatment significantly increased not only the incidence of pain (Fig. 2B), but also the extent of increased mechanical sensitivity in SCI rats (Fig. 2B). The percent reduction in mechanical thresholds gradually increased over time in VEGF165-treated SCI rats (Fig. 2C), in contrast to vehicle-treated SCI rats. These results indicate that exogenous administration of VEGF165 likely amplified and enhanced the processes underlying the development of neuropathic SCI pain.
VEGF and axonal abundance
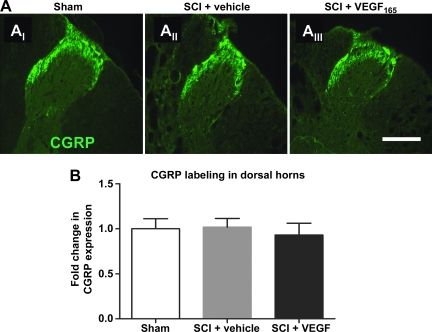
Given that several studies have shown a stimulatory effect of VEGF on axonal growth (Zachary, 2005), and that aberrant axonal growth/sprouting underlies the development of neuropathic pain post-SCI (Hoschouer et al., 2009; Kalous et al., 2007; Macias et al., 2006), we hypothesized that VEGF165 stimulates excessive axonal growth, and thus increases mechanical hypersensitivity in SCI rats (Fig. 2). In previous studies (Hoschouer et al., 2009; Kalous et al., 2007; Macias et al., 2006), researchers also reported that SCI pain was associated with excessive sprouting of pain-processing unmyelinated axons expressing calcitonin gene-related peptide (CGRP). Therefore we analyzed the effect of VEGF165 on CGRP labeling in the dorsal horns in two regions: (1) laminae I and II (which normally contain most of the CGRP-positive axons), and (2) laminae III–V (which normally contain only a small number of CGRP-expressing axons). Our analysis showed that exogenous VEGF165 did not affect CGRP labeling at or near the lesion's epicenter (T6–T10) at 8 weeks after SCI (Fig. 3).
FIG. 3.
(A) A representative example of calcitonin gene-related peptide (CGRP) immunolabeling in sham rats (AI); vehicle-treated spinal cord injury (SCI) rats (AII), and vascular endothelial growth factor (VEGF)165-treated SCI rats (AIII), that were classified as SCI rats that developed pain (scale bar = 100 μm; T6 segment at 8 weeks post-injury). (B) Quantitative analysis of the intensity of CGRP labeling in two segments rostral and two segments caudal from the site of injury (T6–T10; n = 6 animals per group; values normalized to sham = 1).
Our transcriptional analysis also showed that SCI pain was associated not only with higher levels of endogenous VEGF-A mRNA (Nesic et al., 2005), but also with significantly increased expression of neurofilaments (NFs; Fig. 4A; p < 0.05; n = 4 per group; not previously reported), suggesting increased axonal abundance, but also indicating excessive sprouting of myelinated axons, since NFs are the largest cytoskeletal component of myelinated axons. Nonspecific and excessive sprouting of myelinated axons has not yet been associated with neuropathic pain development after SCI. Although the increases in the heavy NF mRNA (NF-H, 1.6-fold) were not as robust as those seen in the light NF mRNA (NF-L, 2.8-fold), the development of allodynia after SCI was associated with an increased presence of large myelinated axons throughout injured cords, encompassing five segments rostral (Fig. 4A) and caudal (not shown) to the site of injury.
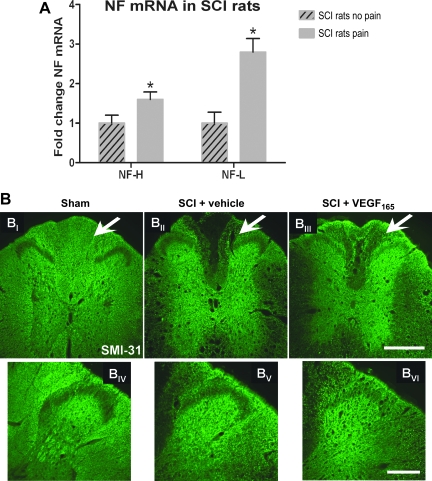
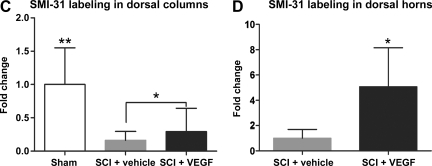
FIG. 4.
(A) Neurofilament (NF)-H and NF-L mRNA were assessed in spinal cord injury (SCI) rats with and without spontaneous pain (n = 4 per group) using DNA microarrays (a method previously described in Nesic et al., 2005). mRNA levels of NF-H and NF-L were significantly increased (*p < 0.05) in five segments rostral from the site of injury. mRNA values were normalized to the levels of mRNAs in SCI rats without pain (set to = 1; mean ± standard deviation). Similarly significant increases in NF mRNAs were also found in five segments caudal from the site of injury (data not shown). (B) A representative example of SMI-31 labeling in sham rats (BI), vehicle-treated SCI rats (BII), and VEGF165-treated SCI rats (BIII), that were classified as SCI rats that developed pain (scale bar = 500 μm; T6 segment at 8 weeks post-injury). Normal labeling for SMI-31 (Sham) was significantly lower in vehicle-treated and VEGF165-treated SCI rats. However, VEGF165-treated SCI rats had visibly more SMI-31-labeled axons in the dorsal columns (marked with arrows), and dorsal horns, than vehicle-treated SCI rats. (BIV–BVI) SMI-31-labeled thick myelinated axons are widely scattered in sham and vehicle-treated dorsal horns, but are visibly increased in the dorsal horns of VEGF165-treated cords (scale bar = 200 μm). (C) Quantitative analysis of SMI-31 labeling in the dorsal columns of spinal cord segments around the site of injury (T6–T10) in three experimental groups (n = 6 animals per group). Sham animals had significantly more SMI-31 labeling compared to both vehicle-treated and VEGF165-treated animals (average ± standard deviation; **p < 0.0001; values normalized to sham = 1). However, VEGF165-treated SCI rats had significantly more SMI-31-labeled axons in the dorsal columns than vehicle-treated SCI rats (*p < 0.01). (D) Semi-quantitative analysis of SMI-31 labeling in the dorsal horns in the same sections used for the analysis shown in C. (average ± standard deviation; *p < 0.05; values normalized to sham = 1). SCI pain was associated with significant twofold increases in SM-31 labeling (p < 0.05) in the dorsal horns, while VEGF165-induced SCI pain was associated with additional significant increases in SM-31 labeling (2.5-fold; p < 0.05; VEGF, vascular endothelial growth factor).
To test whether VEGF165-induced SCI pain was also associated with anincreased abundance of myelinated axons, we quantitatively analyzed SMI-31 labeling in three experimental groups (Fig. 4B and C). SMI-31 antibody primarily recognizes phosphorylated 200-kDa neurofilament (NF-H) contained only in large myelinated axons (Starr et al., 1996). Representative immunofluorescence images of sham, vehicle-treated, and VEGF165-treated SCI rats labeled with SMI-31 are shown in Figure 4B. Sham animals demonstrated intense SMI-31 labeling in all regions (Fig. 4BI), except for the superficial laminae of the dorsal horn, which contains primarily non-myelinated sensory fibers. Chronically injured cords (8 weeks post-contusion), in both vehicle-treated and VEGF165-treated SCI rats, showed a marked (∼80%) reduction in NF-H immunolabeling in the rostral and caudal regions, compared to sham spinal cords (Fig. 4BII). Significant decreases in NF-H labeling, especially those visible in the lateral white matter regions and in the dorsal columns of SCI rats (∼80%; Fig. 4BII), reflected substantial loss of large myelinated axons after contusion injury, as previously reported by Zhang and associates (2000). However, as shown in Figure 4BIII and 4C, VEGF165 treatment resulted in significantly increased NF-H labeling in SCI rats, particularly in the dorsal columns, compared to vehicle-treated rats (29.24%; p < 0.001).
Increased NF-H labeling was also found in laminae I and II of the dorsal horns of VEGF165-treated SCI rats (Fig. 4D). VEGF165-treated SCI rats that were classified as experiencing chronic pain had significantly more myelinated fibers in the dorsal horns (a 2.5-fold increase; p < 0.05) than vehicle-treated SCI rats. Interestingly, the intensity of the SMI-31 labeling in dorsal horns normally devoid of large myelinated fibers was also significantly enhanced (increased twofold; p < 0.05) in SCI rats that developed allodynia compared to uninjured rats. Taken together, these results suggest that SCI pain, which is associated with an increased abundance of SMI-31-positive axons, can be further potentiated by the VEGF165-induced increases in aberrant, nonspecific axonal sprouting seen in pain-processing regions.
However, VEGF165 administration increased neurofilament abundance regardless of the presence of allodynia (e.g., we did not find a significant difference between SMI-31 labeling in allodynic versus non-allodynic SCI rats treated with VEGF165; data not shown). This result suggests that the effects of VEGF on axonal abundance are likely only one of many processes that contribute to the development of SCI pain, a result in agreement with that of a previous study (Nesic et al., 2005).
VEGF isoforms and SCI pain
Here we showed that exogenous administration of one of the VEGF-A isoforms, VEGF165, promotes neuropathic pain in SCI rats. Therefore we investigated whether the endogenous VEGF-A associated with the development of neuropathic pain after SCI (Nesic et al., 2005) is the VEGF165 isoform. To test this hypothesis we administered an antibody that specifically neutralizes VEGF165 (4 μg/mL). It has been shown that this antibody recognizes the endogenous rat VEGF165 isoform (Herrera et al., 2009).
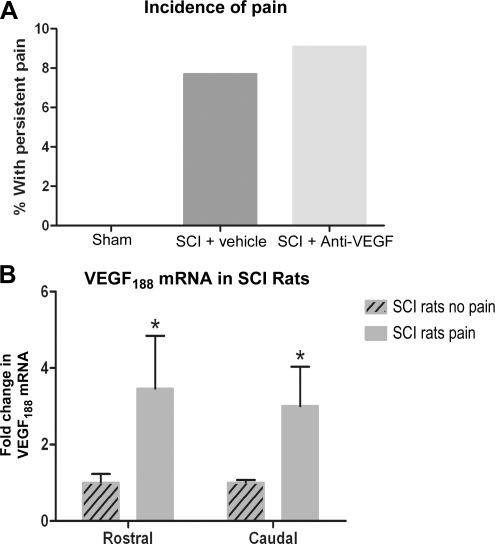
We measured tactile allodynia in three experimental groups: sham (n = 9), vehicle-treated (n = 13), and anti-VEGF165-treated (n = 11) rats, at 4, 6, and 8 weeks after SCI. Using the analysis described in the methods section, we found no difference in pain incidence between SCI rats treated with vehicle (7.7%), and those treated with the anti-VEGF165 antibody (Fig. 5A; 9%), or overall pain levels at any time point (not shown). For example, the average decrease in mechanical thresholds at 8 weeks post-SCI in anti-VEGF-treated SCI rats was 26.1 ± 26%, compared to 21 ± 15.84% in vehicle-treated SCI rats, consistent with the lack of effect of anti-VEGF on SMI-31 immunolabeling (data not shown). Taken together, these data indicate that VEGF isoforms other than VEGF165 are involved in spontaneous pain development (Nesic et al., 2005), and contribute to the non-specific axonal growth seen after SCI (Fig. 4).
FIG. 5.
(A) The incidence of chronic allodynia in spinal cord injury (SCI) rats was not affected by anti-VEGF165 treatment. (B) VEGF188 mRNA levels assessed in SCI rats with and without spontaneous pain (n = 4 per group; mean ± standard deviation) using DNA microarrays were significantly elevated in SCI rats with pain (*p < 0.05; as previously described by Nesic et al., 2005; VEGF, vascular endothelial growth factor).
The DNA microarray analysis (Nesic et al., 2005) also showed that mRNA levels of the VEGF isoform 188 (VEGF188; accession no. L2091) were selectively and significantly increased (Fig. 5C; p < 0.05) in the same SCI rats that developed pain and had increased NF mRNA levels (Fig. 4A). VEGF188 increases associated with allodynia were found in the five segments rostral and caudal to the site of injury, indicating widespread VEGF188 upregulation. The other VEGF isoforms (e.g., 121 or 145) were not quantified by the Affymetrix microarrays we used in our microarray study, and thus any possible changes in their expression related to pain development or excessive axonal growth remain to be investigated.
Discussion
Axonal sprouting and SCI pain
Although collateral axonal sprouting may be of great importance in recovery after SCI (Helgren and Goldberger, 1993; Murray and Goldberger, 1974), the results of several studies have suggested that excessive sprouting of primary afferents, primarily nonmyelinated axons, is one cause of the neuropathic pain seen after SCI (Christensen and Hulsebosch, 1997; Hofstetter et al., 2005; Hoschouer et al., 2009; Krenz and Weaver, 1998; Ondarza et al., 2003; Romero et al., 2000). Excessive sprouting of nonmyelinated sensory axons within dorsal horns was not only found in animal models of SCI (Cameron et al., 2006; Tang et al., 2007), but was also seen in human SCI patients (Ackery et al., 2007). However, our analysis of CGRP-positive unmyelinated pain-processing axons suggests that aberrant sprouting of CGRP axons is not the primary mechanism behind VEGF165-induced pain development in SCI rats.
Although excessive sprouting of myelinated fibers within dorsal horns after SCI has been documented (Di Giovanni et al., 2005; Krenz and Weaver, 1998), its association with neuropathic pain-like behavior has not been established. However, Nakamura and Myers (1999) found that pain after chronic constriction nerve injury was associated with sprouting of myelinated fibers into the dorsal horns, supporting our findings presented here.
Here we showed that both SCI pain (Fig. 4A), and VEGF165-induced SCI pain (Fig. 4B, C, and D), were associated with increased abundance of myelinated fibers throughout the injured cords, including the dorsal horns and dorsal columns (DCs). The increased abundance of SMI-31-labeled axons in VEGF165-treated SCI rats indicates two possibilities: (1) VEGF165 spared axons after SCI, consistent with other reports of the neuroprotective effects of VEGF (Zachary, 2005), and/or (2) VEGF165 induced excessive axonal regeneration/sprouting of surviving axons, a finding in agreement with previous reports of the effects of VEGF on axonal growth (Zachary, 2005). Given that lesions of DC axons can cause motor deficits, while sparing of DC axons improves locomotor recovery after SCI (Brodal, 1992; Nakashima et al., 2008), and that motor recovery of SCI rats treated with VEGF165 did not improve (Fig. 2B), we conclude that increased SMI-31 labeling in VEGF165-treated SCI rats reflected predominantly nonspecific, aberrant sprouting of myelinated axons, although some axonal sparing could not be excluded. As thick myelinated axons are normally only scarcely present in dorsal horns (Fig. 4BIV), our results confirm that axonal sprouting underlying SCI pain (Fig. 4BVI) is aberrant and nonspecific, and thus can contribute to the pathological alterations seen in pain processing.
If SMI-31-labeled axons are collaterals of the primary myelinated afferents within dorsal horns, they should convey touch sensation via thick Aβ fibers and thin Aδ myelinated axons ending in laminae III–V or I, respectively. Our results showed that SMI-31-labeled myelinated fibers increase throughout laminae I–IV in VEGF165-treated SCI rats, thus indicating that nonspecific connections established by touch-sensing myelinated primary afferents may contribute to the decreased mechanical thresholds seen after SCI.
Thick myelinated DC axons relay tactile information from the fore- and hindlimbs to the gracile and cuneate nuclei. These pathways have been recognized as important in the pathogenesis of the tactile allodynia caused by nerve injury (Bian et al., 1998; Sun et al., 2001; Sung et al., 1998; Terayama et al., 2008), and in mechanical, but not thermal, allodynia seen after hemisection injury (Kim et al., 2005). However, a possible role of DC axons in the development of mechanical allodynia after contusion SCI has not been studied.
Alternative splicing of the human VEGF-A gene gives rise to at least six different transcripts, encoding isoforms of 121, 145, 165, 183, 189, and 206 amino acid residues (the corresponding rat VEGF isoforms are one amino acid shorter). All VEGF-A isoforms activate two receptors, VEGFR1 and VEGFR2, and some activate neuropilins (Ferrara et al., 2003). Activation of both VEGFRs and neuropilins has been implicated in the regulation of axonal growth. For example, it has been shown that the effect of VEGF-A on the growth of sensory axons is mediated through the activation of VEGFR2 (Sondell et al., 2000). When binding to axonal neuropilins (Agudo et al., 2005), VEGF competes with semaphorins, which are known to have negative effects on axonal guidance and growth (Hou et al., 2008). It has already been shown that overexpression of semaphorin 3A in injured spinal cords significantly inhibits excessive sprouting of sensory unmyelinated fibers and reduces mechanical allodynia (Cameron et al., 2006; Rabchevsky, 2006; Tang et al., 2004). Therefore it is possible that excess VEGF in VEGF165-treated injured cords binds to neuropilins, outcompeting repulsive semaphorins and promoting excessive axonal growth, but this remains to be investigated.
VEGF165-induced versus SCI pain
Here we showed that exogenous VEGF administration significantly increased the incidence of pain development after SCI. This confirms that the VEGF-A upregulation seen in SCI rats that spontaneously develop allodynia (Nesic et al., 2005) may play an important role in the development of SCI pain. Given that spontaneous SCI allodynia was associated with an increased abundance of myelinated axons (Fig. 4A and D), and that exogenous VEGF-A additionally increased aberrant sprouting of myelinated axons (Fig. 4B, C, and D), we conclude that increases in endogenous VEGF-A contribute to SCI pain development by promoting excessive growth of myelinated axons, especially within pain-processing regions. However, the effect of VEGF165 on axonal growth alone cannot explain the development of chronic allodynia, since we did not find a significant difference in axonal abundance between VEGF165-treated SCI rats with and without pain. This result supports previous findings (Nesic et al., 2005), that there is a long list of molecules and processes, including aberrant axonal sprouting, that all work in concert to cause the pathological alterations in pain processing in injured spinal cords. For example, another possible mediator of the effect of VEGF165 on SCI pain is amplified glial activation, given that VEGF is known to induce glial activation (Krum et al., 2002; Forstreuter et al., 2002), and SCI pain has been associated with increased glial activation (Hulsebosch et al., 2009). Our analysis of astrocytic and microglial activation at and around the site of injury at 8 weeks post-SCI showed no effect of VEGF165 treatment (data not shown). However, to address the question whether VEGF165 induces glial activation and thus triggers pain-like behavior in SCI rats requires a detailed analysis of the time course and spatial distribution of glial markers, which is beyond the scope of this article, and thus will be presented elsewhere. Our future report will also include the possible contributions of infiltrating inflammatory cells to the SCI pain triggered by VEGF165.
VEGF-A is likely just one of the players in the complex interaction of many factors that underlie the development of SCI pain (e.g., excessive sprouting of myelinated fibers is unlikely to be the only factor contributing to VEGF-induced allodynia, and therefore a direct correlation between axonal growth and allodynia cannot be expected).
This complexity also implies that individual variability among those processes (including different degrees of VEGF-A increases or glial activation) cumulatively may render some SCI rats more susceptible to the development of mechanical allodynia than others (Figs. 1 and 2), a result in agreement with that of a previous study (Nesic et al., 2005), and with the variable incidence of pain seen in human SCI patients.
Our experiments with VEGF165 neutralizing antibody also suggest that VEGF165 is likely not the endogenous isoform that is involved in the development of SCI pain in rats (Fig. 5A). This is consistent with findings of a recent study (Herrera et al., 2009), that showed that endogenous levels of VEGF165 decrease after SCI, and thus it is unlikely to be involved in the development of pain after SCI. Microarray analysis (Fig. 5B) indicated that one of the possible VEGF isoforms involved in pain development may be VEGF188. Although increases in VEGF188 have been found after cerebral ischemia (Pichiule et al., 2003), the role of this VEGF isoform in SCI remains to be investigated. Unfortunately, VEGF188-specific antibodies for use in neutralization experiments that would confirm the results of the microarray study are not yet commercially available. However, the identification and inhibition of axonal VEGF receptors that are activated by both VEGF isoforms (165 and 188) may offer new therapeutic strategies to alleviate post-SCI pain.
Acknowledgments
Part of this work was supported by the National Institutes of Health (NIH)/National Institute for Neurological Disorders and Stroke (grant NS045624 to P.A.N.). The 7-T MRI scanner was funded by the NIH/National Center for Research Resources under the High-End Instrumentation Program (grant S10 RR17205-01 to P.A.N.). It was also supported in part by NIH/NINDS grant NS058417 to O.N., and Mission Connect, a project of the TIRR Foundation, to O.N. The NIH is not responsible for the contents of this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- Ackery A.D. Norenberg M.D. Krassioukov A. Calcitonin gene-related peptide immunoreactivity in chronic human spinal cord injury. Spinal Cord. 2007;45:678–686. doi: 10.1038/sj.sc.3102020. [DOI] [PubMed] [Google Scholar]
- Agudo M. Robinson M. Cafferty W. Bradbury E.J. Kilkenny C. Hunt S.P. McMahon S.B. Regulation of neuropilin 1 by spinal cord injury in adult rats. Mol. Cell Neurosci. 2005;28:475–484. doi: 10.1016/j.mcn.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Baastrup C. Finnerup N.B. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–475. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bian D. Ossipov M.H. Zhong C. Malan T.P., Jr. Porreca F. Tactile allodynia, but not thermal hyperalgesia, of the hindlimbs is blocked by spinal transection in rats with nerve injury. Neurosci. Lett. 1998;241:79–82. doi: 10.1016/s0304-3940(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Bolay H. Moskowitz M.A. Mechanisms of pain modulation in chronic syndromes. Neurology. 2002;59:S2–S7. doi: 10.1212/wnl.59.5_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- Brodal P. The Central Nervous System: Structure and Function. Oxford University Press; New York: 1992. [Google Scholar]
- Cameron A.A. Smith G.M. Randall D.C. Brown D.R. Rabchevsky A.G. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J. Neurosci. 2006;26:2923–2932. doi: 10.1523/JNEUROSCI.4390-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M.D. Hulsebosch C.E. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 1997;147:463–475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S. Faden A.I. Yakovlev A. Duke-Cohan J.S. Finn T. Thouin M. Knoblach S. De Biase A. Bregman B.S. Hoffman E.P. Neuronal plasticity after spinal cord injury: identification of a gene cluster driving neurite outgrowth. FASEB J. 2005;19:153–154. doi: 10.1096/fj.04-2694fje. [DOI] [PubMed] [Google Scholar]
- Dijkers M. Bryce T. Zanca J. Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J. Rehabil. Res. Dev. 2009;46:13–29. [PubMed] [Google Scholar]
- DomBourian M.G. Turner N.A. Gerovac T.A. Vemuganti R. Miranpuri G.S. Tureyen K. Satriotomo I. Miletic V. Resnick D.K. B1 and TRPV-1 receptor genes and their relationship to hyperalgesia following spinal cord injury. Spine (Phila. Pa 1976) 2006;31:2778–2782. doi: 10.1097/01.brs.0000245865.97424.b4. [DOI] [PubMed] [Google Scholar]
- Drew G.M. Siddall P.J. Duggan A.W. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004;109:379–388. doi: 10.1016/j.pain.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Gerber H.P. LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Finnerup N.B. Johannesen I.L. Sindrup S.H. Bach F.W. Jensen T.S. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord. 2001;39:256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- Fischer S. Clauss M. Wiesnet M. Renz D. Schaper W. Karliczek G.F. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am. J. Physiol. 1999;276:C812–C820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- Forstreuter F. Lucius R. Mentlein R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J. Neuroimmunol. 2002;132:93–98. doi: 10.1016/s0165-5728(02)00315-6. [DOI] [PubMed] [Google Scholar]
- Hains B.C. Waxman S.G. Sodium channel expression and the molecular pathophysiology of pain after SCI. Prog. Brain Res. 2007;161:195–203. doi: 10.1016/S0079-6123(06)61013-3. [DOI] [PubMed] [Google Scholar]
- Hains B.C. Klein J.P. Saab C.Y. Craner M.J. Black J.A. Waxman S.G. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgren M.E. Goldberger M.E. The recovery of postural reflexes and locomotion following low thoracic hemisection in adult cats involves compensation by undamaged primary afferent pathways. Exp. Neurol. 1993;123:17–34. doi: 10.1006/exnr.1993.1137. [DOI] [PubMed] [Google Scholar]
- Herrera J.J. Chacko T. Narayana P.A. Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J. Neurosci. Res. 2008;86:443–447. doi: 10.1002/jnr.21481. [DOI] [PubMed] [Google Scholar]
- Herrera J.J. Nesic O. Narayana P.A. Reduced vascular endothelial growth factor expression in contusive spinal cord injury. J. Neurotrauma. 2009;26:995–1003. doi: 10.1089/neu.2008.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter C.P. Holmstrom N.A. Lilja J.A. Schweinhardt P. Hao J. Spenger C. Wiesenfeld-Hallin Z. Kurpad S.N. Frisen J. Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Hoschouer E.L. Yin F.Q. Jakeman L.B. L1 cell adhesion molecule is essential for the maintenance of hyperalgesia after spinal cord injury. Exp. Neurol. 2009;216:22–34. doi: 10.1016/j.expneurol.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S.T. Jiang S.X. Smith R.A. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int. Rev. Cell Mol. Biol. 2008;267:125–181. doi: 10.1016/S1937-6448(08)00603-5. [DOI] [PubMed] [Google Scholar]
- Hulsebosch C.E. From discovery to clinical trials: treatment strategies for central neuropathic pain after spinal cord injury. Curr. Pharm. Des. 2005;11:1411–1420. doi: 10.2174/1381612053507864. [DOI] [PubMed] [Google Scholar]
- Hulsebosch C.E. Hains B.C. Crown E.D. Carlton S.M. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R.R. Kohno T. Moore K.A. Woolf C.J. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kalous A. Osborne P.B. Keast J.R. Acute and chronic changes in dorsal horn innervation by primary afferents and descending supraspinal pathways after spinal cord injury. J. Comp. Neurol. 2007;504:238–253. doi: 10.1002/cne.21412. [DOI] [PubMed] [Google Scholar]
- Kim J. Back S.K. Yoon Y.W. Hong S.K. Na H.S. Dorsal column lesion reduces mechanical allodynia in the induction, but not the maintenance, phase in spinal hemisected rats. Neurosci. Lett. 2005;379:218–222. doi: 10.1016/j.neulet.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Krenz N.R. Weaver L.C. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998;85:443–458. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- Krum J.M. Mani N. Roseustein J.M. Angiogenic and astroglial responses to vascular endothelial growth factor administration in the adult rat brain. Neuroscience. 2002;110:589–604. doi: 10.1016/s0306-4522(01)00615-7. [DOI] [PubMed] [Google Scholar]
- Macias M.Y. Syring M.B. Pizzi M.A. Crowe M.J. Alexanian A.R. Kurpad S.N. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp. Neurol. 2006;201:335–348. doi: 10.1016/j.expneurol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Mills C.D. Hulsebosch C.E. Increased expression of metabotropic glutamate receptor subtype 1 on spinothalamic tract neurons following spinal cord injury in the rat. Neurosci. Lett. 2002;319:59–62. doi: 10.1016/s0304-3940(01)02551-4. [DOI] [PubMed] [Google Scholar]
- Mills C.D. Fullwood S.D. Hulsebosch C.E. Changes in metabotropic glutamate receptor expression following spinal cord injury. Exp. Neurol. 2001;170:244–257. doi: 10.1006/exnr.2001.7721. [DOI] [PubMed] [Google Scholar]
- Murray M. Goldberger M.E. Restitution of function and collateral sprouting in the cat spinal cord: the partially hemisected animal. J. Comp. Neurol. 1974;158:19–36. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- Nakamura S. Myers R.R. Myelinated afferents sprout into lamina II of L3–5 dorsal horn following chronic constriction nerve injury in rats. Brain Res. 1999;818:285–290. doi: 10.1016/s0006-8993(98)01291-8. [DOI] [PubMed] [Google Scholar]
- Nakashima S. Arnold S.A. Mahoney E.T. Sithu S.D. Zhang Y.P. D'Souza S.E. Shields C.B. Hagg T. Small-molecule protein tyrosine phosphatase inhibition as a neuroprotective treatment after spinal cord injury in adult rats. J. Neurosci. 2008;28:7293–7303. doi: 10.1523/JNEUROSCI.1826-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic O. Lee J. Johnson K.M. Ye Z. Xu G.Y. Unabia G.C. Wood T.G. McAdoo D.J. Westlund K.N. Hulsebosch C.E. Regino Perez-Polo J. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J. Neurochem. 2005;95:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- Nesic O. Lee J. Unabia G.C. Johnson K. Ye Z. Vergara L. Hulsebosch C.E. Perez-Polo J.R. Aquaporin 1—a novel player in spinal cord injury. J. Neurochem. 2008;105:628–640. doi: 10.1111/j.1471-4159.2007.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondarza A.B. Ye Z. Hulsebosch C.E. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp. Neurol. 2003;184:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Peng X.M. Zhou Z.G. Glorioso J.C. Fink D.J. Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann. Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Pichiule P. Agani F. Chavez J.C. Xu K. LaManna J.C. HIF-1 alpha and VEGF expression after transient global cerebral ischemia. Adv. Exp. Med. Biol. 2003;530:611–617. doi: 10.1007/978-1-4615-0075-9_60. [DOI] [PubMed] [Google Scholar]
- Rabchevsky A.G. Segmental organization of spinal reflexes mediating autonomic dysreflexia after spinal cord injury. Prog. Brain Res. 2006;152:265–274. doi: 10.1016/S0079-6123(05)52017-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M.I. Rangappa N. Li L. Lightfoot E. Garry M.G. Smith G.M. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. 2000;20:4435–4445. doi: 10.1523/JNEUROSCI.20-12-04435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall P.J. McClelland J.M. Rutkowski S.B. Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Sondell M. Sundler F. Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur. J. Neurosci. 2000;12:4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- Starr R. Attema B. DeVries G.H. Monteiro M.J. Neurofilament phosphorylation is modulated by myelination. J. Neurosci. Res. 1996;44:328–337. doi: 10.1002/(SICI)1097-4547(19960515)44:4<328::AID-JNR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sung B. Na H.S. Kim Y.I. Yoon Y.W. Han H.C. Nahm S.H. Hong S.K. Supraspinal involvement in the production of mechanical allodynia by spinal nerve injury in rats. Neurosci. Lett. 1998;246:117–119. doi: 10.1016/s0304-3940(98)00235-3. [DOI] [PubMed] [Google Scholar]
- Sun H. Ren K. Zhong C.M. Ossipov M.H. Malan T.P. Lai J. Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain. 2001;90:105–111. doi: 10.1016/s0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- Tan A.M. Stamboulian S. Chang Y.W. Zhao P. Hains A.B. Waxman S.G. Hains B.C. Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J. Neurosci. 2008;28:13173–13183. doi: 10.1523/JNEUROSCI.3142-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.Q. Heron P. Mashburn C. Smith G.M. Targeting sensory axon regeneration in adult spinal cord. J. Neurosci. 2007;27:6068–6078. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.Q. Tanelian D.L. Smith G.M. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J. Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terayama R. Omura S. Fujisawa N. Yamaai T. Ichikawa H. Sugimoto T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience. 2008;153:1245–1255. doi: 10.1016/j.neuroscience.2008.03.041. [DOI] [PubMed] [Google Scholar]
- Tusher V.G. Tibshirani R. Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S.G. Hains B.C. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 2006;29:207–215. doi: 10.1016/j.tins.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Widenfalk J. Lipson A. Jubran M. Hofstetter C. Ebendal T. Cao Y. Olson L. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120:951–960. doi: 10.1016/s0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- Woolf C.J. Mannion R.J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Yezierski R.P. Pain following spinal cord injury: the clinical problem and experimental studies. Pain. 1996;68:185–194. doi: 10.1016/s0304-3959(96)03178-8. [DOI] [PubMed] [Google Scholar]
- Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- Zhang S.X. Underwood M. Landfield A. Huang F.F. Gison S. Geddes J.W. Cytoskeletal disruption following contusion injury to the rat spinal cord. J. Neuropathol. Exp. Neurol. 2000;59:287–296. doi: 10.1093/jnen/59.4.287. [DOI] [PubMed] [Google Scholar]