Abstract
Nuclear receptors (NRs) comprise the second largest protein family targeted by currently available drugs, acting via specific ligand interactions within the ligand binding domain (LBD). Recently, farnesyl pyrophosphate (FPP) was shown to be a unique promiscuous NR ligand, activating a subset of NR family members and inhibiting wound healing in skin. The current study aimed at visualizing the unique basis of FPP interaction with multiple receptors in order to identify general structure–activity relationships that operate across the NR family. Docking of FPP to the 3D structures of the LBDs of a diverse set of NRs consistently revealed an electrostatic FPP pyrophosphate contact with an NR arginine conserved in the NR family, a hydrophobic farnesyl contact with NR helix-12 and a ligand binding pocket volume between 300 and 430 Å3 as the minimal requirements for FPP activation of any NR. Lack of any of these structural features appears to render a given NR resistant to FPP activation. We used these structure–activity relationships to rationally design and successfully engineer several mutant human estrogen receptors that retain responsiveness to estradiol but no longer respond to FPP.
Keywords: farnesyl pyrophosphate, nuclear receptors, protein engineering, rational design, structure–activity relationships
Thirteen percent of FDA-approved drugs available on the market today elicit their responses through nuclear receptors (NRs) (Overington et al., 2006). NRs are ligand inducible transcription factors that are directly modulated by ligand binding and can both activate and repress gene expression (Gronemeyer et al., 2004). NRs generally contain three domains: an N-terminal activation function domain 1, a DNA binding domain (DBD) and a ligand binding domain (LBD). Crystallographic structures of LBDs in complex with different ligands suggest that the basis of ligand-induced NR activation lies in a conformational change in the position of the C-terminal helix (H12) of the LBD, which acquires an extended conformation in the unliganded form and a more compact conformation in the liganded form, with slight changes in the conformation of helix-3 (H3) and a loop known as the phi loop upon ligand binding (Renaud et al., 1995; Wurtz et al., 1996). The liganded conformation exhibits a hydrophobic groove that is not exposed in the unliganded conformation. Co-activators or co-repressors bind to this groove resulting in receptor activation or repression, respectively (Ding et al., 1998; Voegel et al., 1998). By this mechanism, NR-regulated gene expression activity is coupled to and exquisitely sensitive to the subtleties of ligand binding and ligand orientation in the ligand binding pocket (LBP).
These structural subtleties of NR ligand–receptor interactions are disease relevant. Estradiol (also known as E2) is the native ligand of the estrogen receptor (ER). Selective estrogen receptor modulators (SERMs) elicit their responses by binding within the ER LBP and inducing SERM-specific conformational changes in ER (Levenson and Jordan, 1998). SERMS such as tamoxifene and raloxifene (RAL), which elicit anti-estrogen action in breast tissue and pro-estrogen responses in the bone and vascular tissues, are used for breast cancer and osteoporosis treatments (Lonard and Smith, 2002; Park and Jordan, 2002; Greene et al., 2004; Singh et al., 2008). The differential profiles of SERMs compared to E2 can be explained by different atomic contacts maps between these ligands and ER isoforms upon ligand binding, resulting in different ER conformations and differential recruitment of co-activators or co-repressors in a tissue-specific manner (Brzozowski et al., 1997; Shiau et al., 1998).
Disturbances of individual atomic contacts between ER and its ligands also underlie the development of drug resistance. For example, RAL resistance in breast cancer patients is attributed to the ERβ D351Y mutation (Jensen and Jordan, 2003). Interaction of D351 with alkylaminoethoxy side chain of RAL allows ERβ to adopt an antagonist conformation. However, Y351 interacts with RAL's alkylaminoethoxy side chain in such a way that helix-12 (H12) now adopts an agonist conformation allowing RAL to serve as an estrogen instead of an anti-estrogen. Thus, single-point mutations in the LBD of ERs can selectively ‘knock-out’ or ‘knock-in’ responsiveness to one or more of the multitude of ligands that can activate or antagonize this NR.
Farnesyl pyrophosphate (FPP) is an important metabolic intermediate of the cholesterol biosynthetic pathway. FPP is further converted to squalene via squalene synthase. Chemically, FPP is an organic molecule with a highly flexible hydrocarbon chain and a polar pyrophosphate group. The presence of this flexible hydrocarbon chain allows FPP to adopt different conformations and shapes. Previous in vitro studies have shown that FPP activates a subset of NR family members (Das et al., 2007), and that FPP inhibits epithelialization and wound healing through the glucocorticoid receptor in skin (Vukelic et al., 2010). FPP is the prototype of a ligand exhibiting a significant degree of promiscuity in its ability to activate different NR family members without clearly preferring a single one. These prior studies did not establish the mechanism of this FPP activation profile vis-à-vis several NR family members, but given that most known NR-activating ligands, such as E2, thyroxine and glucocorticoids, operate by binding in the LBP of NRs, we hypothesized that FPP too elicited its activity by interaction within the LBP of the NRs. As FPP is also a promiscuous NR ligand, testing this hypothesis provided us with the added opportunity to identify general structure–activity relationships that operate over the diverse conformational characteristics of a broad range of NR family members.
Crystallography, though the gold standard for visualizing the specific atomic contact maps of protein:ligand interactions would be a laborious and expensive process for studying the interaction of FPP with the potentially dozens of NRs with which it might interact. RAR and TR antagonists were previously identified using computational molecular docking algorithms (Schapira et al., 2000, 2001, 2003). These computational approaches appear accurate enough to allow high-throughput visualization of NR ligand interactions for the purposes of discerning their structural complexes. Previous computational molecular docking of FPP to a large library of NR structures have revealed the 3D activating conformation of FPP in the group of NRs it activates (Das et al., 2007). We hypothesized that docking to a large NR library could assess the NR–FPP structural interactions and comparison of these results with in vitro activation patterns of those same NRs by FPP would uncover the underlying NR ligand structure–activity relationships. A close correlation of docking results with in vitro NR activation measurements simultaneously established the reliability of our docking approach as a NR molecular design tool. We then utilized this tool to design hERα variants with only their FPP responsiveness ‘knocked-out’ and their E2 responsiveness preserved.
Results
Structural determinants of NR activation by FPP
A suspected FPP activating conformation was visualized previously by in silico docking: a common docked pose was identified in nearly all the activated NRs and was absent from all the NRs that were not activated by FPP (Das et al., 2007) (Supplementary data, Table S1). The appearance of this common conformation of FPP in the LBP of any crystallographic form of any given NR was a nearly 100% sensitive and specific indicator that the NR would be activated by FPP in vitro. The absence of this conformation of FPP from the LBP of all the crystallographic forms of any given NR was a 100% sensitive and specific indicator that the NR in question would not be activated by FPP in vitro. This observation strongly suggested that the common conformation observed was the activating conformation of FPP across a subset of NRs. We hypothesized that we could examine this conformation and identify the common contacts and structural features in the LBP that support activation by FPP.
Across all the NRs activated by FPP, extensive hydrophobic contacts were observed between the terminal carbons of the farnesyl group of FPP and the H11–H12 loop and/or H12 residues (Supplementary data, Table S2). These contacts are consistent with the commonly appreciated model of NR activation (Wurtz et al., 1996, 1998). These hydrophobic contacts serve as tethers for the H11–H12 loop and the H12 helix, holding this fragment to the top of the pocket in the receptor-activated conformation like the lid of a jar: the tethering of the H11–H12 loop and H12 allows the binding of co-activator peptide at the co-activator groove, which is otherwise blocked by the un-tethered H12. Therefore, these hydrophobic contacts are crucial for the receptor activation. As expected, these contacts were found to be absent from all the lowest energy docked conformations of FPP in complex with NRs from the non-activated group. Interestingly, several dockings of the non-activated group exhibited FPP docked within and making extensive contacts with other surfaces of the LBP, but in these conformations, contacts between FPP and the H11–H12 loop and H12 were absent (data not shown). Hence, the LBP of any given NR may be structurally complementary with FPP binding without being structurally complementary with the activation of that NR by FPP. Put another way, FPP docking within the LBP is sensitive but not specific for NR activation by FPP, while an observed docking contact between FPP and the H11–H12 loop or H12 is both sensitive and highly specific for the activation of any given NR by FPP.
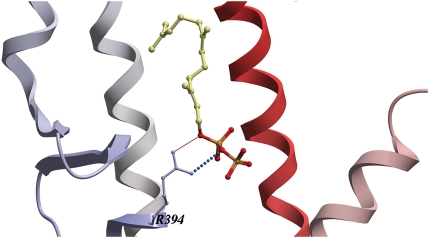
The docking also revealed a preferred orientation of FPP throughout the activated NR group in which FPP is anchored within the LBP by a salt bridge between its pyrophosphate group and a positively charged arginine side chain (equivalent to hERα R394) at the ‘bottom’ of the LBP (Fig. 1). This arginine is completely conserved across the activated NR group (Supplementary data, Figure S1A), but is also partially conserved in the non-activated NR group (Supplementary data, Figure S1B) and is thus a sensitive but not specific determinant of FPP activation.
Fig. 1.
An arginine responsible for FPP binding in the activated group. A close up view of the docked FPP-ERα complex showing a salt bridge between the R394 from the ERα LBP and the negatively charged pyrophosphate group of FPP. FPP's hydrocarbon backbone and pyrophosphate group are represented in yellow–grey and red–yellow, respectively. ERα backbone is depicted as a ribbon colored from N- to C-termini in a gradient from blue to red. [Images created using ICM graphics tools.]
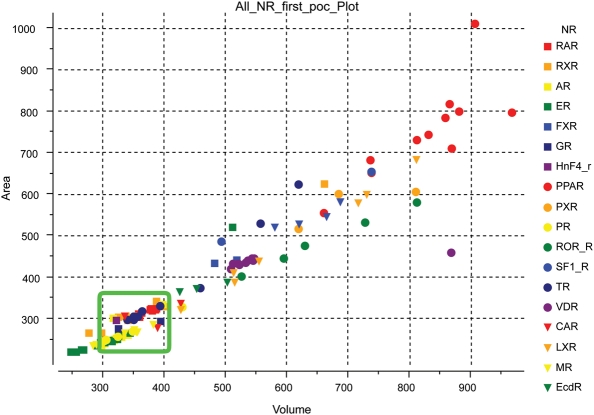
In order to identify the specificity determinant that must be operating in conjunction with the arginine and H12 contacts, we estimated the LBP volumes and areas for all the NRs in both activated and non-activated groups. A clear distinction was found between the FPP-activated and FPP-non-activated groups (Fig. 2). For the FPP-activated group, the LBP volume varied from 300 to 400 Å3. In contrast, the LBP volumes for the FPP-non-activated group were either smaller or larger than 300–400 Å3, generally varying between 400 and 850 Å3.
Fig. 2.
Plot of the LBP shapes/sizes seen in the published crystal structures of NRs. Area is plotted on the Y-axis and volume is plotted on the X-axis. The volumes of the NRs activated by FPP ranges between 300 and 400 Å3 (green rectangle). NRs not activated by FPP exhibit LBPs with volumes below 300 Å3 or above 400 Å3. [Picture created using ICM graphics tools.]
In comparing these results to the independent in vitro results, the combination of H12 and pivotal arginine contacts with an LBP volume between 300 and 400 Å3 is nearly 100% sensitive and specific for predicting whether any given NR will be activated by FPP.
Blind predictions of NR activation by FPP
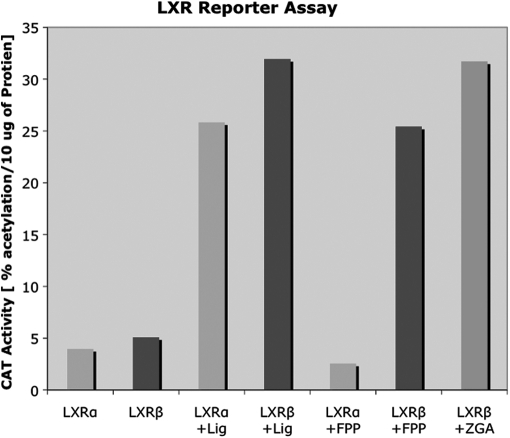
Blind predictions are a stringent test of this prediction. We previously blindly predicted androgen and progesterone receptors (AR and PR respectively) activation by FPP using the presence of these structural features (Das et al., 2007). The liver X receptor beta (LXRβ) had not previously been tested in vitro for FPP activation, but did have available crystal structures. Notably, the alpha isoform of LXR was not found to be activated by FPP in vitro in a previous study (Das et al., 2007). Therefore, LXRβ was also assumed to be resistant to activation by FPP and hence was never tested in vitro. However, FPP docking studies with six LXRβ crystal structures in agonist conformations (PDBs: 1upv, 1upw, 1pqc, 1pq6, 1pq9 and 1p8d) indicated that one of the these crystal structures (PDB: 1pq9) supported an appropriately oriented docked FPP conformation contacting H12, the key arginine contact within an appropriate LBP size, a LBP volume of 420.9 Å3 and hydrophobic contacts between the FPP's hydrocarbon tail and the LXRβ H12. Despite having an LBP a little larger than 400 Å3 in this case, LXRβ was proposed to be activated by FPP as the docked conformation of FPP is supported by all other parameters required for NR activation by FPP. LXRβ was indeed found to be activated by FPP in cells (Fig. 3). Thus, docking and the structure–activity relationships we have mapped are sufficiently accurate to blindly predict activation by FPP in three different cases.
Fig. 3.
Activation of LXRβ (human) by FPP. HeLa cells were transfected with Gal4-LBD chimeras of LXRα or LXRβ along with a Gal4 responsive CAT reporter (pMC110). The cells were incubated either with 20 µM 25-hydroxycholesterol (a synthetic ligand of LXRα and β indicated as ‘Lig’ in the figure), 10 µM FPP or 100 µM ZGA (a synthetic inhibitor that increases the amount of FPP in the cells). Forty hours later, the cells were harvested and analyzed for CAT activity. CAT activity (percent acetylation/10 µg protein) is plotted on the Y-axis and the receptor type and ligand applied are plotted on the X-axis. The results represent an average of two experiments.
Rational design and engineering of ERα variants with altered response towards FPP
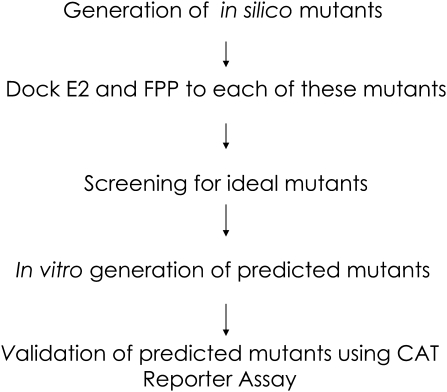
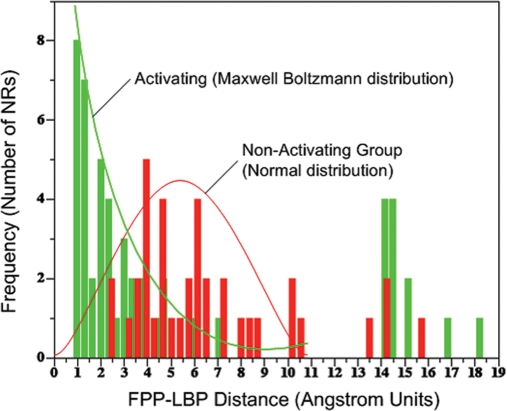
The structure–activity relationships we identified appeared accurate enough to use in molecular design, specifically as tools to ‘knock-out’ or ‘knock-in’ ligand specificity for any NR with any ligand. As a test of this hypothesis, we endeavored to design an hERα variant that is activated by E2 and not by FPP, since the atomic contact maps of FPP activation (inferred from our docking studies) and E2 (from the literature) were available to us. We designed a systematic docking based in silico mutagenesis screen to identify point mutations that would preserve E2 activation while eliminating FPP activation (Fig. 4). The screen takes advantage of our finding that a docking result with a lowest energy conformation exhibiting a distance between the center of mass of the docked FPP conformation and the geometric center of the NR LPB (the ‘FPP–LBP distance’) of ≤2 Å tends to represent a conformation of FPP that contacts H12 and the key arginine in its docked pose (Fig. 5; Supplementary data, Table S3). Systematic hERα mutants were created in silico for all 23 residues found in the receptor LBP, followed by E2 and FPP docking to each of these mutants. The E2 and FPP docked complexes were screened for those that exhibited an E2–LBP distance of ≤2 Å and an FPP–LBP distance of >2 Å for all eight conformations of agonist-bound hERα available in the database (PDBs: 1qku, 1g50, 1l2i, 1ere, 1pcg, 1x7e, 1x7r, 1gwr). Based upon this distance score, the screen predicted four promising sets of mutations: G521I/M/R, L525W/Y, E353S and R394C/D/E/K/I/S/T/V. We filtered out the G521I/M/R mutants, as glycine is a known helix breaker and hence its mutation would likely disrupt the 3D structure of the protein. Accordingly, five mutants, R394 A/D/E/K/T, were chosen for testing in vitro. Additionally, a L540T mutant was generated as an internal control to study the importance of H12 residues in the activation of hERα by FPP.
Fig. 4.
Schematic of the procedure to rationally design an ERα that no longer responds to FPP while retaining responsiveness to estradiol (E2). This schematic is general to the design of any NR to be activated or not by any ligand.
Fig. 5.
Histogram of the distributions of distances (in Å) between the center of mass of FPP located in its docked conformation and the geometric center of the LBP (FPP–LBP distance) for all the NRs tested in vitro. The histogram of distances corresponding to the NRs group activated by FPP is shown in green and describes a distribution similar to a Boltzmann distribution. The histogram of distances corresponding to the FPP-non-activated group of NRs is shown in red and clearly describes a separate population exhibiting a normal distribution. An FPP–LBP distance of ≤2 Å represents a hyperplane between the two distributions that distinguish the activated from non-activated population with nearly 100% sensitivity and specificity. Thus, a high-throughput screen of many NR mutants can be performed automatically as a molecular engineering tool in silico to find those mutations that either disturb FPP docking beyond 2 Å or rescue FPP docking to within 2 Å. The use of this cutoff may be general to the engineering of the interaction of any ligand with any NR.
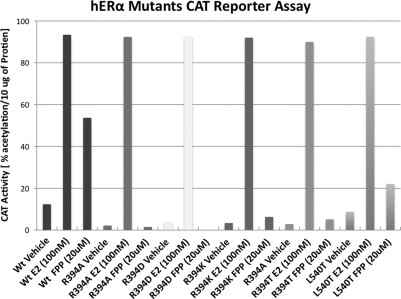
To assess the response of E2 and FPP towards each of these hERα mutants, cell-based chloramphenicol acetyltransferase (CAT) reporter assays were carried out for each mutant. Wild-type hERα receptor was used as a control. Receptor activation by E2 and FPP was determined indirectly by measuring the amount of CAT protein synthesized by the cells. For this purpose, the Gal4-responsive CAT reporter (pMC110) and one of the Gal4-DBD-hERα-LBD wild type, R394A/D/E/K/T and L540T mutant chimeras were transiently expressed in HeLa cells, which were then incubated with E2 or FPP. Addition of E2 resulted in the stimulation of the CAT reporter gene by wild-type hERα as well as by R394A/D/K/T and L540T mutants (Fig. 6). Addition of FPP resulted in the stimulation of the CAT reporter gene by hERα wild type, but not by the R394A/D/K/T mutants; minimal stimulation was induced by the L540T mutants. These results confirm that R394 and the H12 contact through L540 are independently required for FPP activation. Mutation of the hydrophobic H12 contact resulted in a less dramatic reduction, suggesting that this contact is the less potent requirement for FPP activation of NRs.
Fig. 6.
Response of hERα wild type, mutants R394A/D/K/T and L540T in the CAT reporter assay. CAT activity, an indirect measure of the receptor response, is shown on the X-axis.
Discussion
Our results demonstrate that computational molecular docking, as used here, is a highly accurate tool for evaluating structure–activity relationships in the NR family. Previously, using this in silico docking approach, we were able to independently segregate NRs into two groups on the basis of docking alone: those predicted to be activated by FPP and those predicted not to be activated by FPP (Das et al., 2007). These two groups correlated almost perfectly with the in vitro FPP activation data. In this work, we went a step further and validated this docking approach in blind predictions and in a novel protein engineering experiment. The results suggest that any structurally compatible ligand making both strong atomic contacts within the LBP and a hydrophobic contact with the H11–H12 loop or H12 can activate an NR, and that the conserved elements that support these contacts are present across certain members of the NR family for some ligands such as FPP.
The approach demonstrates that NR structural elements can be correlated directly with NR activation without the need for ligand binding studies. Using binding data as a proxy for activation in identifying structure–activity relationships may in fact be misleading as (i) the additional parameter of affinity may have to be taken into account, and (ii) ligands may bind in the LBP of NRs and not activate the NR if they do not contact H12. Although this conclusion may seem counterintuitive, this and other factors make the correlation of binding with docking and NR structural elements a challenging approach, while this study demonstrates that 3D structural features correlate simply and directly with receptor activation measurements.
The conserved arginine, which we found to be specific for FPP binding, was previously suggested to be involved in the binding of native and non-native ligands to the steroid receptor LBPs, and their subsequent activation, on the basis of its atomic network in crystal structures. The previously reported E2-bound ERα LBD crystal structure indicates the involvement of R394 in E2 binding by forming an indirect H-bond through water (Brzozowski et al., 1997; Tanenbaum et al., 1998). The hERα R394 equivalents such as R752 in androgen receptor (Sack et al., 2001), R611 in glucocorticoid receptor (Kauppi et al., 2003), R817 in mineralocorticoid receptor (Bledsoe et al., 2005) and R766 in PR (Williams and Sigler, 1998) have been reported to participate in H-bond network formation for the binding of dihydroxytestosterone (DHT), dexamethasone, aldosterone and hydrocortisone, and progesterone, respectively. The non-responsiveness of hERαR394A/D/K/T mutants towards FPP provides compelling evidence that R394 is crucial for FPP binding, but that this residue may not be essential in the activation of NRs by other hormones: certainly E2 but possibly DHT, dexamethasone, aldosterone and hydrocortisone as well.
Our study is the first to demonstrate the strict requirement of a pocket-size constraint for NR ligands across different receptors. The results clearly show that LBP size is critical for activation by FPP and suggest that bridging the potent arginine contact with H12 is the reason for the pocket-size constraint. Previous studies have noted the importance of pocket volume for the individual activation of NRs by ligands (Bogan et al., 1998), but these studies are limited in scope as the inferences are based on different ligands with different NRs. Here we were able to take advantage of a rare promiscuous ligand to directly investigate pocket-size variation in different NRs for the same ligand. Notably, our study revealed that the pertinent feature that allows FPP to bind to the LBPs of different sizes is the presence of a highly flexible, hydrophobic (purely carbon) backbone. Due to this intrinsic flexibility and chemical homogeneity, FPP adopts different conformations and poses within different LBPs and therefore is compatible with a range of volumes without disturbing its H12 contact that is needed for receptor activation. FPP is constrained in its ability to activate receptors in the presence of the key arginine only by its maximal extended length and its minimal collapsed volume, the range of which is suggested by our results to span volumes ranging from 300 to 430 Å3. A previously unrecognized sub-cluster of NRs compatible with at least the function of FPP activation via a defined pocket-size range has therefore been identified in this study.
The pocket-size constraint is simply an alternative way of looking at the overall map of FPP-receptor contacts required for NR activation by FPP, a map that is itself a manifestation of the flexible and hydrophobic/electronegative nature of FPP. The map, which we have deciphered in this study, shows that (i) a strong electrostatic contact is required between some portion of the NR LBP molecular surface and the electronegative pyrophosphate group of FPP in order to dock FPP firmly within the LBP of the NR, and (ii) independently, the shape of the NR LBP must be narrow and small enough to orient the FPP terminal carbon to promote an H11–H12 loop or H12 contact. The latter contact, identified as Leu540 in ER, is known to be a mediator of ligand-dependent NR activation in many NRs, thus it is not FPP specific. As with other NR ligands, most of the ligand affinity is determined by other extensive contacts within the LBP, and this one contact, although the critical switch for NR activation, is hydrophobic and insufficient to support activation on its own. Thus, the substitution of a single amino acid within this contact region would be expected to reduce but not necessarily abolish activation, as the ligand would still bind in the LBP and still be positioned to make less optimal hydrophobic contacts with backbone or other side chain carbons in the H11–H12 loop/H12 region. Indeed, this view is supported by our observation that mutation of Leu540 in H11–H12 loop/H12 region of ER, the contact site for FPP in ER, has a less potent effect than mutation of the arginine critical for FPP binding in the LBP. The Leu540T mutation we tested confirms this aspect of our map and model of FPP activation of diverse NRs.
Our predicted structural determinants of FPP-induced NR activation have been able to correctly discriminate between the two LXR isoforms. Our docking results predicted the activation of LXRβ by FPP and this prediction was found to be correct in a cell-based assay. Notably, these two isoforms share a high sequence similarity (78%) and the residue differences are located far away from the LBP (Alberti et al., 2000; Williams et al., 2003). These two features pose challenges in the discovery of LXRβ-specific ligands (Baihua Hu et al., 2009). LXRs are considered an important therapeutic target for treating atherosclerosis (Schultz et al., 2000; Bennett et al., 2006). A concerted effort is going to develop novel agonists of LXRs (Collins et al., 2002; Hu et al., 2006, 2009). However, the undesirable effects caused by LXRα agonists restrict the use of non-selective LXR agonists as potential drug targets. It has been proposed that an LXRβ-specific agonist might reduce these side effects (Bradley et al., 2007). Our results show that FPP is an LXRβ-specific agonist, and our results reveal both a method and the structural features that can identity LXRβ-specific agonists in general.
Finally, our results demonstrate that the large library of crystallographic NR structures sufficiently samples the dynamic range of NR conformations to support accurate docking both as a structure evaluation tool and as a molecular design tool for any NR with any chemical agonist. All of the designs succeeded in generating hERα's without FPP responsiveness while retaining E2 responsiveness, and the entire design process was computer automated. Simply by changing the input ligand and input receptor, NR LBD mutations that knock-in or knock-out any ligand for any NR could theoretically be identified from the screen we have devised.
Materials and methods
In silico systematic mutagenesis and docking
In silico ERα mutants were generated in the eight agonist conformations available (PDBs: 1l2i, 1gwr, 1x7r, 1g50, 1ere, 1qku, 1x7e and 1pcg). For this purpose, only the receptor (excluding the bound ligand) was rendered in 3D and prepared for in silico mutagenesis in a similar way as described before (Das et al., 2007). Residues found within the LBP radius of 5 Å were chosen by first superimposing each receptor conformation (chain 1) on another ERα agonist conformation (chain 1, PDB: 3erd) and then selecting the residues surrounding the diethylstilbestrol complexed with 3erd receptor. Each residue was then mutated systematically and the side chain of the mutated residue was minimized by systematic search. Each of these minimized mutated receptor objects was used for E2 and FPP docking as described previously (Das et al., 2007).
In vitro mutagenesis to generate hERα mutants
We obtained CMV-ERα full length from Dr Michael Garabedian (NYU School of Medicine) and performed in vitro mutagenesis to generate the hERα R394A/D/E/K/T and L540T mutants. The in vitro mutagenesis was performed using QuikChange Site-Directed mutagenesis kit (Stratagene, La Jolla, CA, USA). The oligonucleotides used in the mutagenesis were: forward 5′-GATTGGTCTCGTCTGGGCCTCCATGGAGCACCCAG-3′ and reverse 5′-CTGGGTGCTCCATGGAGGCCCAGACGAGACCAATC-3′ for R394A, forward 5′-GATTGGTCTCGTCTGGGATTCCATGGAGCACCCAG-3′ and reverse 5′-CTGGGTGCTCCATGGAATCCCAGACGAGACCAATC-3′ for R394D, forward 5′-GATTGGTCTCGTCTGGGAATCCATGGAGCACCCAG-3′ and reverse 5′-CTGGGTGCTCCATGGATTCCCAGACGAGACCAATC-3′ for R394E, forward 5′-GATTGGTCTCGTCTGGAAATCCATGGAGCACCCAG-3′ and 5′-CTGGGTGCTCCATGGATTTCCAGACGAGACCAATC-3′ for R394K, forward 5-GATTGGTCTCGTCTGGACCTCCATGGAGCACCCAG-3 and reverse 5-CTGGGTGCTCCATGGAGGTCCAGACGAGACCAATC-3 for R394T and forward 5′-CCCCTCTATGACCTGACTCTGGAGATGCTGGAC-3′ and reverse 5′-GTCCAGCATCTCCAGAGTCAGGTCATAGAGGGG-3′ for L540T.
For the transactivation assay, a Gal4 responsive reporter pMC110, as described previously (Casanova et al., 1994), was used. In addition, Gal4-DBD-ERα-LBD wild-type and mutant chimeras were generated using PCR-based cloning. PCR fragments were generated using primers forward 5′-CGCGGATCCGCGATCAAACGCTCTAAG-3′ and reverse 5′-CCGCTCGAGCGGGACCGTGGCAGGGAA-3′ and then subcloned into BamHI and XhoI sites in the modified multiple cloning region of a Gal4-DBD fusion vector, pSG424M described previously (Sadowski and Ptashne, 1989). The sequence of each construct was confirmed by automated sequencing.
Transfection in mammalian cells
HeLa cells were seeded at 150 000 cells per well in 12-well plates. Twenty-four hours later, transfections were performed in phenol red free DMEM using Lipofectamine 2000 (Invitrogen). Five hours post-transfection, the media was supplemented with bovine calf serum previously treated with AG1X8 resin and charcoal (to remove estrogens) to achieve a final 10% serum concentration. The appropriate ligands as described in Fig. 6 were added at the same time. Transfections were performed in duplicate using 100 ng of pMC110 reporter and 300 ng of one of the Gal4-DBD-ERα-LBD wild-type or mutant chimeras per well. Twenty-four hours later, the cells were harvested and assayed for CAT activity, as previously described (Mahajan and Samuels, 2000).
Supplementary data
Funding
This work was supported by grants from the National Institutes of Health (DP2 OD004631 to T.C. and DK 16636 to H.H.S.) and a grant from the Entertainment Industry Foundation (EIF) to H.H.S.
Supplementary Material
Acknowledgements
We gratefully acknowledge Sondra Maureen Nemetski for critical review of the manuscript. We are thankful to Kay Yeung for her technical assistance in CAT reporter assays. We would also like to thank Raquel Cintron for her assistance with cell culture. We would like to extend our thanks to Dr M. Garabedian for providing the full-length CMV-hERα clone.
Footnotes
Edited by Michael Sternberg
References
- Alberti S., Steffensen K.R., Gustafsson J.A. Gene. 2000;243:93–103. doi: 10.1016/s0378-1119(99)00555-7. doi:S0378-1119(99)00555-7[pii] [DOI] [PubMed] [Google Scholar]
- Baihua Hu R.U., Collini M., Quinet E., Feingold I., Goos-Nilson A., Wihelmsson A., Nambi P., Wrobel J. Bioorg. Med. Chem. 2009;17:3519–3727. doi: 10.1016/j.bmc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Bennett D.J., Cooke A.J., Edwards A.S. Recent Pat. Cardiovasc. Drug Discov. 2006;1:21–46. doi: 10.2174/157489006775244245. doi:10.2174/157489006775244245. [DOI] [PubMed] [Google Scholar]
- Bledsoe R.K., Cooke A.J., Edwards A.S., et al. J. Biol. Chem. 2005;280:31283–31293. doi: 10.1074/jbc.M504098200. doi:M504098200[pii]10.1074/jbc.M504098200. [DOI] [PubMed] [Google Scholar]
- Bogan A.A., Cohen F.E., Scanlan T.S. Nat. Struct. Biol. 1998;5:679–681. doi: 10.1038/1372. doi:10.1038/1372. [DOI] [PubMed] [Google Scholar]
- Bradley M.N., Hong C., Chen M., et al. J. Clin. Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. doi:10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski A.M., Pike A.C., Dauter Z., Hubbard R.E., Bonn T., Engstrom O., Ohman L., Greene G.L., Gustafsson J.A., Carlquist M. Nature. 1997;389:753–758. doi: 10.1038/39645. doi:10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Casanova J., Helmer E., Selmi-Ruby S., Qi J.S., Au-Fliegner M., Desai-Yajnik V., Koudinova N., Yarm F., Raaka B.M., Samuels H.H. Mol. Cell Biol. 1994;14:5756–5765. doi: 10.1128/mcb.14.9.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J.L., Fivush A.M., Watson M.A., et al. J. Med. Chem. 2002;45:1963–1966. doi: 10.1021/jm0255116. doi:jm0255116[pii] [DOI] [PubMed] [Google Scholar]
- Das S., Schapira M., Tomic-Canic M., Goyanka R., Cardozo T., Samuels H.H. Mol. Endocrinol. 2007;21:2672–2686. doi: 10.1210/me.2007-0080. doi:me.2007-0080[pii]10.1210/me.2007-0080. [DOI] [PubMed] [Google Scholar]
- Ding X.F., Anderson C.M., Ma H., Hong H., Uht R.M., Kushner P.J., Stallcup M.R. Mol. Endocrinol. 1998;12:302–313. doi: 10.1210/mend.12.2.0065. doi:10.1210/me.12.2.302. [DOI] [PubMed] [Google Scholar]
- Greene G.L., Shiau A.K., Nettles K.W. Ernst Schering Res. Found. Workshop. 2004;46:33–45. doi: 10.1007/978-3-662-05386-7_3. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Gustafsson J.A., Laudet V. Nat. Rev. Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. doi:10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- Hu B., Collini M., Unwalla R., et al. J. Med. Chem. 2006;49:6151–6154. doi: 10.1021/jm0609566. doi:10.1021/jm0609566. [DOI] [PubMed] [Google Scholar]
- Hu B., Unwalla R., Collini M., Quinet E., Feingold I., Goos-Nilsson A., Wihelmsson A., Nambi P., Wrobel J. Bioorg. Med. Chem. 2009;17:3519–3527. doi: 10.1016/j.bmc.2009.04.012. doi:S0968-0896(09)00357-5[pii]10.1016/j.bmc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Jensen E.V., Jordan V.C. Clin. Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- Kauppi B., Jakob C., Farnegardh M., et al. J. Biol. Chem. 2003;278:22748–22754. doi: 10.1074/jbc.M212711200. doi:10.1074/jbc.M212711200M212711200[pii] [DOI] [PubMed] [Google Scholar]
- Levenson A.S., Jordan V.C. Cancer Res. 1998;58:1872–1875. [PubMed] [Google Scholar]
- Lonard D.M., Smith C.L. Steroids. 2002;67:15–24. doi: 10.1016/s0039-128x(01)00133-7. doi:S0039128X01001337[pii] [DOI] [PubMed] [Google Scholar]
- Mahajan M.A., Samuels H.H. Mol. Cell Biol. 2000;20:5048–5063. doi: 10.1128/mcb.20.14.5048-5063.2000. doi:10.1128/MCB.20.14.5048-5063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington J.P., Al-Lazikani B., Hopkins A.L. Nat. Rev. Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. doi:nrd2199[pii]10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Park W.C., Jordan V.C. Trends Mol. Med. 2002;8:82–88. doi: 10.1016/s1471-4914(02)02282-7. doi:S1471491402022827[pii] [DOI] [PubMed] [Google Scholar]
- Renaud J.P., Rochel N., Ruff M., Vivat V., Chambon P., Gronemeyer H., Moras D. Nature. 1995;378:681–689. doi: 10.1038/378681a0. doi:10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- Sack J.S., Kish K.F., Wang C., et al. Proc. Natl Acad. Sci. USA. 2001;98:4904–4909. doi: 10.1073/pnas.081565498. doi:10.1073/pnas.08156549898/9/4904[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I., Ptashne M. Nucleic Acids Res. 1989;17:7539. doi: 10.1093/nar/17.18.7539. doi:10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Raaka B.M., Samuels H.H., Abagyan R. Proc. Natl Acad. Sci. USA. 2000;97:1008–1013. doi: 10.1073/pnas.97.3.1008. doi:10.1073/pnas.97.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Raaka B.M., Samuels H.H., Abagyan R. BMC Struct. Biol. 2001;1:1. doi: 10.1186/1472-6807-1-1. doi:10.1186/1472-6807-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M., Raaka B.M., Das S., Fan L., Totrov M., Zhou Z., Wilson S.R., Abagyan R., Samuels H.H. Proc. Natl Acad. Sci. USA. 2003;100:7354–7359. doi: 10.1073/pnas.1131854100. doi:10.1073/pnas.1131854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J.R., Tu H., Luk A., et al. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. doi:10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau A.K., Barstad D., Loria P.M., Cheng L., Kushner P.J., Agard D.A., Greene G.L. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. doi:S0092-8674(00)81717-1[pii] [DOI] [PubMed] [Google Scholar]
- Singh M.N., Martin-Hirsch P.L., Martin F.L. Med. Sci. Monit. 2008;14:RA144–148. doi:867954[pii] [PubMed] [Google Scholar]
- Tanenbaum D.M., Wang Y., Williams S.P., Sigler P.B. Proc. Natl Acad. Sci. USA. 1998;95:5998–6003. doi: 10.1073/pnas.95.11.5998. doi:10.1073/pnas.95.11.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegel J.J., Heine M.J., Tini M., Vivat V., Chambon P., Gronemeyer H. EMBO J. 1998;17:507–519. doi: 10.1093/emboj/17.2.507. doi:10.1093/emboj/17.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukelic S., Stojadinovic O., Pastar I., Vouthounis C., Krzyzanowska A., Das S., Samuels H.H., Tomic-Canic M. J. Biol. Chem. 2010;285:1980–1988. doi: 10.1074/jbc.M109.016741. doi:M109.016741[pii]10.1074/jbc.M109.016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.P., Sigler P.B. Nature. 1998;393:392–396. doi: 10.1038/30775. doi:10.1038/30775. [DOI] [PubMed] [Google Scholar]
- Williams S., Bledsoe R.K., Collins J.L., et al. J. Biol. Chem. 2003;278:27138–27143. doi: 10.1074/jbc.M302260200. doi:10.1074/jbc.M302260200M302260200[pii] [DOI] [PubMed] [Google Scholar]
- Wurtz J.M., Bourguet W., Renaud J.P., Vivat V., Chambon P., Moras D., Gronemeyer H. Nat. Struct. Biol. 1996;3:87–94. doi: 10.1038/nsb0196-87. doi:10.1038/nsb0196-87. [DOI] [PubMed] [Google Scholar]
- Wurtz J.M., Egner U., Heinrich N., Moras D., Mueller-Fahrnow A. J. Med. Chem. 1998;41:1803–1814. doi: 10.1021/jm970406v. doi:10.1021/jm970406vjm970406v[pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.