Abstract
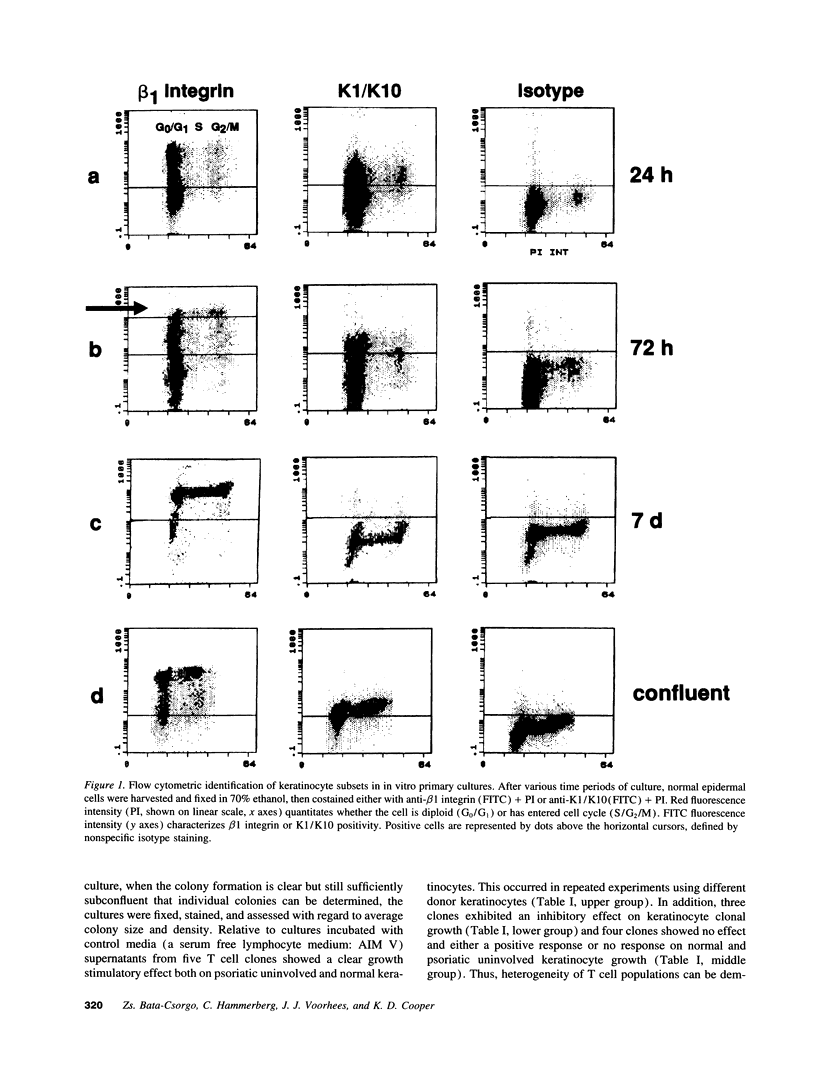
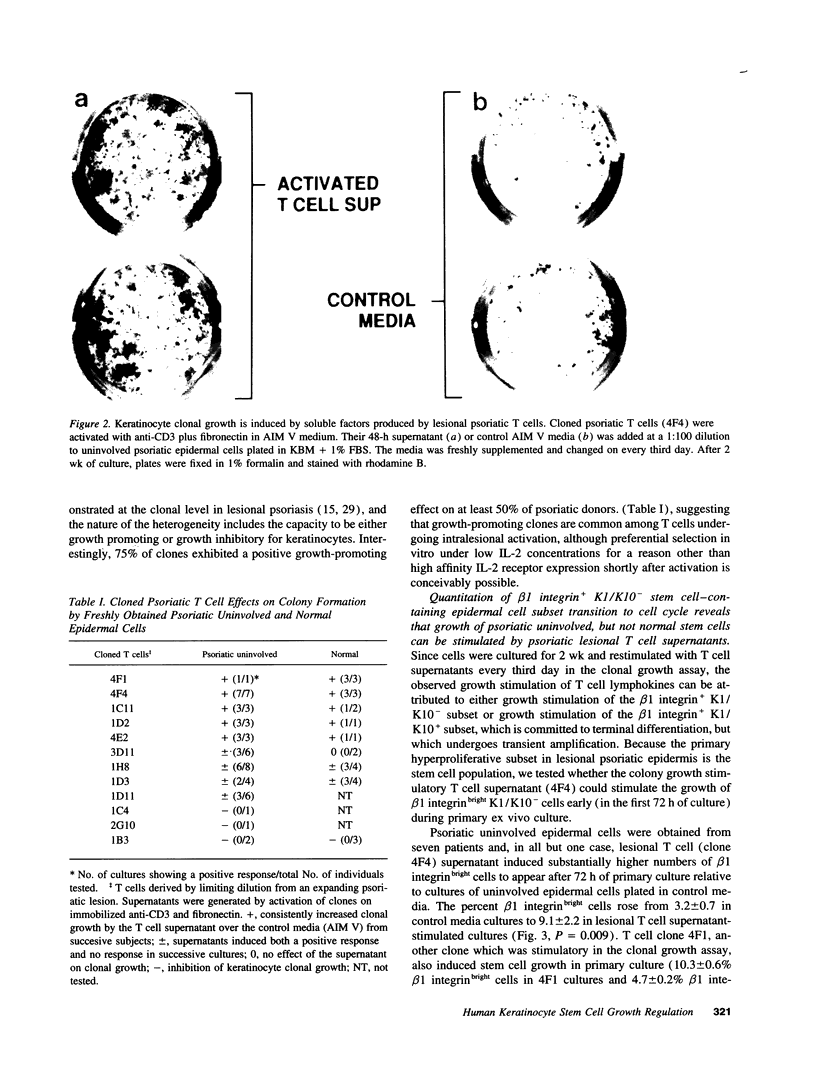
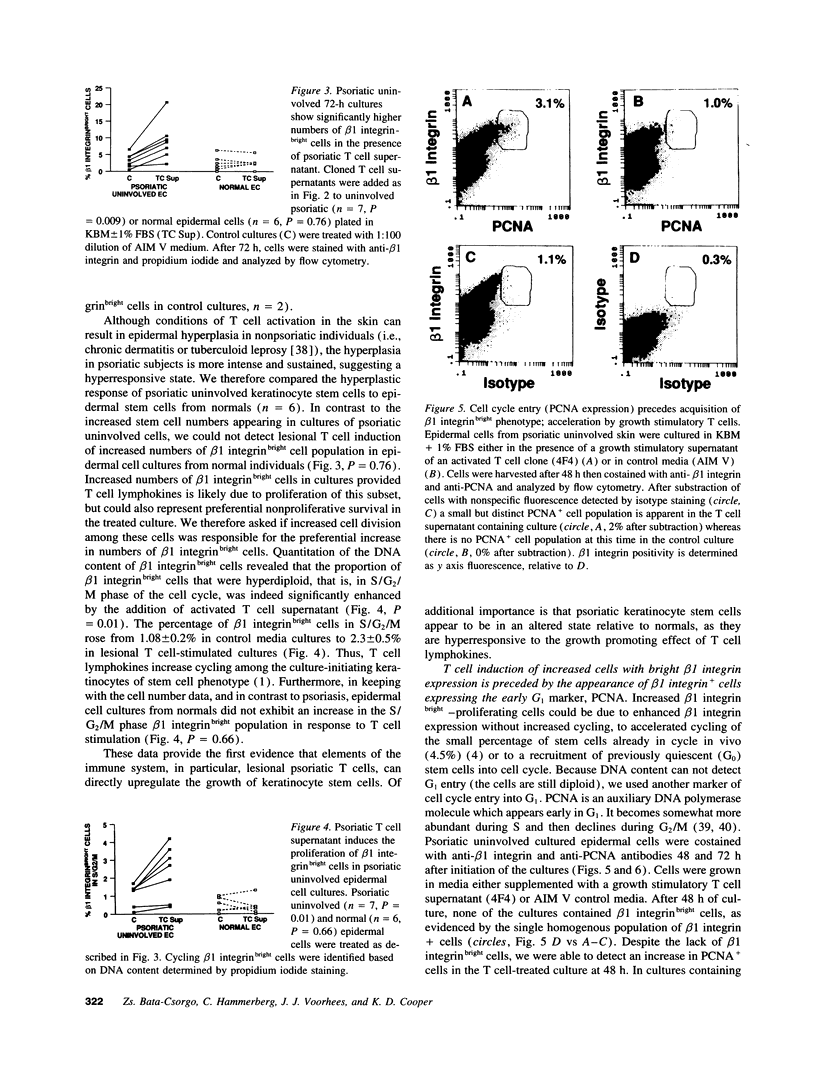
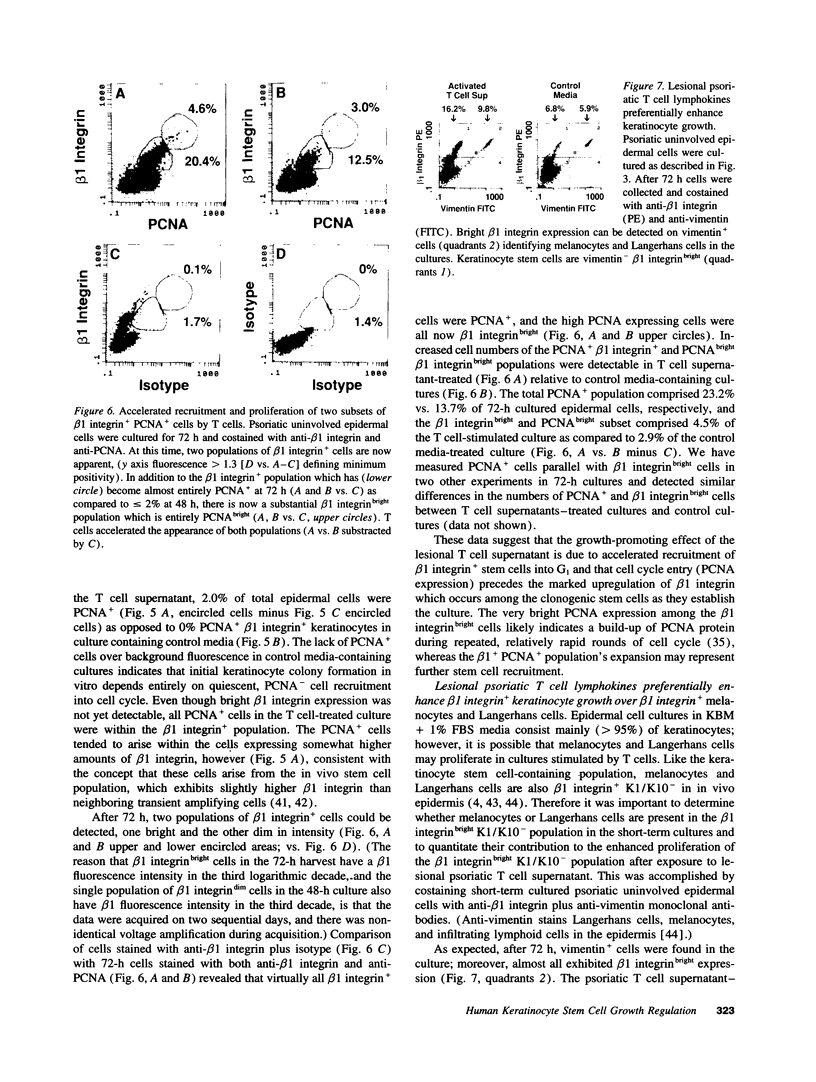
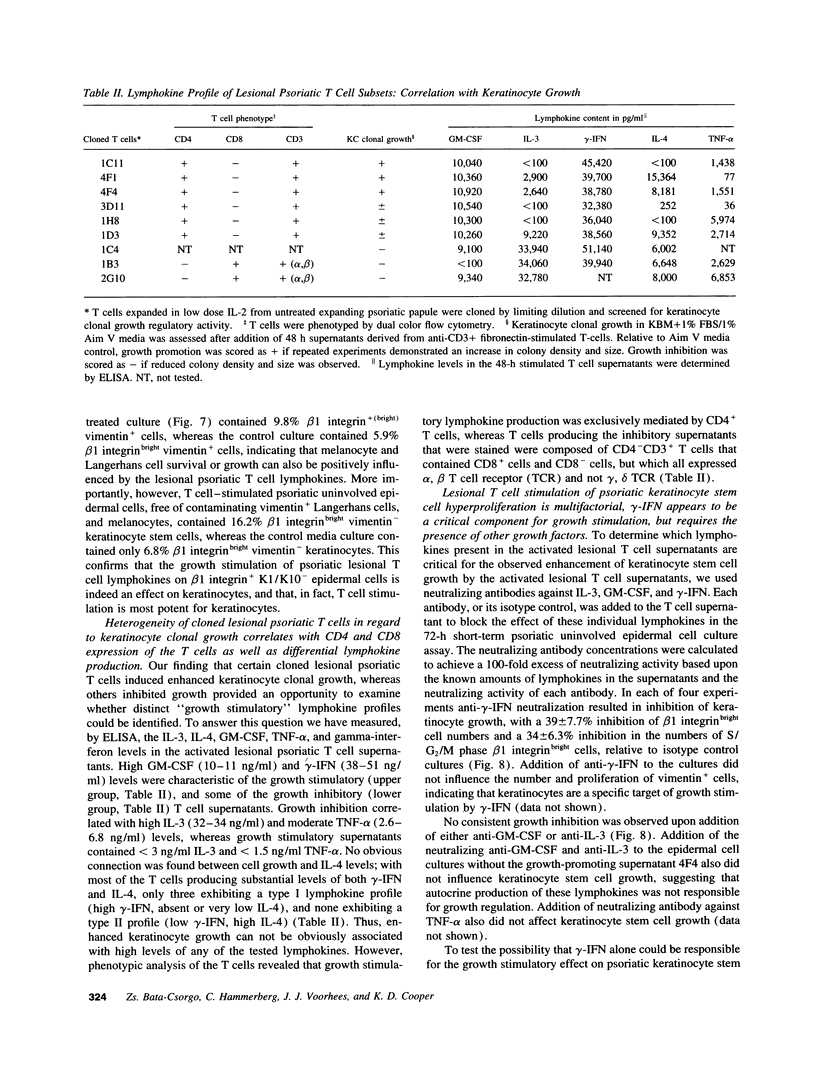
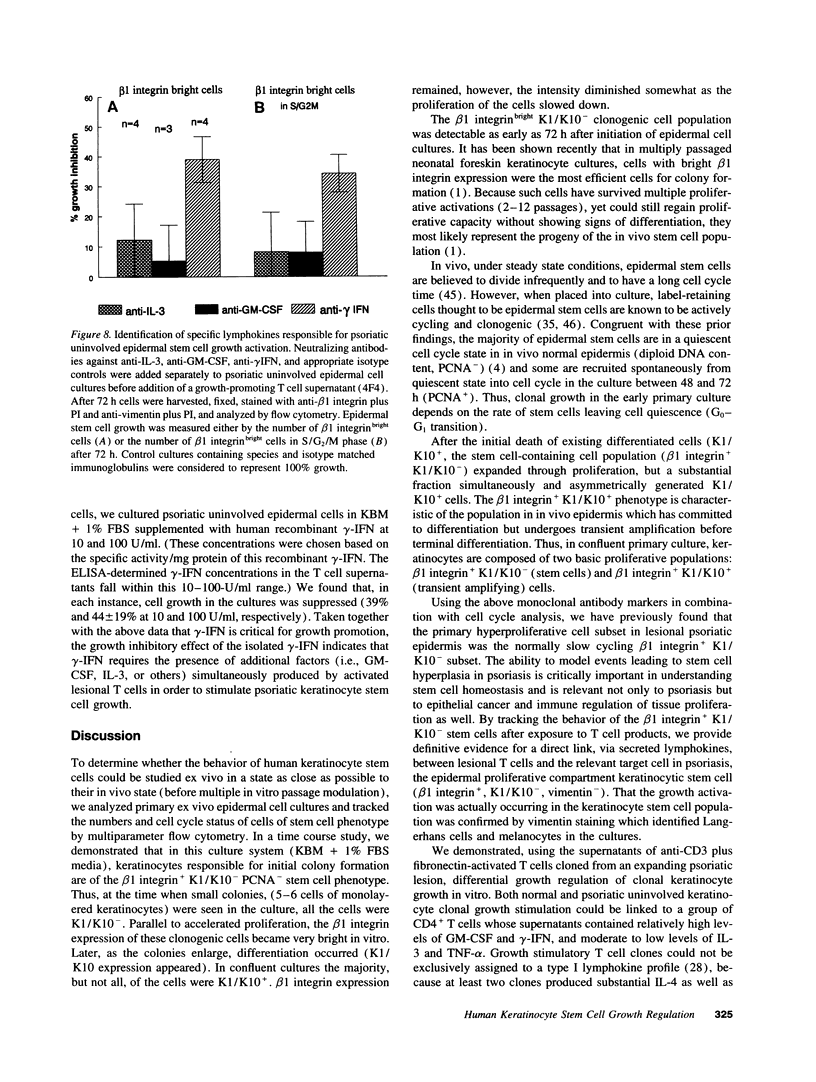
Flow cytometric analysis of primary ex vivo keratinocyte cultures demonstrated that stem cells, (beta 1 integrin+, keratin 1/keratin 10 [K1/K10-], proliferating cell nuclear antigen [PCNA-] [Bata-Csorgo, Zs., C. Hammerberg, J. J. Voorhees, and K. D. Cooper. 1993. J. Exp. Med. 178:1271-1281]) establish such cultures. This methodology also enabled the quantitation of synchronized recruitment of these cells from G0 into G1 of the cell cycle (PCNA expression), which preceded bright beta 1 integrin expression. (beta 1 integrinbright expression has been shown to be a characteristic feature of keratinocyte stem cells in culture (Jones, P. H., and F. M. Watt. 1993. Cell. 73:713-724). Using the above assay, we determined whether lesional T lymphocytes in psoriasis could be directly responsible for the induction of the stem cell hyperproliferation that is characteristic of this disease. Indeed, CD4+ T lymphocytes, cloned from lesional psoriatic skin and stimulated by immobilized anti-CD3 plus fibronectin, promoted psoriatic uninvolved keratinocyte stem cell proliferation via soluble factors. This induction appeared to be through accelerated recruitment of stem cells from their quiescent state (G0) into cell cycle. By contrast, normal keratinocyte stem cells exhibited no such growth stimulation. Supernatants exhibiting growth induction all contained high levels of GM-CSF and gamma-IFN, low IL-3 and TNF-alpha, and variable IL-4. Only anti-gamma-IFN antibody was able to neutralize growth stimulatory activity of the supernatants on psoriatic uninvolved keratinocyte stem cells. However, because recombinant gamma-IFN alone inhibited growth in this assay, these data suggest that, in psoriasis, gamma-IFN acts cooperatively with other growth factors in the immune induction of cell cycle progression by the normally quiescent stem cell keratinocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baadsgaard O., Gupta A. K., Taylor R. S., Ellis C. N., Voorhees J. J., Cooper K. D. Psoriatic epidermal cells demonstrate increased numbers and function of non-Langerhans antigen-presenting cells. J Invest Dermatol. 1989 Feb;92(2):190–195. doi: 10.1111/1523-1747.ep12276718. [DOI] [PubMed] [Google Scholar]
- Baadsgaard O., Tong P., Elder J. T., Hansen E. R., Ho V., Hammerberg C., Lange-Vejlsgaard G., Fox D. A., Fisher G., Chan L. S. UM4D4+ (CDw60) T cells are compartmentalized into psoriatic skin and release lymphokines that induce a keratinocyte phenotype expressed in psoriatic lesions. J Invest Dermatol. 1990 Sep;95(3):275–282. doi: 10.1111/1523-1747.ep12484908. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Powles A. V., Lambert S., Valdimarsson H., Fry L. A prospective study of the Koebner reaction and T lymphocytes in uninvolved psoriatic skin. Acta Derm Venereol. 1988;68(5):430–434. [PubMed] [Google Scholar]
- Baker B. S., Powles A. V., Valdimarsson H., Fry L. An altered response by psoriatic keratinocytes to gamma interferon. Scand J Immunol. 1988 Dec;28(6):735–740. doi: 10.1111/j.1365-3083.1988.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Swain A. F., Fry L., Valdimarsson H. Epidermal T lymphocytes and HLA-DR expression in psoriasis. Br J Dermatol. 1984 May;110(5):555–564. doi: 10.1111/j.1365-2133.1984.tb04678.x. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5390–5394. doi: 10.1073/pnas.82.16.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bata-Csorgo Z., Hammerberg C., Voorhees J. J., Cooper K. D. Flow cytometric identification of proliferative subpopulations within normal human epidermis and the localization of the primary hyperproliferative population in psoriasis. J Exp Med. 1993 Oct 1;178(4):1271–1281. doi: 10.1084/jem.178.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. D., Hulsebosch H. J., Krieg S. R., Bakker P. M., Cormane R. H. Immunocompetent cells in psoriasis. In situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275(3):181–189. doi: 10.1007/BF00510050. [DOI] [PubMed] [Google Scholar]
- Braun-Falco O., Schmoeckel C. The dermal inflammatory reaction in initial psoriatic lesions. Arch Dermatol Res. 1977 Mar 25;258(1):9–16. doi: 10.1007/BF00582862. [DOI] [PubMed] [Google Scholar]
- Caux C., Moreau I., Saeland S., Banchereau J. Interferon-gamma enhances factor-dependent myeloid proliferation of human CD34+ hematopoietic progenitor cells. Blood. 1992 May 15;79(10):2628–2635. [PubMed] [Google Scholar]
- Chang E. Y., Hammerberg C., Fisher G., Baadsgaard O., Ellis C. N., Voorhees J. J., Cooper K. D. T-cell activation is potentiated by cytokines released by lesional psoriatic, but not normal, epidermis. Arch Dermatol. 1992 Nov;128(11):1479–1485. [PubMed] [Google Scholar]
- Christophers E., Parzefall R., Braun-Falco O. Initial events in psoriasis: quantitative assessment. Br J Dermatol. 1973 Oct;89(4):327–334. doi: 10.1111/j.1365-2133.1973.tb02986.x. [DOI] [PubMed] [Google Scholar]
- Cooper K. D., Hammerberg C., Baadsgaard O., Elder J. T., Chan L. S., Sauder D. N., Voorhees J. J., Fisher G. IL-1 activity is reduced in psoriatic skin. Decreased IL-1 alpha and increased nonfunctional IL-1 beta. J Immunol. 1990 Jun 15;144(12):4593–4603. [PubMed] [Google Scholar]
- Cooper K. D., Hammerberg C., Baadsgaard O., Elder J. T., Chan L. S., Taylor R. S., Voorhees J. J., Fisher G. Interleukin-1 in human skin: dysregulation in psoriasis. J Invest Dermatol. 1990 Nov;95(5 Suppl):24S–26S. doi: 10.1111/1523-1747.ep12505698. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G., Cheng S. Z., Dong G., Sun T. T., Lavker R. M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989 Apr 21;57(2):201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- De Luca M., Pellegrini G., Bondanza S., Cremona O., Savoia P., Cancedda R., Marchisio P. C. The control of polarized integrin topography and the organization of adhesion-related cytoskeleton in normal human keratinocytes depend upon number of passages in culture and ionic environment. Exp Cell Res. 1992 Sep;202(1):142–150. doi: 10.1016/0014-4827(92)90413-3. [DOI] [PubMed] [Google Scholar]
- Ellis C. N., Gorsulowsky D. C., Hamilton T. A., Billings J. K., Brown M. D., Headington J. T., Cooper K. D., Baadsgaard O., Duell E. A., Annesley T. M. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986 Dec 12;256(22):3110–3116. [PubMed] [Google Scholar]
- Fierlbeck G., Rassner G., Müller C. Psoriasis induced at the injection site of recombinant interferon gamma. Results of immunohistologic investigations. Arch Dermatol. 1990 Mar;126(3):351–355. [PubMed] [Google Scholar]
- Gottlieb A. B., Luster A. D., Posnett D. N., Carter D. M. Detection of a gamma interferon-induced protein IP-10 in psoriatic plaques. J Exp Med. 1988 Sep 1;168(3):941–948. doi: 10.1084/jem.168.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths C. E., Voorhees J. J., Nickoloff B. J. Characterization of intercellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989 Apr;20(4):617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Baadsgaard O., Ellis C. N., Voorhees J. J., Cooper K. D. Lymphocytes and macrophages of the epidermis and dermis in lesional psoriatic skin, but not epidermal Langerhans cells, are depleted by treatment with cyclosporin A. Arch Dermatol Res. 1989;281(4):219–226. doi: 10.1007/BF00431054. [DOI] [PubMed] [Google Scholar]
- Hancock G. E., Kaplan G., Cohn Z. A. Keratinocyte growth regulation by the products of immune cells. J Exp Med. 1988 Oct 1;168(4):1395–1402. doi: 10.1084/jem.168.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer R., Denhardt D. T. Cell cycle-regulated and proliferation stimulus-responsive genes. Crit Rev Eukaryot Gene Expr. 1991;1(4):247–300. [PubMed] [Google Scholar]
- Jones P. H., Watt F. M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993 May 21;73(4):713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Nusrat A., Sarno E. N., Job C. K., McElrath J., Porto J. A., Nathan C. F., Cohn Z. A. Cellular responses to the intradermal injection of recombinant human gamma-interferon in lepromatous leprosy patients. Am J Pathol. 1987 Aug;128(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Walsh G., Guido L. S., Meyn P., Burkhardt R. A., Abalos R. M., Barker J., Frindt P. A., Fajardo T. T., Celona R. Novel responses of human skin to intradermal recombinant granulocyte/macrophage-colony-stimulating factor: Langerhans cell recruitment, keratinocyte growth, and enhanced wound healing. J Exp Med. 1992 Jun 1;175(6):1717–1728. doi: 10.1084/jem.175.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Witmer M. D., Nath I., Steinman R. M., Laal S., Prasad H. K., Sarno E. N., Elvers U., Cohn Z. A. Influence of delayed immune reactions on human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1986 May;83(10):3469–3473. doi: 10.1073/pnas.83.10.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki P., Vanderlaan M., Dolbeare F., Gray J., Tan E. M. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res. 1986 Sep;166(1):209–219. doi: 10.1016/0014-4827(86)90520-3. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Sun T. T. Epidermal stem cells. J Invest Dermatol. 1983 Jul;81(1 Suppl):121s–127s. doi: 10.1111/1523-1747.ep12540880. [DOI] [PubMed] [Google Scholar]
- Lavker R. M., Sun T. T. Heterogeneity in epidermal basal keratinocytes: morphological and functional correlations. Science. 1982 Mar 5;215(4537):1239–1241. doi: 10.1126/science.7058342. [DOI] [PubMed] [Google Scholar]
- Marcelo C. L., Duell E. A., Rhodes L. M., Dunham W. R. In vitro model of essential fatty acid deficiency. J Invest Dermatol. 1992 Dec;99(6):703–708. doi: 10.1111/1523-1747.ep12614196. [DOI] [PubMed] [Google Scholar]
- Morganroth G. S., Chan L. S., Weinstein G. D., Voorhees J. J., Cooper K. D. Proliferating cells in psoriatic dermis are comprised primarily of T cells, endothelial cells, and factor XIIIa+ perivascular dendritic cells. J Invest Dermatol. 1991 Mar;96(3):333–340. doi: 10.1111/1523-1747.ep12465237. [DOI] [PubMed] [Google Scholar]
- Morhenn V. B., Abel E. A., Mahrle G. Expression of HLA-DR antigen in skin from patients with psoriasis. J Invest Dermatol. 1982 Feb;78(2):165–168. doi: 10.1111/1523-1747.ep12506332. [DOI] [PubMed] [Google Scholar]
- Morhenn V. B. Keratinocyte proliferation in wound healing and skin diseases. Immunol Today. 1988 Apr;9(4):104–107. doi: 10.1016/0167-5699(88)91278-9. [DOI] [PubMed] [Google Scholar]
- Morris R. J., Potten C. S. Slowly cycling (label-retaining) epidermal cells behave like clonogenic stem cells in vitro. Cell Prolif. 1994 May;27(5):279–289. doi: 10.1111/j.1365-2184.1994.tb01425.x. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Basham T. Y., Merigan T. C., Morhenn V. B. Antiproliferative effects of recombinant alpha- and gamma-interferons on cultured human keratinocytes. Lab Invest. 1984 Dec;51(6):697–701. [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Elder J. T., Fisher G. J., Voorhees J. J. Decreased growth inhibition by recombinant gamma interferon is associated with increased transforming growth factor-alpha production in keratinocytes cultured from psoriatic lesions. Br J Dermatol. 1989 Aug;121(2):161–174. doi: 10.1111/j.1365-2133.1989.tb01795.x. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Chamchick N., Thivolet J., Wijdenes J., Morel P., Revillard J. P. CD4 antibody treatment of severe psoriasis. Lancet. 1991 Aug 3;338(8762):321–321. doi: 10.1016/0140-6736(91)90465-2. [DOI] [PubMed] [Google Scholar]
- Placek W., Haftek M., Thivolet J. Sequence of changes in psoriatic epidermis. Immunocompetent cell redistribution precedes altered expression of keratinocyte differentiation markers. Acta Derm Venereol. 1988;68(5):369–377. [PubMed] [Google Scholar]
- Poizot-Martin I., Dhiver C., Mawas C., Olive D., Gastaut J. A. Are CD4 antibodies and peptide T new treatments for psoriasis? Lancet. 1991 Jun 15;337(8755):1477–1477. doi: 10.1016/0140-6736(91)93166-7. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Morris R. J. Epithelial stem cells in vivo. J Cell Sci Suppl. 1988;10:45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- Prinz J., Braun-Falco O., Meurer M., Daddona P., Reiter C., Rieber P., Riethmüller G. Chimaeric CD4 monoclonal antibody in treatment of generalised pustular psoriasis. Lancet. 1991 Aug 3;338(8762):320–321. doi: 10.1016/0140-6736(91)90464-z. [DOI] [PubMed] [Google Scholar]
- Schlaak J. F., Buslau M., Jochum W., Hermann E., Girndt M., Gallati H., Meyer zum Büschenfelde K. H., Fleischer B. T cells involved in psoriasis vulgaris belong to the Th1 subset. J Invest Dermatol. 1994 Feb;102(2):145–149. doi: 10.1111/1523-1747.ep12371752. [DOI] [PubMed] [Google Scholar]
- Singer K. H., Tuck D. T., Sampson H. A., Hall R. P. Epidermal keratinocytes express the adhesion molecule intercellular adhesion molecule-1 in inflammatory dermatoses. J Invest Dermatol. 1989 May;92(5):746–750. doi: 10.1111/1523-1747.ep12722441. [DOI] [PubMed] [Google Scholar]
- Stingl G., Wolff K., Diem E., Baumgartner G., Knapp W. In situ identification of lymphoreticular cells in benign and malignant infiltrates by membrane receptor sites. J Invest Dermatol. 1977 Aug;69(2):231–235. doi: 10.1111/1523-1747.ep12506348. [DOI] [PubMed] [Google Scholar]
- Strange P., Cooper K. D., Hansen E. R., Fisher G., Larsen J. K., Fox D., Krag C., Voorhees J. J., Baadsgaard O. T-lymphocyte clones initiated from lesional psoriatic skin release growth factors that induce keratinocyte proliferation. J Invest Dermatol. 1993 Nov;101(5):695–700. doi: 10.1111/1523-1747.ep12371678. [DOI] [PubMed] [Google Scholar]
- Uyemura K., Yamamura M., Fivenson D. F., Modlin R. L., Nickoloff B. J. The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J Invest Dermatol. 1993 Nov;101(5):701–705. doi: 10.1111/1523-1747.ep12371679. [DOI] [PubMed] [Google Scholar]
- Volc-Platzer B., Valent P., Radaszkiewicz T., Mayer P., Bettelheim P., Wolff K. Recombinant human interleukin 3 induces proliferation of inflammatory cells and keratinocytes in vivo. Lab Invest. 1991 Apr;64(4):557–566. [PubMed] [Google Scholar]
- Watt F. M. Epidermal stem cells in culture. J Cell Sci Suppl. 1988;10:85–94. doi: 10.1242/jcs.1988.supplement_10.7. [DOI] [PubMed] [Google Scholar]
- Weinshenker B. G., Bass B. H., Ebers G. C., Rice G. P. Remission of psoriatic lesions with muromonab-CD3 (orthoclone OKT3) treatment. J Am Acad Dermatol. 1989 Jun;20(6):1132–1133. doi: 10.1016/s0190-9622(89)80200-2. [DOI] [PubMed] [Google Scholar]
- Zambruno G., Manca V., Santantonio M. L., Soligo D., Giannetti A. VLA protein expression on epidermal cells (keratinocytes, Langerhans cells, melanocytes): a light and electron microscopic immunohistochemical study. Br J Dermatol. 1991 Feb;124(2):135–145. doi: 10.1111/j.1365-2133.1991.tb00422.x. [DOI] [PubMed] [Google Scholar]