Abstract
Although members of the fibroblast growth factor (FGF) family and their receptors have well-established roles in embryogenesis, their contributions to adult physiology remain relatively unexplored. Here, we use real-time quantitative PCR to determine the mRNA expression patterns of all 22 FGFs, the seven principal FGF receptors (FGFRs), and the three members of the Klotho family of coreceptors in 39 different mouse tissues. Unsupervised hierarchical cluster analysis of the mRNA expression data reveals that most FGFs and FGFRs fall into two groups the expression of which is enriched in either the central nervous system or reproductive and gastrointestinal tissues. Interestingly, the FGFs that can act as endocrine hormones, including FGF15/19, FGF21, and FGF23, cluster in a third group that does not include any FGFRs, underscoring their roles in signaling between tissues. We further show that the most recently identified Klotho family member, Lactase-like, is highly and selectively expressed in brown adipose tissue and eye and can function as an additional coreceptor for FGF19. This FGF atlas provides an important resource for guiding future studies to elucidate the physiological functions of FGFs in adult animals.
We determine the mRNA expression patterns of the fibroblast growth factors, their receptors and associated proteins in 39 adult mouse tissues.
Fibroblast growth factors (FGFs) are polypeptides found in metazoan organisms ranging from nematodes to humans. They were discovered as mitogens for fibroblasts (1) and today are recognized as having myriad effects including prominent roles in embryonic development and organogenesis (2). The human/mouse FGF gene family comprises 22 members (FGF1–23), with FGF15 being the mouse ortholog of human FGF19 (we will refer to this member as FGF15/19 unless referring specifically to either the mouse or human ortholog). Based on phylogenetic analysis, the mouse and human FGF families can be divided into seven subfamilies: the FGF1 subfamily consisting of FGF1 and FGF2; the FGF4 subfamily consisting of FGF4, -5, and -6; the FGF7 subfamily consisting of FGF3, -7, -10, and -22; the FGF8 subfamily consisting of FGF8, -17, and -18; the FGF9 subfamily consisting of FGF9, -16, and -20; the FGF11 subfamily consisting of FGF11, -12, -13, and -14; and, the FGF15/19 subfamily consisting of FGF15/19, -21, and -23 (3,4).
With the exception of FGF11 subfamily members, the FGFs are secreted proteins that mediate their biological responses by binding to and activating cell surface FGFRs. Members of the FGF11 subfamily, also known as FGF homologous factors, are functionally distinct from the other FGFs in that they interact with the cytoplasmic C-terminal tails of voltage-gated sodium channels to modulate channel gating and subcellular trafficking (5). The mammalian FGFR genes (FGFR1–FGFR4) encode single-pass membrane-spanning tyrosine kinases with an extracellular ligand-binding domain, a transmembrane domain, and an intracellular kinase domain. The ligand-binding domain is composed of three Ig-like domains (D1–D3). The region encompassing D2 and D3 of the FGFRs mediates FGF binding affinity and specificity. FGFR1, -2, and -3 have two alternative exons (b and c) encoding the second half of D3 that are spliced in a tissue-specific fashion (6,7,8). This splicing event plays a crucial role in dictating FGF-FGFR binding specificity. Consequently, there are a total of seven principal FGFRs, termed FGFR1b, -1c, -2b, -2c, -3b, -3c, and -4, each activated by a unique subset of FGFs.
Most of the 18 secreted FGFs interact with high affinity with heparan sulfate glycosaminoglycans (HSGAGs), which are either membrane anchored or present in the extracellular matrix (7). FGFRs also interact with HSGAGs via D2. These interactions are necessary for efficient FGF-FGFR binding and dimerization (8). The binding to HSGAGs also limits the diffusion of FGFs from their cells of origin, thereby restricting them to either autocrine or paracrine actions (7,9). Members of the FGF15/19 subfamily, however, are atypical in that they interact only weakly or not at all with HSGAGs, permitting them to escape from the extracellular matrix and function in an endocrine manner (10). To compensate for their poor HSGAG binding affinity, these endocrine FGFs require Klotho proteins as coreceptors (11). Klothos are type I transmembrane glycoproteins with extracellular regions that contain β-glycosidase-like domains. Klotho was discovered as a suppressor of aging in mice (12). Disruption of the Klotho gene causes a premature aging phenotype (12), whereas transgenic mice that overexpress Klotho exhibit increased resistance to oxidative stress (13) and a significant extension of lifespan (14). Recent studies have revealed multiple functions of Klotho, most notably as a coreceptor for FGF23 (15,16). β-Klotho was identified based on sequence similarity to Klotho (17). It is required as a coreceptor for FGF15/19 and FGF21 (10,18,19,20). Recently, the Lactase-like (Lctl) gene, which is also referred to as Klotho/lactase-phlorizin hydrolase-related protein, was shown to encode a third member of the Klotho family (21). Unlike Klotho and β-Klotho, Lctl contains only a single β-glycosidase-like domain in the extracellular region. It has not been determined whether Lctl functions as a coreceptor for endocrine FGF signaling.
FGFs have well-established roles in the regulation of cell proliferation, migration, differentiation, and apoptosis during embryonic development. However, surprisingly little is known about FGF expression and function in adults. In this report, we use real-time quantitative PCR (qPCR) to determine the expression profile of all 22 FGFs, the seven principal FGFRs, and three Klotho family members in 39 different mouse tissues and two mouse strains.
Results and Discussion
The mRNA levels of the FGFs and their receptors in 39 tissues derived from adult C57/Bl6 and 129x1/SvJ mice were measured by qPCR. All tissues were from male mice except for the female reproductive organs as previously described (22). Primer sets were designed and validated as described in Materials and Methods, and their sequences are provided in Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org. The similarity of gene profiles between the two mouse strains was assessed by hierarchical cluster analysis, which showed that all genes clustered together in the two strains (Supplemental Fig. 1).
FGF gene subfamilies
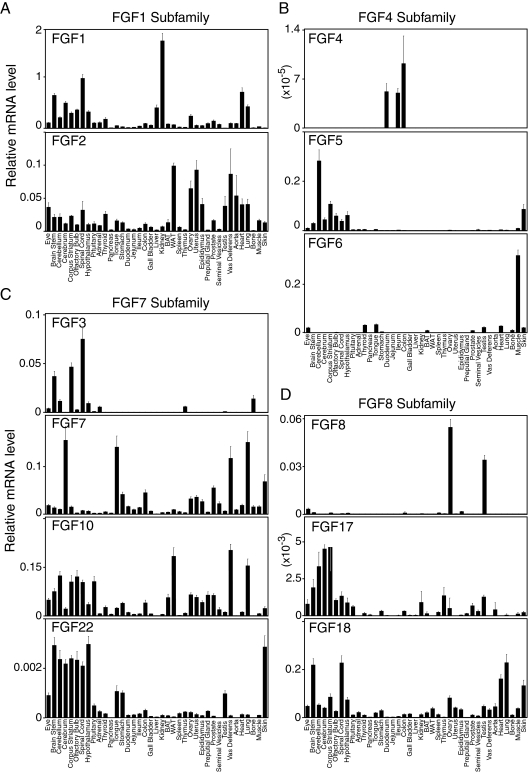
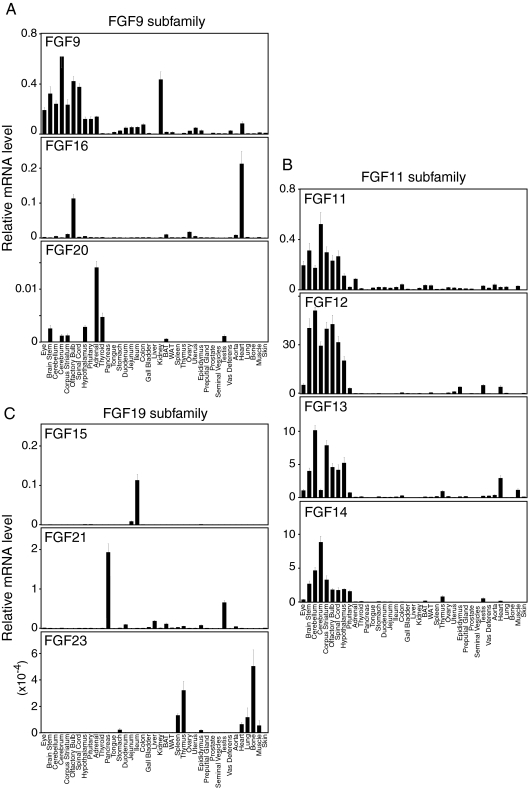
The anatomical profiles of all FGF mRNAs in C57/Bl6 mice grouped according to phylogenetic subfamily are depicted in Figs. 1 and 2. Bar graphs indicate normalized mRNA levels as arbitrary units, which were obtained by dividing the averaged, efficiency-corrected values for FGF mRNA gene expression by that for 18S rRNA expression for each sample as described elsewhere (23). The resulting values were multiplied by 105 for graphic representation. For the purposes of discussion, we consider relative mRNA values equal to or greater than 0.1 arbitrary unit to be high expression whereas mRNAs with qPCR cycle times equal to or greater than 34 are considered undetectable.
Figure 1.
Anatomic expression profiles of FGF-1, -4, -7, and -8 subfamilies in mouse tissues from C57/Bl6 mice. qPCR data are presented as the mean of triplicate measurements ± sd.
Figure 2.
Anatomic expression profiles of FGF-9, -11, and -19 subfamilies in mouse tissues from C57/Bl6 mice. qPCR data are presented as the mean of triplicate measurements ± sd.
FGF1 subfamily (FGF1, 2)
Fgf1 and Fgf2 are both broadly expressed with absolute mRNA levels of Fgf1 generally much higher than those of Fgf2 (Fig. 1A). Fgf1 mRNA is highly expressed throughout the central nervous system (CNS) and in kidney, heart, liver, and lung. Fgf2 mRNA is most abundant in white adipose tissue, heart, lung, aorta, and both male and female reproductive tissues (Fig. 1A).
FGF4 subfamily (FGF4, -5, and -6)
FGF4 was originally identified as a rearranged human oncogene after transfection of DNA from Kaposi’s sarcoma into NIH 3T3 cell (24,25). FGF4 protects mice from lethal irradiation (26) and is implicated in maintaining pluripotency of human embryonic stem cells (27). Fgf4 is detected, albeit at very low absolute levels, in duodenum, ileum, and colon (Fig. 1B) (28), suggesting that it may function in maintaining intestinal stem cells. Fgf5 is highly expressed throughout the CNS and is also present in the skin (Fig. 1B) (29), where it regulates hair growth (30,31). Mutations in the Fgf5 gene are associated with hair length phenotypes in canines (30,32). FGF6 is best known for its role in skeletal muscle physiology including muscle regeneration (33) (reviewed in Ref. 34). Consistent with this, Fgf6 is highly expressed in muscle (Fig. 1B).
FGF7 subfamily (FGF3, -7, -10, and -22)
Fgf3, the product of the int-2 protooncogene (35), is most abundantly expressed in the CNS including the spinal cord, corpus striatum, and brain stem (Fig. 1C). FGF7, also known as keratinocyte growth factor, is involved in protecting and repairing epithelial tissues (36). Fgf7 mRNA levels are highest in cerebrum, lung, vas deferens, tongue, and skin (Fig. 1C). Fgf10 mRNA is abundant in the CNS, vas deferens, and lung. It is also highly expressed in white adipose tissue, where it functions as an adipocyte growth factor (37,38). Fgf22 mRNA is highest in the CNS and skin but only at very low absolute levels (Fig. 1C) (39).
FGF8 family (FGF8, -17, and -18)
FGF8 was first identified as an androgen-inducible growth factor present in steroid-dependent cancers (40,41). Recently, FGF8 was implicated in GnRH deficiency and GnRH neuron development in the hypothalamus (42). Fgf8 mRNA levels are highest in ovary and testes (Fig. 1D) (43,44). Fgf17 mRNA is present at only very low levels but is highest in brain (Fig. 1D) (45,46), which correlates with its function in social behavior (47). Fgf18 mRNA is highly expressed in the brain stem, spinal cord, lung, heart, and skin (Fig. 1D).
FGF9 subfamily (FGF9, -16, and -20)
FGF9 was originally purified as a glia-activating factor from a cultured human glioma cell line (48). In the CNS, FGF9 is produced mainly by neurons and is a potent mitogen and survival factor for numerous cell types (49). Fgf9 is broadly expressed with high levels throughout the brain and in kidney (Fig. 2A) (50,51). Fgf16 is highly expressed in olfactory bulb and in heart (Fig. 2A), where it is required for cardiomyocyte proliferation (52). FGF20 is a neurotrophic factor expressed in the substantia nigra pars compacta (53,54) and is associated with Parkinson’s disease (55). Fgf20 is expressed at low levels in a limited set of tissues including adrenal (Fig. 2A).
Intracellular FGFs: FGF11 subfamily (FGF11, -12, -13, and -14)
The intracellular FGFs are all very abundantly expressed in the CNS (Fig. 2B) (56,57). This subfamily has the highest overall expression level among all the FGF subfamilies. Mutations in these genes are associated with neurological abnormalities (5).
FGF15/19 subfamily (FGF15/19, -21, and -23)
FGF15/19, FGF21, and FGF23 are atypical FGFs in that they function as endocrine hormones, they are the transcriptional targets of nuclear receptors, and in the adult mammal they have unique roles in governing lipid, carbohydrate, and mineral metabolism (58). Although Fgf15 mRNA is broadly expressed in the CNS during development (59), it is not detectable in adult CNS. Rather Fgf15 is highly and selectively expressed in ileum (Fig. 2C), where it is released into the blood to regulate bile acid homeostasis (60). FGF21 regulates carbohydrate and fatty acid metabolism and is induced by fasting and ketogenic diets in liver (61,62,63). In addition to liver, Fgf21 is very highly expressed in pancreas and testis (Fig. 2C) (64,65). Fgf23 is expressed, albeit at very low absolute levels, in bone and thymus (Fig. 2C) (66,67,68). FGF23 is secreted from the bone to regulate phosphate and calcium homeostasis (69,70).
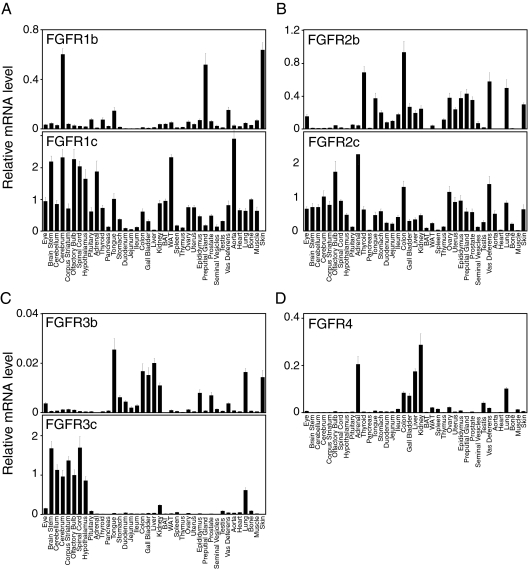
FGF receptors
Fgfr1 and Fgfr2 are both very broadly expressed (Fig. 3, A and B). The Fgfr1c isoform is highly expressed in most tissues, whereas Fgfr1b mRNA levels are high only in the cerebrum, tongue, preputial gland, vas deferens, and skin (Fig. 3A) (71). For Fgfr2, the “c” isoform is predominant in the CNS, whereas Fgfr2b and Fgfr2c are expressed at comparable levels in most other tissues (Fig. 3B).
Figure 3.
Anatomic expression profiles of FGFRs in mouse tissues from C57/Bl6 mice. qPCR data are presented as the mean of triplicate measurements ± sd.
The Fgfr3b and Fgfr3c isoforms have very different expression patterns. Fgfr3b is most abundant in gastrointestinal (GI) tissues, liver, kidney, lung, and skin but only at very low absolute levels. In contrast, Fgfr3c is highly expressed throughout the CNS and in lung and kidney (Fig. 3C).
Fgfr4 is highly expressed in kidney, liver, and adrenal (Fig. 3D) (72). Fgfr4 is also highly expressed in the lung (Fig. 3D), where it cooperates with Fgfr3 to promote the formation of alveoli during postnatal lung development (73).
Klotho family coreceptors
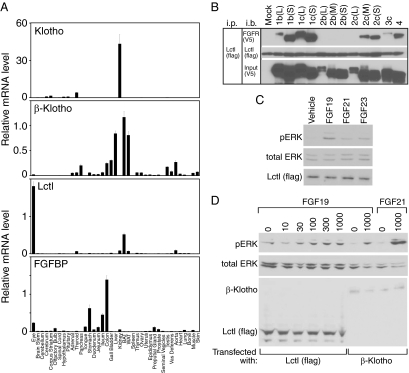
Klotho
Klotho binds to FGFR1c, FGFR3c, and FGFR4 to form a receptor complex for FGF23 (15,16). Klotho is very abundantly expressed in kidney (Fig. 4A), where it has well-established roles in regulating phosphate, calcium, and vitamin D metabolism (12,74,75). Klotho is also expressed, albeit at much lower levels, throughout the CNS and in thyroid (Fig. 4A) (12).
Figure 4.
Anatomic expression profile of Klotho family proteins and FGF binding protein (FGFBP1), and Lctl activity analysis. A, qPCR data are presented as the mean of triplicate measurements ± sd for tissues from C57/Bl6 mice. B, Lysates of 293 cells transfected with expression vectors for different FGFR isoforms and Flag-tagged Lctl were immunoprecipitated with Lctl using anti-Flag antibody and probed for either FGFRs using anti-V5 antibody or for Flag. Antibodies used for immunoprecipitation (ip) and immunoblotting (i.b.) are indicated. C, HEK293 cells were transfected with an expression plasmid for Flag-tagged Lctl and treated with FGF19 (1000 ng/ml), FGF21 (1000 ng/ml), FGF23 (500 ng/ml), or vehicle alone. Phosphorylated ERK (pERK), total ERK (tERK), and Flag-tagged Lctl were measured by immunoblotting. D, HEK293 cells were transfected with expression plasmids for Flag-tagged Lctl or β-Klotho and treated with FGF19 or FGF21 at the indicated concentrations. Phosphorylated ERK (pERK), total ERK (tERK), β-Klotho, and Flag-tagged Lctl were measured by immunoblotting.
β-Klotho
Mice defective in β-Klotho have increased bile acid synthesis and small gall bladders, phenotypes that are also manifest in mice lacking FGFR4 or FGF15 (60,76,77,78). β-Klotho interacts with FGFR4 to form a functional receptor for FGF15/19 (10,18,79,80). β-Klotho also complexes with FGFR1, -2, -3, and -4 to form receptors for FGF21 (10,18,19,20,81). β-Klotho expression is high in enterohepatic tissues including liver, gall bladder, colon, and pancreas (Fig. 4A) (17). It is also highly expressed in both white and brown adipose tissue (Fig. 4A), which is consistent with known effects of FGF15/19 and FGF21 on these tissues (63,82,83).
Lctl
A third member of the Klotho family, Lctl, was previously shown to be highly expressed in kidney and skin (21). Although we detected Lctl mRNA in kidney, we did not detect it in skin (Fig. 4A). The reason for this discrepancy is not known. In our profiling studies, Lctl is highly expressed in only brown adipose tissue and eye (Fig. 4A). Unlike Klotho and β-Klotho, Lctl has only a single β-glycosidase-like domain, raising the question as to whether it functions as a coreceptor for the endocrine FGFs. To address this issue, we first tested for interactions between Lctl and the FGFRs. Human embryonic kidney (HEK)293 cells, which do not express any of the Klotho family members, were transfected with expression plasmids for Lctl and the various FGFR isoforms, and coimmunoprecipitation experiments were performed. Lctl interacted efficiently with FGFR1b and -1c and FGFR2c and -4 (Fig. 4B). This interaction pattern is similar to that between Klotho and the FGFRs (15).
We next examined whether Lctl-FGFR complexes can be activated by the endocrine FGFs. In HEK293 cells transfected with an Lctl expression plasmid, FGF19 treatment caused ERK1/2 phosphorylation whereas FGF21 and FGF23 did not (Fig. 4C). This effect of FGF19 was dependent on the presence of Lctl (data not shown and Refs. 15 and 18). In dose-response analysis done in HEK293 cells transfected with an Lctl expression plasmid, FGF19 caused ERK1/2 phosphorylation at concentrations as low as 30 ng/ml (Fig. 4D, lanes 1–6). The efficacy of ERK1/2 phosphorylation caused by FGF19 was comparable in HEK293 cells transfected with either Lctl or β-Klotho (compare lanes 6 and 8) and less than that caused by FGF21 in HEK293 cells transfected with β-Klotho (compare lanes 6 and 10). That FGF19 can signal through Lctl-FGFR complexes is intriguing in light of the known effects of FGF15/19 in tissues in which Lctl is expressed, namely brown adipose tissue and eye. In obese mice, FGF15/19 causes weight loss by activating thermogenesis in brown adipose tissue (82,83). FGF15/19 regulates patterning and growth of the retina and the differentiation and growth of the lens in both zebrafish (84) and chicken (85).
FGF-binding protein 1 (FGFBP1)
FGFBP1 was originally cloned from A431 cancer cell lines as a protein that binds reversibly to FGF1 and FGF2 (86). More recently it was shown to also bind FGF7, -10, and -22 (87). FGFBP1 facilitates the release of FGFs from the extracellular matrix and their presentation to FGFRs, thus enhancing FGF activity (88). Fgfbp1 mRNA is abundant in the GI tract, with highest levels in the colon, stomach, and ileum (Fig. 4A). Fgfbp1 is also highly expressed in the eye (Fig. 4A).
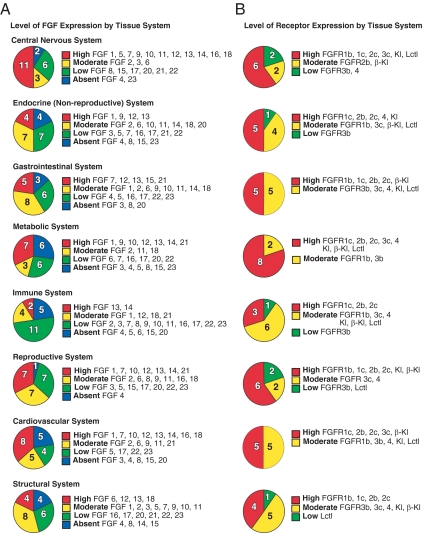
Distribution of FGFs and FGFRs in tissue systems
The distribution of the FGFs and receptors across tissue systems is summarized in Fig. 5, A and B, and discussed below.
Figure 5.
Tissue distribution of FGFs and FGFRs in adult mouse. A, FGF expression by tissue system. B, FGFR and Klotho coreceptor expression by tissue system. Expression levels in different tissue systems are indicated. Normalized FGF mRNA expression levels were defined as absent if the cycle time value was more than 34, low if the level was below 0.01 arbitrary units, moderate if the level was between 0.01 and 0.1, and high if the level was equal to or greater than 0.1 arbitrary units. Tissue systems are defined as CNS (eye, brainstem, cerebellum, cerebrum, corpus striatum, olfactory bulb, spinal cord, hypothalamus, and pituitary), endocrine (adrenal and thyroid), GI (tongue, stomach, duodenum, jejunum, ileum, colon, pancreas, and gall bladder), metabolic (liver, kidney, brown and white adipose tissue), immune (spleen and thymus), reproductive (ovary, uterus, epididymus, preputial gland, prostate, seminal vesicles, testis, and vas deferens), respiratory (aorta, heart, and lung), and structural (muscle, bone, and skin). Tissues were pooled from C57/Bl6 mice (n = 6).
CNS (eye, brainstem, cerebellum, cerebrum, corpus striatum, olfactory bulb, spinal cord, hypothalamus, pituitary)
The FGFs play prominent roles in the CNS (89). For example, during the past decade, much evidence has emerged supporting the involvement of the FGF system in psychiatric disorders (90,91). However, most of this work has been restricted to FGF1 and FGF2. We show that many of the FGFs are abundantly expressed in adult CNS (Fig. 5A). The expression of some of the FGFs, including Fgf5 and Fgf9 and the intracellular FGFs (FGF11 subfamily), is enriched in the CNS. The high levels of intracellular FGF mRNAs in the CNS correlate well with their function in controlling neuronal excitability (92). Whereas most of the FGFs are expressed at high levels across different parts of the CNS, others are enriched in particular parts of the brain such as Fgf7 in cerebellum and Fgf16 in olfactory bulb.
Fgfr1, Fgfr2, and Fgfr3 are also highly expressed in the CNS (Fig. 5B). Interestingly, the c isoforms of these receptors are predominant in brain, with only the cerebellum expressing high levels of a b isoform, namely Fgfr1b. Among the Klotho family, only Klotho is highly expressed in brain tissues. Lctl is highly expressed in eye.
Endocrine system (adrenal, thyroid)
Fgf1, which acts as a thymocyte mitogen and is elevated in thyroid hyperplasia and neoplasia (93,94,95), is highly expressed in adult thyroid as is Fgf9. Although Fgf12 and Fgf13 are both highly expressed in thyroid gland according to our strict definition, their expression in thyroid is much lower than in other tissues. Fgf20 is selectively expressed in adrenal, although its absolute expression level is low.
Both the adrenal and thyroid express Fgfr1c and Fgfr2c at high levels. The adrenal also expresses Fgfr4 whereas the thyroid gland expresses Fgfr2b. Although Klotho mRNA appears to be high in thyroid, we cannot exclude the possibility that this reflects tissue contamination from parathyroid gland, where Klotho is known to be abundantly expressed (96). FGF23 acts on the parathyroid gland to suppress production and secretion of PTH (96).
GI system (tongue, stomach, duodenum, jejunum, ileum, colon, gall bladder, pancreas)
Fgf7 is highly expressed in tongue as is Fgf15 in ileum and Fgf21 in pancreas. As in the thyroid gland, Fgf12 and Fgf13 are expressed in GI tissues but only at levels that are much lower than those in other tissues. Fgf4 is selectively expressed in the GI tract albeit only at very low levels.
Fgfr1c, Fgfr2b, and Fgfr2c are highly expressed in multiple GI tissues, whereas Fgfr1b is highly expressed in tongue. Fgfr3b and Fgfr4 are expressed in GI tissues, including colon and gall bladder, but at relatively low absolute levels. β-Klotho is highly expressed in colon, gall bladder, and pancreas. These findings are consistent with FGF15/19 causing gall bladder filling (76) and proliferation in colon (97,98), and FGF21 blocking chemically induced pancreatitis (65). Interestingly, Fgfbp1 is selectively expressed in the GI system with high expression levels in stomach, ileum, and colon.
Metabolic system (liver, kidney, and brown and white adipose tissue)
Fgf1 is very highly expressed in liver, and both Fgf1 and Fgf9 are abundant in kidney. Fgf10 is highly expressed in white adipose tissue, where it promotes adipogenesis (38). Both Fgf13 and Fgf14 are highly expressed in brown adipose tissue. Fgf21 is highly expressed in liver, pancreas, and brown adipose tissue, and Fgf12 mRNA levels are high in white adipose tissue and kidney.
Fgfr1c is the predominant FGFR in brown and white adipose tissue and kidney. Fgfr2b, Fgfr2c, and Fgfr4 are major FGFRs in liver. Fgfr3c is highly expressed in kidney as is Fgfr4. Klotho is expressed at high levels in the kidney, where it serves as a coreceptor for FGF23 (15,16). β-Klotho is highly expressed in liver and both white and brown adipose tissue, where it likely serves as a coreceptor for FGF15/19 and FGF21 (63,82,83). As described above, Lctl is highly expressed in brown adipose tissue, where it may contribute to the effects of FGF15/19 on metabolic rate (82).
Immune system (spleen, thymus)
Although Fgf7 and Fgf10 are required for development of the thymus (99,100), expression of these and most of the other FGFs and FGFRs is relatively low in adult thymus and spleen (Fig. 5, A and B). Fgf13 and Fgf14 are both highly expressed in thymus. Fgfr1c is highly expressed in spleen as are Fgfr2b and Fgfr2c in thymus.
Reproductive system (ovary, uterus, epididymus, preputial gland, prostate, seminal vesicles, testis, vas deferens)
FGF signaling regulates the proliferation and differentiation of testicular cells, the induction of spermatogenesis, and the development of epididymus and prostate (reviewed in Ref. 101). The male reproductive duct system (epididymus and vas deferens) and accessory glands (seminal vesicles, prostate gland, and preputial gland) have similar FGF and FGFR expression patterns. All these tissues express Fgf1, Fgf2, Fgf7, and Fgf10. The intracellular FGFs, Fgf12, Fgf13, and Fgf14, and the endocrine FGF, Fgf21, are highly expressed in the testis. FGF7 and FGF10 were previously shown to be important in the growth and development of the reproductive ducts and accessory glands by acting on FGFR2b (reviewed in Refs. 102 and 103), which is highly expressed in epididymus, preputial gland, prostate, and vas deferens. Fgfr1b, Fgfr1c, and Fgfr2c are also expressed at high levels in male reproductive tissues.
In the female reproductive tissues, Fgf1, Fgf12, and Fgf13 are highly expressed in ovary, whereas Fgf13 is also highly expressed in uterus. Fgfr1c, Fgfr2b, and Fgfr2c are highly expressed in both ovary and uterus.
Among the Klotho family of proteins, Klotho is highly expressed in ovary and testes as is β-Klotho in testis, implying roles for the endocrine FGFs in reproductive function.
Cardiovascular system (heart, aorta, lung)
Fgf1, Fgf12, and Fgf18 are highly expressed in both heart and lung, whereas Fgf13, Fgf14, and Fgf16 are expressed in heart, and Fgf7 and Fgf10 are expressed in lung. FGF7 is implicated in the repair of lung tissue (104,105,106) whereas FGF10 and FGF18 are implicated in lung development (107,108). Among the FGFs, only Fgf13 is highly expressed in aorta. Heart, lung, and aorta all express Fgfr1c at high levels, whereas lung also expresses Fgfr2b, Fgfr2c, and Fgfr3c, and aorta expresses Fgfr2c. Notably, FGFR2b is a receptor for FGF7 and 10 (109,110). Interestingly, β-Klotho is abundantly expressed in aorta, suggesting that FGF15 and/or FGF21 may affect blood vessel physiology in adult mice. FGF15 is known to be required for proper development of the cardiac outflow tract (111).
Structural system (bone, muscle, skin)
Fgf6 and Fgf13 are highly expressed in muscle, and Fgf12, Fgf13, and Fgf18 are abundantly expressed in skin. Although bone expresses only very low levels of FGFs, it is a major source of FGF23, which regulates phosphate, calcium, and vitamin D metabolism (112). Skin expresses Fgfr1b and Fgfr2b as well as Fgfr2c. Bone and muscle express Fgfr1c whereas bone also expresses Fgfr2c. None of the Klotho family proteins are highly expressed in structural tissues.
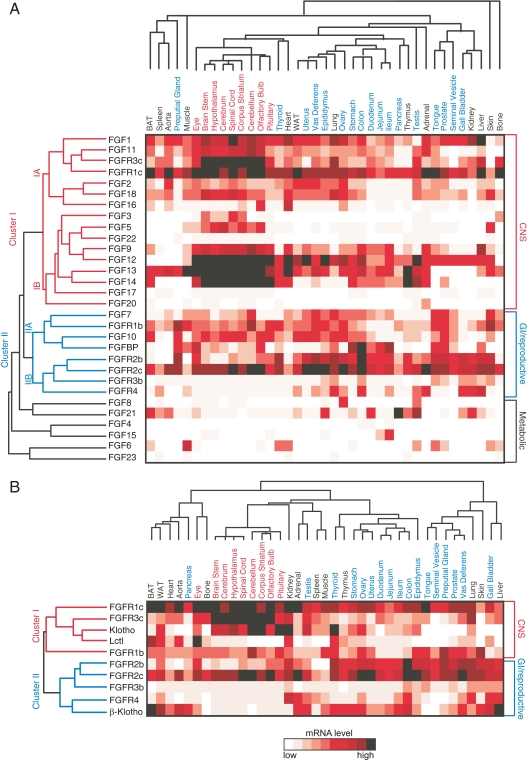
Hierarchical clustering of FGFs and FGFRs
To further investigate the potential physiological functions of FGFs and their receptors, unsupervised hierarchical clustering of the expression data was performed. Clustering of the FGFs and FGFRs resulted in a dendrogram with two main branches (Fig. 6A). Cluster I represents FGFs and FGFRs that are abundantly expressed in CNS, whereas Cluster II includes ones that are abundant in reproductive and GI tissues. Cluster I includes Fgfr1c and Fgfr3c, the FGF1 and FGF9 subfamilies, and Fgf3 and Fgf22 from the FGF7 subfamily. Based on published FGF-FGFR interaction data (113), these findings are consistent with FGF1 and 2 signaling through FGFR1c, and FGF1, 2, 9, 16, and 20 acting through FGFR3c.
Figure 6.
Cluster analysis of FGF and FGFRs. A, Unsupervised hierarchical clustering of FGFs and FGFRs based on tissue expression. qPCR data were clustered using average linkage with Pearson correlation coefficient using Matrix 1.28 software as described in Materials and Methods. B, Unsupervised hierarchical clustering of FGFRs and Klotho family coreceptors based on tissue expression. qPCR data were clustered using average linkage with Pearson correlation coefficient using Matrix 1.28 software.
Cluster II includes Fgfr1b, Fgfr2b, Fgfr2c, Fgfr3b, and Fgfr4 together with Fgf7 and Fgf10 from the FGF7 subfamily and Fgfbp1 (Fig. 6A). FGF7 and 10 bind to FGFR2b with highest affinity but also interact with FGFR1b (113). FGF10 was shown to induce proliferation in L6 myoblasts in an FGFR1b-dependent manner (71). It is interesting that Fgfbp1 clusters with Fgf7 and Fgf10, because FGFBP1 interacts with both these FGFs and enhances their ability to promote epithelial repair processes (87).
Notably, the endocrine FGFs segregate from all of the FGFRs (Fig. 6A), suggesting that their major function is to signal across tissues. Interestingly, each of the endocrine FGFs clustered with a canonical FGF: Fgf21 with Fgf8 (high expression in testis), Fgf15 with Fgf4 (high expression in GI tissues), and Fgf23 with Fgf6 (high expression in muscle and bone). Whether there is functional significance to this colocalization remains to be investigated.
Hierarchical clustering was also performed with expression data for the FGFRs and Klotho family members (Fig. 6B). Klotho clustered most closely with Fgfr1c and Fgfr3c, which it interacts with to form receptors for FGF23 (16). The coexpression of Klotho, Fgfr1c, and Fgfr3c throughout the CNS raises the interesting possibility that FGF23 acts on these tissues. Similarly, the clustering of Lctl with Fgfr1c suggests that these two proteins may form a receptor for FGF15/19 in tissues such as brown adipose tissue and eye.
β-Klotho clustered most closely with Fgfr4, with which it forms a high-affinity receptor complex for FGF15/19 in tissues including liver, colon, and gall bladder. The coexpression of β-Klotho and Fgfr4 in adrenal suggests that FGF15/19 might also influence steroidogenesis. β-Klotho is known to form complexes with FGFR1c and FGFR3c that interact efficiently with FGF21 (19,20,81). Although β-Klotho did not cluster with either Fgfr1c or Fgfr3c, its coexpression with these receptors in brown and white adipose tissue, skin, ovary, and testis suggests that these tissues may be targets for FGF21. In this regard, it is interesting that Fgf21 is also highly expressed in testis, where it may act in a paracrine fashion.
In summary, we have surveyed genes encoding FGFs, FGFRs and associated proteins for their expression across 39 different adult mouse tissues. Although it is difficult to compare our results with those of previous publications due to the variety of techniques employed and the different definitions of what constitutes meaningful expression, in Supplemental Tables 2 and 3 we have overlaid our data with those in the literature. For the purposes of this comparison we have limited the literature references to those in which gene expression is measured in mouse or rat tissues. Two general conclusions emerge from these tables: First, we detected expression of FGFs and related genes in nearly all tissues where they have been previously reported. Notable discrepancies include Lctl and Fgfbp1 in skin. The basis for these differences is unclear. Second, we detected expression of FGFs and related genes in many tissues where they had not been previously described. An important strength of our approach is that the data are both quantitative and comprehensive, thus permitting direct comparison of gene expression across tissues in a manner that was not previously possible. Although the current study is comprehensive in the number of tissues examined, an important limitation is that whole tissues were used. Thus, genes expressed in small subsets of cells within whole tissues are likely to be missed. Determining the specific cell types in which the FGFs and associated proteins are expressed is an important future step. Nevertheless, this atlas provides a useful resource and hypothesis-generating tool for those studying FGF action in adult animals.
Materials and Methods
Animals and tissue collection
All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center, which assures that all animal use adheres to federal regulations as published in the Animal Welfare Act (AWA), the Guide for the Care and Use of Laboratory Animals (Guide), the Public Health Service Policy, and the US Government Principles Regarding the Care and Use of Animals. C57/BL6 and 129×1/SvJ mice (6 wk of age) were purchased from The Jackson Laboratory (Bar Harbor, ME), and were killed at 8–9 wk of age killed at lights on. Tissues were harvested as previously described (22).
RNA preparation and cDNA synthesis
All tissues, except pancreas, were snap frozen on liquid nitrogen and stored at −80 C until RNA extraction using RNA Stat60 (TelTest, Friendswood, TX). Pancreas RNA was prepared immediately due to high ribonuclease content. Total RNA was pooled in equal quantities for each tissue (n = 6). RNA pools from male mice were used for all tissues except ovary and uterus. Genomic DNA contamination was eliminated by deoxyribonuclease (DNAse) treatment using DNAse I (Roche, Indianapolis, IN). cDNA for qPCR assays was prepared from 2.4 μg DNased RNA using SuperScript RT III (Invitrogen, Carlsbad, CA) in a final volumr of 100 μl. After cDNA synthesis, ribonuclease-free water was added to increase the sample volume to 300 μl. Before pooling, RNA samples from difficult-to-dissect tissues were assayed for tissue-specific markers to ensure their tissue fidelity as described elsewhere (22).
Primer design and qPCR
Individual FGFs, FGFR, Klothos, and 18S rRNA expression levels were measured in triplicate wells of a 384-well reaction plate with 10 ng cDNA per well on an Applied Biosystems 7900HT (Applied Systems, Foster City, CA) with SYBR Green chemistry. Primer concentrations were 75 nm for 18S rRNA and 150 nm for all others. Primer sets were designed and validated as described elsewhere (23). For FGFRs, primer pairs were designed to detect receptor b and c isoforms selectively. Primer pairs are provided in Supplemental Table 1. Universal cDNA standards generated from mouse RNA (BD CLONTECH, Palo Alto, CA) were used for all analyses, except for FGF3, -4, -5, -6, -8, -15, -16, -17, -21, -23, and Lctl because of their limited expression.
qPCR data were analyzed by ABI instrument software SDS2.1 (ABI Advanced Technologies, Inc., Columbia, MD). Baseline values of amplification plots were set automatically, and threshold values were kept constant to obtain normalized cycle time and linear regression data. mRNA with cycle times equal to or greater than 34 were determined to be below the limit of detection. PCR efficiencies were calculated from the slope of the resulting standard curves. Normalized mRNA levels are expressed as arbitrary units and were obtained by dividing the averaged, efficiency corrected values for mRNA expression by that for 18s rRNA (22). The resulting values were multiplied by 105 for graphic representation. Error bars represent experimental error and were calculated based on the sds of the average value from triplicate sample wells (22).
Hierarchical clustering
Unsupervised clustering was performed on the normalized RNA levels by calculating the Pearson’s centered correlation coefficient followed by average linkage analysis using Matrix 1.28 software (gift from Dr. Luc Girard, University of Texas Southwestern Medical Center, Dallas, TX) as described elsewhere (22). For pair wise correlation, Pearson correlation r values were computed between the expression pattern of every pair of genes. Data were hierarchical clustered with GEPAS web resource using unweighted pair group method with arithmetic mean and Pearson coefficient distance.
Expression vectors
cDNA containing the mouse Lctl was cloned into the pEF1 vector (Invitrogen) with a FLAG-epitope tag at the C terminus. Expression vectors for mouse FGFRs were described previously (15).
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting experiments were performed as described elsewhere (15). HEK293 cells were maintained in DMEM supplemented with 10% fetal bovine serum. Subconfluent HEK293 cells were transfected with expression vectors for Lctl and FGFRs 36 h before the experiments by using Lipofectamine (Invitrogen) as carrier. Cells were lysed in buffer containing phosphatase and proteinase inhibitors as previously described (22). After a portion of each cell lysate sample was saved for immunoblotting with anti-V5 antibody, the cell lysates were incubated with anti-V5-agarose beads (Sigma-Aldrich, St. Louis, MO) at 4 C for 3 h. The beads were washed four times with Tris-buffered saline containing 1% Triton X-100 (TBST); bead-bound proteins were eluted with Laemmli sample buffer, electrophoresed, and then transferred to Hybond C Extra membrane (Amersham Biosciences, Piscataway, NJ). The protein blots were incubated with anti-V5 antibody (Invitrogen). Chemiluminescence signals were developed with the SuperSignal West Dura system (Pierce Chemical Co., Rockford, IL).
Lctl functional analysis
Subconfluent HEK293 cells were transfected with expression vectors for Flag-tagged Lctl or β-Klotho using Lipofectamine as carrier (Invitrogen). After 36 h, cells were serum starved overnight and then treated with recombinant human FGF19, -21, or -23 for 15 min. Cell lysates were subjected to immunoblot analysis using anti-phospho-p44/42 MAPK (ERK1/2) antibody (Cell Signaling Technology, Beverly, MA), anti-ERK antibody (Cell Signaling), or anti-Flag.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants DK067158, P20RR20691, and 1RL1GM084436-01 (to S.A.K. and D.J.M.), U19DK62434 (to D.J.M.), AG19712 (to M.K.), and DE13686 (to M.M.); grants from the Robert A. Welch Foundation (Grant I-1558 to S.A.K.; Grant I-1275 to D.J.M.); grants from the Genzyme Renal Innovations Program (to M.K.) and the Howard Hughes Medical Institute (to D.J.M.). David J. Mangelsdorf is an Investigator of the Howard Hughes Medical Institute. Klementina Fon Tacer was supported by Fulbright Fellowship. Angie L. Bookout was supported by National Institutes of Health Training Grant GM007062.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 28, 2010
Abbreviations: CNS, Central nervous system; DNAse, deoxyribonuclease; FGF, fibroblast growth factor; FGFBP, FGF-binding protein; FGFR, fibroblast growth factor receptor; GI, gastrointestinal; HEK, human embryonic kidney; HSGAGs, heparan sulfate glycosaminoglycans; qPCR, quantitative real-time PCR.
References
- Gospodarowicz D 1974 Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature 249:123–127 [DOI] [PubMed] [Google Scholar]
- Itoh N 2007 The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biol Pharm Bull 30:1819–1825 [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM 2004 Evolution of the Fgf and Fgfr gene families. Trends Genet 20:563–569 [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM 2008 Functional evolutionary history of the mouse Fgf gene family. Dev Dyn 237:18–27 [DOI] [PubMed] [Google Scholar]
- Goldfarb M 2005 Fibroblast growth factor homologous factors: evolution, structure, and function. Cytokine Growth Factor Rev 16:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Williams LT 1993 Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res 60:1–41 [DOI] [PubMed] [Google Scholar]
- Ornitz DM 2000 FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22:108–112 [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA 2005 Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev 16:107–137 [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Goetz R 2005 A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Curr Opin Struct Biol 15:506–516 [DOI] [PubMed] [Google Scholar]
- Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt RJ, Yu X, White KE, Inagaki T, Kliewer SA, Yamamoto M, Kurosu H, Ogawa Y, Kuro-o M, Lanske B, Razzaque MS, Mohammadi M 2007 Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 27:3417–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Kuro-O M 2009 Endocrine fibroblast growth factors as regulators of metabolic homeostasis. Biofactors 35:52–60 [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI 1997 Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt KP, Kuro-o M 2005 Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 280:38029–38034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M 2005 Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M 2006 Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T 2006 Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774 [Google Scholar]
- Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI 2000 Molecular cloning and expression analyses of mouse βklotho, which encodes a novel Klotho family protein. Mech Dev 98:115–119 [DOI] [PubMed] [Google Scholar]
- Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M 2007 Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282:26687–26695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M 2007 βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA 104:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T 2008 βKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol 22:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Fujimori T, Hayashizaki Y, Nabeshima Y 2002 Identification of a novel mouse membrane-bound family 1 glycosidase-like protein, which carries an atypical active site structure. Biochim Biophys Acta 1576:341–345 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ 2006 Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF 2006 High-throughput real-time quantitative reverse transcription PCR. Curr Protoc Mol Biol 15.8.1–15.8.28 [DOI] [PubMed] [Google Scholar]
- Delli Bovi P, Basilico C 1987 Isolation of a rearranged human transforming gene following transfection of Kaposi sarcoma DNA. Proc Natl Acad Sci USA 84:5660–5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Bovi P, Curatola AM, Kern FG, Greco A, Ittmann M, Basilico C 1987 An oncogene isolated by transfection of Kaposi’s sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell 50:729–737 [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hirai K, Yamamoto H, Tanooka H, Sakamoto H, Iwamoto T, Takahashi T, Terada M, Ochiya T 2004 HST-1/FGF-4 plays a critical role in crypt cell survival and facilitates epithelial cell restitution and proliferation. Oncogene 23:3681–3688 [DOI] [PubMed] [Google Scholar]
- Mayshar Y, Rom E, Chumakov I, Kronman A, Yayon A, Benvenisty N 2008 Fibroblast growth factor 4 and its novel splice isoform have opposing effects on the maintenance of human embryonic stem cell self-renewal. Stem Cells 26:767–774 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Ochiya T, Takahama Y, Ishii Y, Osumi N, Sakamoto H, Terada M 2000 Detection of spatial localization of Hst-1/Fgf-4 gene expression in brain and testis from adult mice. Oncogene 19:3805–3810 [DOI] [PubMed] [Google Scholar]
- Haub O, Drucker B, Goldfarb M 1990 Expression of the murine fibroblast growth factor 5 gene in the adult central nervous system. Proc Natl Acad Sci USA 87:8022–8026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehler JS, David VA, Schäffer AA, Bajema K, Eizirik E, Ryugo DK, Hannah SS, O'Brien SJ, Menotti-Raymond M 2007 Four independent mutations in the feline fibroblast growth factor 5 gene determine the long-haired phenotype in domestic cats. J Hered 98:555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Kato T, Takimoto H, Masui S, Oshima H, Ozawa K, Suzuki S, Imamura T 1998 Localization of rat FGF-5 protein in skin macrophage-like cells and FGF-5S protein in hair follicle: possible involvement of two Fgf-5 gene products in hair growth cycle regulation. J Invest Dermatol 111:963–972 [DOI] [PubMed] [Google Scholar]
- Drögemüller C, Rüfenacht S, Wichert B, Leeb T 2007 Mutations within the FGF5 gene are associated with hair length in cats. Anim Genet 38:218–221 [DOI] [PubMed] [Google Scholar]
- Floss T, Arnold HH, Braun T 1997 A role for FGF-6 in skeletal muscle regeneration. Genes Dev 11:2040–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand A-S, Laziz I, Chanoine C 2006 FGF6 in myogenesis. Biochim Biophys Acta 1763:773–778 [DOI] [PubMed] [Google Scholar]
- Dickson C, Acland P, Smith R, Dixon M, Deed R, MacAllan D, Walther W, Fuller-Pace F, Kiefer P, Peters G 1990 Characterization of int-2: a member of the fibroblast growth factor family. J Cell Sci Suppl 13:87–96 [DOI] [PubMed] [Google Scholar]
- Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM 2000 KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol 279:L1146–L1158 [DOI] [PubMed] [Google Scholar]
- Beer HD, Florence C, Dammeier J, McGuire L, Werner S, Duan DR 1997 Mouse fibroblast growth factor 10: cDNA cloning, protein characterization, and regulation of mRNA expression. Oncogene 15:2211–2218 [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Emoto H, Konishi M, Mikami T, Ohuchi H, Nakao K, Itoh N 1999 FGF-10 is a growth factor for preadipocytes in white adipose tissue. Biochem Biophys Res Commun 258:109–112 [DOI] [PubMed] [Google Scholar]
- Beyer TA, Werner S, Dickson C, Grose R 2003 Fibroblast growth factor 22 and its potential role during skin development and repair. Exp Cell Res 287:228–236 [DOI] [PubMed] [Google Scholar]
- Tanaka A, Miyamoto K, Minamino N, Takeda M, Sato B, Matsuo H, Matsumoto K 1992 Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc Natl Acad Sci USA 89:8928–8932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valve E, Martikainen P, Seppänen J, Oksjoki S, Hinkka S, Anttila L, Grenman S, Klemi P, Härkönen P 2000 Expression of fibroblast growth factor (FGF)-8 isoforms and FGF receptors in human ovarian tumors. Int J Cancer 88:718–725 [DOI] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N 2008 Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest 118:2822–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur CA, Shankar DB, Shackleford GM 1995 Fgf-8, activated by proviral insertion, cooperates with the Wnt-1 transgene in murine mammary tumorigenesis. J Virol 69:2501–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratini Jr J, Teixeira AB, Costa IB, Glapinski VF, Pinto MG, Giometti IC, Barros CM, Cao M, Nicola ES, Price CA 2005 Expression of fibroblast growth factor-8 and regulation of cognate receptors, fibroblast growth factor receptor-3c and -4, in bovine antral follicles. Reproduction 130:343–350 [DOI] [PubMed] [Google Scholar]
- Hoshikawa M, Ohbayashi N, Yonamine A, Konishi M, Ozaki K, Fukui S, Itoh N 1998 Structure and expression of a novel fibroblast growth factor, FGF-17, preferentially expressed in the embryonic brain. Biochem Biophys Res Commun 244:187–191 [DOI] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL 2007 Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci USA 104:7652–7657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, Rubenstein JL, Mucke L 2008 Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav 7:344–354 [DOI] [PubMed] [Google Scholar]
- Naruo K, Seko C, Kuroshima K, Matsutani E, Sasada R, Kondo T, Kurokawa T 1993 Novel secretory heparin-binding factors from human glioma cells (glia-activating factors) involved in glial cell growth. Purification and biological properties. J Biol Chem 268:2857–2864 [PubMed] [Google Scholar]
- Garcès A, Nishimune H, Philippe JM, Pettmann B, deLapeyrière O 2000 FGF9: a motoneuron survival factor expressed by medial thoracic and sacral motoneurons. J Neurosci Res 60:1–9 [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Naruo K, Seko C, Matsumoto S, Kondo T, Kurokawa T 1993 Molecular cloning of a novel cytokine cDNA encoding the ninth member of the fibroblast growth factor family, which has a unique secretion property. Mol Cell Biol 13:4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T, Kondo T, Nakamura S, Kirino T, Kurokawa T, Ikeda K 1998 Neuronal localization of fibroblast growth factor-9 immunoreactivity in human and rat brain. Brain Res 783:179–187 [DOI] [PubMed] [Google Scholar]
- Hotta Y, Sasaki S, Konishi M, Kinoshita H, Kuwahara K, Nakao K, Itoh N 2008 Fgf16 is required for cardiomyocyte proliferation in the mouse embryonic heart. Dev Dyn 237:2947–2954 [DOI] [PubMed] [Google Scholar]
- Ohmachi S, Mikami T, Konishi M, Miyake A, Itoh N 2003 Preferential neurotrophic activity of fibroblast growth factor-20 for dopaminergic neurons through fibroblast growth factor receptor-1c. J Neurosci Res 72:436–443 [DOI] [PubMed] [Google Scholar]
- Ohmachi S, Watanabe Y, Mikami T, Kusu N, Ibi T, Akaike A, Itoh N 2000 FGF-20, a novel neurotrophic factor, preferentially expressed in the substantia nigra pars compacta of rat brain. Biochem Biophys Res Commun 277:355–360 [DOI] [PubMed] [Google Scholar]
- Mizuta I, Tsunoda T, Satake W, Nakabayashi Y, Watanabe M, Takeda A, Hasegawa K, Nakashima K, Yamamoto M, Hattori N, Murata M, Toda T 2008 Calbindin 1, fibroblast growth factor 20, and α-synuclein in sporadic Parkinson’s disease. Hum Genet 124:89–94 [DOI] [PubMed] [Google Scholar]
- Hartung H, Feldman B, Lovec H, Coulier F, Birnbaum D, Goldfarb M 1997 Murine FGF-12 and FGF-13: expression in embryonic nervous system, connective tissue and heart. Mech Dev 64:31–39 [DOI] [PubMed] [Google Scholar]
- Wang Q, McEwen DG, Ornitz DM 2000 Subcellular and developmental expression of alternatively spliced forms of fibroblast growth factor 14. Mech Dev 90:283–287 [DOI] [PubMed] [Google Scholar]
- Moore DD 2007 Physiology. Sister act. Science 316:1436–1438 [DOI] [PubMed] [Google Scholar]
- Gimeno L, Brûlet P, Martínez S 2003 Study of Fgf15 gene expression in developing mouse brain. Gene Expr Patterns 3:473–481 [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA 2005 Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2:217–225 [DOI] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E 2007 Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437 [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA 2007 Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB 2005 FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nakatake Y, Konishi M, Itoh N 2000 Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 1492:203–206 [DOI] [PubMed] [Google Scholar]
- Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J 2006 Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55:2470–2478 [DOI] [PubMed] [Google Scholar]
- Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T 2001 Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98:6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Yoshioka M, Itoh N 2000 Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun 277:494–498 [DOI] [PubMed] [Google Scholar]
- Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD 2003 Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278:37419–37426 [DOI] [PubMed] [Google Scholar]
- Kolek OI, Hines ER, Jones MD, LeSueur LK, Lipko MA, Kiela PR, Collins JF, Haussler MR, Ghishan FK 2005 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol 289:G1036–G1042 [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T 2004 Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer HD, Vindevoghel L, Gait MJ, Revest JM, Duan DR, Mason I, Dickson C, Werner S 2000 Fibroblast growth factor (FGF) receptor 1-IIIb is a naturally occurring functional receptor for FGFs that is preferentially expressed in the skin and the brain. J Biol Chem 275:16091–16097 [DOI] [PubMed] [Google Scholar]
- Partanen J, Mäkelä TP, Eerola E, Korhonen J, Hirvonen H, Claesson-Welsh L, Alitalo K 1991 FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J 10:1347–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M, Xu X, Ohyama K, Deng CX 1998 FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 125:3615–3623 [DOI] [PubMed] [Google Scholar]
- Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y 2007 α-Klotho as a regulator of calcium homeostasis. Science 316:1615–1618 [DOI] [PubMed] [Google Scholar]
- Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y 2003 Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17:2393–2403 [DOI] [PubMed] [Google Scholar]
- Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, Suino-Powell K, Xu HE, Richardson JA, Gerard RD, Mangelsdorf DJ, Kliewer SA 2006 Identification of a hormonal basis for gallbladder filling. Nat Med 12:1253–1255 [DOI] [PubMed] [Google Scholar]
- Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y 2005 Impaired negative feedback suppression of bile acid synthesis in mice lacking βKlotho. J Clin Invest 115:2202–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Wang F, Kan M, Jin C, Jones RB, Weinstein M, Deng CX, McKeehan WL 2000 Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J Biol Chem 275:15482–15489 [DOI] [PubMed] [Google Scholar]
- Lin BC, Wang M, Blackmore C, Desnoyers LR 2007 Liver-specific activities of FGF19 require Klotho β. J Biol Chem 282:27277–27284 [DOI] [PubMed] [Google Scholar]
- Wu X, Ge H, Gupte J, Weiszmann J, Shimamoto G, Stevens J, Hawkins N, Lemon B, Shen W, Xu J, Veniant MM, Li YS, Lindberg R, Chen JL, Tian H, Li Y 2007 Co-receptor requirements for fibroblast growth factor-19 signaling. J Biol Chem 282:29069–29072 [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, Hale JE, Coskun T, Shanafelt AB 2008 FGF-21/FGF-21 receptor interaction and activation is determined by βKlotho. J Cell Physiol 215:1–7 [DOI] [PubMed] [Google Scholar]
- Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA 2004 Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145:2594–2603 [DOI] [PubMed] [Google Scholar]
- Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA 2002 Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143:1741–1747 [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Miyake A, Nakagawa Y, Mido T, Yoshikawa M, Konishi M, Itoh N 2008 Fgf19 is required for zebrafish lens and retina development. Dev Biol 313:752–766 [DOI] [PubMed] [Google Scholar]
- Kurose H, Okamoto M, Shimizu M, Bito T, Marcelle C, Noji S, Ohuchi H 2005 FGF19-FGFR4 signaling elaborates lens induction with the FGF8-L-Maf cascade in the chick embryo. Dev Growth Differ 47:213–223 [DOI] [PubMed] [Google Scholar]
- Wu DQ, Kan MK, Sato GH, Okamoto T, Sato JD 1991 Characterization and molecular cloning of a putative binding protein for heparin-binding growth factors. J Biol Chem 266:16778–16785 [PubMed] [Google Scholar]
- Beer HD, Bittner M, Niklaus G, Munding C, Max N, Goppelt A, Werner S 2005 The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: implications for epithelial repair. Oncogene 24:5269–5277 [DOI] [PubMed] [Google Scholar]
- Abuharbeid S, Czubayko F, Aigner A 2006 The fibroblast growth factor-binding protein FGF-BP. Int J Biochem Cell Biol 38:1463–1468 [DOI] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O 2003 Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res 313:139–157 [DOI] [PubMed] [Google Scholar]
- Riva MA, Molteni R, Bedogni F, Racagni G, Fumagalli F 2005 Emerging role of the FGF system in psychiatric disorders. Trends Pharmacol Sci 26:228–231 [DOI] [PubMed] [Google Scholar]
- Akil H, Evans SJ, Turner CA, Perez J, Myers RM, Bunney WE, Jones EG, Watson SJ 2008 The fibroblast growth factor family and mood disorders. Novartis Found Symp 289:94–96; discussion 97–100, 193–195 [DOI] [PubMed] [Google Scholar]
- Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, Giza J, Tchetchik D, Kelley K, Vega A, Matthews G, Rossi P, Ornitz DM, D'Angelo E 2007 Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron 55:449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggo MC, Hopkins JM, Franklyn JA, Johnson GD, Sanders DS, Sheppard MC 1995 Expression of fibroblast growth factors in thyroid cancer. J Clin Endocrinol Metab 80:1006–1011 [DOI] [PubMed] [Google Scholar]
- Logan A, Black EG, Gonzalez AM, Buscaglia M, Sheppard MC 1992 Basic fibroblast growth factor: an autocrine mitogen of rat thyroid follicular cells? Endocrinology 130:2363–2372 [DOI] [PubMed] [Google Scholar]
- Thompson SD, Franklyn JA, Watkinson JC, Verhaeg JM, Sheppard MC, Eggo MC 1998 Fibroblast growth factors 1 and 2 and fibroblast growth factor receptor 1 are elevated in thyroid hyperplasia. J Clin Endocrinol Metab 83:1336–1341 [DOI] [PubMed] [Google Scholar]
- Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J 2007 The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers LR, Pai R, Ferrando RE, Hotzel K, Le T, Ross J, Carano R, D'Souza A, Qing J, Mohtashemi I, Ashkenazi A, French DM 2007 Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene 27:85–97 [DOI] [PubMed] [Google Scholar]
- Pai R, Dunlap D, Qing J, Mohtashemi I, Hotzel K, French DM 2008 Inhibition of fibroblast growth factor 19 reduces tumor growth by modulating β-catenin signaling. Cancer Res 68:5086–5095 [DOI] [PubMed] [Google Scholar]
- Erickson M, Morkowski S, Lehar S, Gillard G, Beers C, Dooley J, Rubin JS, Rudensky A, Farr AG 2002 Regulation of thymic epithelium by keratinocyte growth factor. Blood 100:3269–3278 [DOI] [PubMed] [Google Scholar]
- Revest JM, Suniara RK, Kerr K, Owen JJ, Dickson C 2001 Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol 167:1954–1961 [DOI] [PubMed] [Google Scholar]
- Chaffer CL, Dopheide B, Savagner P, Thompson EW, Williams ED 2007 Aberrant fibroblast growth factor receptor signaling in bladder and other cancers. Differentiation 75:831–842 [DOI] [PubMed] [Google Scholar]
- Cotton LM, O'Bryan MK, Hinton BT 2008 Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr Rev 29:193–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N 2000 FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277:643–649 [DOI] [PubMed] [Google Scholar]
- Post M, Souza P, Liu J, Tseu I, Wang J, Kuliszewski M, Tanswell AK 1996 Keratinocyte growth factor and its receptor are involved in regulating early lung branching. Development 122:3107–3115 [DOI] [PubMed] [Google Scholar]
- Ulich TR, Yi ES, Longmuir K, Yin S, Biltz R, Morris CF, Housley RM, Pierce GF 1994 Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest 93:1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach H, Kasper M, Koslowski R, Pan T, Schuh D, Müller M, Mason RJ 2000 Alveolar epithelial type II cell apoptosis in vivo during resolution of keratinocyte growth factor-induced hyperplasia in the rat. Histochem Cell Biol 114:49–61 [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S 1999 Fgf10 is essential for limb and lung formation. Nat Genet 21:138–141 [DOI] [PubMed] [Google Scholar]
- Ohbayashi N, Hoshikawa M, Kimura S, Yamasaki M, Fukui S, Itoh N 1998 Structure and expression of the mRNA encoding a novel fibroblast growth factor, FGF-18. J Biol Chem 273:18161–18164 [DOI] [PubMed] [Google Scholar]
- Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, Chan AM, Aaronson SA 1992 Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci USA 89:246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Finch PW, Aaronson SA 1998 Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7). J Biol Chem 273:13230–13235 [DOI] [PubMed] [Google Scholar]
- Vincentz JW, McWhirter JR, Murre C, Baldini A, Furuta Y 2005 Fgf15 is required for proper morphogenesis of the mouse cardiac outflow tract. Genesis 41:192–201 [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamashita T 2007 FGF23 is a hormone-regulating phosphate metabolism–unique biological characteristics of FGF23. Bone 40:1190–1195 [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM 2006 Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem 281:15694–15700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.