Abstract
In the past 50 years, we have witnessed explosive growth in the understanding of normal and neoplastic lymphoid cells. B-cell, T-cell, and natural killer (NK)–cell neoplasms in many respects recapitulate normal stages of lymphoid cell differentiation and function, so that they can be to some extent classified according to the corresponding normal stage. Likewise, the molecular mechanisms involved the pathogenesis of lymphomas and lymphoid leukemias are often based on the physiology of the lymphoid cells, capitalizing on deregulated normal physiology by harnessing the promoters of genes essential for lymphocyte function. The clinical manifestations of lymphomas likewise reflect the normal function of lymphoid cells in vivo. The multiparameter approach to classification adopted by the World Health Organization (WHO) classification has been validated in international studies as being highly reproducible, and enhancing the interpretation of clinical and translational studies. In addition, accurate and precise classification of disease entities facilitates the discovery of the molecular basis of lymphoid neoplasms in the basic science laboratory.
Introduction
Mature B-cell and T/natural killer (NK)–cell neoplasms are clonal tumors of B cells, T cells, or NK cells that in many respects recapitulate stages of normal B-cell or T-cell differentiation. Although classifications of lymphoid malignancies have historically treated lymphomas and leukemias separately, this distinction is now appreciated as artificial. However, as the precursor neoplasms—lymphoblastic lymphomas/leukemias and acute myeloid leukemias—are closely related from a clinical and biological perspective, they will not be covered in this review. In addition, plasma cell neoplasms often have been treated separately from other lymphoid neoplasms, but this segregation is equally artificial, and the World Health Organization (WHO) classification of lymphoid neoplasms1 includes plasma disorders. A recent review by Kyle and Rajkumar in this series comprehensively discussed plasma cell myeloma,2 and thus we will not include it further in this review.
As immunologists and pathologists have come to understand the ontogeny of lymphoid cells, it has been tempting to base the classification of lymphoid malignancies strictly on the corresponding normal stage. However, some neoplasms—for example, hairy cell leukemia—do not clearly correspond to a normal B-cell differentiation stage, and some clinically distinctive neoplasms may exhibit immunophenotypic heterogeneity, such as hepatosplenic T-cell lymphoma, which is derived from either α/β or γ/δ T cells. Thus, the normal counterpart of the neoplastic cell cannot at this time be the sole basis for classification.
Cancers are increasingly recognized as genetic diseases, with precise molecular alterations often defining entities. However, progress in identifying the molecular pathogenesis of lymphoid malignancies has in most instances followed an accurate description of the disease by pathologists, based on morphologic, immunophenotypic and clinical parameters (Table 1). This paradigm is exemplified by both mantle cell lymphoma (MCL)3,4 and anaplastic large cell lymphoma (ALCL),5 in which careful pathologic descriptions preceded the recognition of CCND1 and ALK, respectively, as genes critical to their pathogenesis. However, the process is iterative, and once the underlying molecular cause is known, we gain access to new diagnostic tools that help better define the borderlines of the disease (Figure 1). Thus, the use of monoclonal antibodies reactive in routine sections to cyclin D1 and the ALK tyrosine kinase helped define the morphologic spectrum of MCL and ALCL. This partnership between the pathologist and the molecular biologist is crucial in the classification of disease, and should be equally applicable to other organ systems.
Table 1.
Pathogenetic insights based on a disease-oriented approach to lymphoma classification
| Lymphomas associated with infectious agents | |
| Nasal, cutaneous and systemic NK/T-cell lymphomas | EBV |
| Adult T-cell leukemia/lymphoma | HTLV1 |
| Marginal zone lymphomas | H pylori, B burgdorferi, C jejuni, Hepatitis C, and others |
| Primary effusion lymphoma, LBCL associated with multicentric CD | HHV-8/ KSHV |
| Plasmablastic, Burkitt, DLBCL, CHL | EBV (subset of cases) |
| Lymphomas with deregulation of apoptosis and survival pathways | |
| Follicular lymphoma | BCL2/IGH@ |
| MALT lymphomas | API2/MALT1 and variants |
| Lymphomas with deregulation of the cell cycle | |
| Mantle cell lymphoma | CCND1/IGH@ |
| Burkitt's lymphoma | MYC/IGH@ and variants |
| Lymphomas with deregulation of cell signaling or transcriptional regulation | |
| Anaplastic large cell lymphoma | NPM/ALK and variants |
| Diffuse large B-cell lymphomas | BCL6, NFκB, Stat6 |
| Lymphomas associated with host susceptibility factors, congenital or acquired | |
| Enteropathy-associated T-cell lymphoma | Genetics, gliadin allergy |
| Extranodal and systemic EBV + T/NK cell lymphomas | Genetics, host response to EBV |
| Hepatosplenic T-cell lymphoma | Immunosuppression combined with chronic antigenic stimulation |
| Lymphomatoid granulomatosis | Partial immune dysfunction and EBV |
| Burkitt lymphoma | Polyclonal B-cell activation with or without immunosuppression (malaria, HIV) |
| Posttransplantation and other iatrogenic lymphoproliferative disorders | Iatrogenic immunosuppression |
LBCL indicates large B-cell lymphoma.
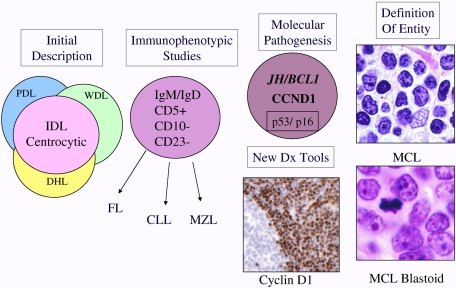
Figure 1.
Schematic diagram illustrating evolution of the entity MCL. MCL was recognized in Kiel classification and modified Rappaport classification as centrocytic lymphoma and lymphocytic lymphoma of intermediate differentiation (IDL), respectively.6 Precise criteria for the distinction from other morphologically similar lymphomas were lacking. The recognition of a characteristic immunophenotype (CD5+, CD23−, CD10−, monoclonal B cell) helped better define the entity. The identification of the t(11;14) resulting in CCNDI/IGH translocation in virtually all cases of MCL led to the use of cyclin D1 detection by immunohistochemistry for diagnosis. In addition, secondary genetic events such as p53 and p16 deletion/mutation were identified in high-grade variants of MCL, which had been recognized histologically as the “blastoid” subtype. The data derived from immunophenotypic and genetic studies are integrated, culminating in our current definition of the disease. PDL indicates poorly differentiated lymphocytic; WDL, well-differentiated lymphocytic; DHL, diffuse histiocytic lymphoma; and MZL, marginal zone lymphoma.
Historical background: the early years
The first description of what we now recognize as a lymphoma is generally attributed to Thomas Hodgkin in 1832 (Table 2).46 The first use of the term lymphosarcoma is attributed to Rudolf Virchow in 1863 (reviewed in Trumper et al8). In 1898 and 1902, Carl Sternberg and Dorothy Reed independently described the characteristic binucleate and multinucleate giant cells that came to be called the Reed-Sternberg or Sternberg-Reed cell.7 Sternberg felt that Hodgkin disease was an inflammatory process, related to tuberculosis, but Reed disagreed.47 She made several other key observations: the general health of the patient before the onset of the disease is usually excellent; there was an early peak among children and young adults; and Hodgkin disease usually presents as painless progressive cervical adenopathy without leukemia. She proposed that the pathologic picture is sufficient for diagnosis. Having considerable success as a fellow in pathology, she sought a teaching post at Johns Hopkins Medical School. Her chairman, William Welch, answered, “No women had ever held a teaching position in the school, and there would be great opposition to it.”47 In her diary she noted, “Suddenly, as I saw what I had to face in acceptance of injustice—I knew that I couldn't take it.”47 She left pathology and took up a career in public health.
Table 2.
Milestones in the evolution of the classification of lymphoid neoplasms
| Year | Reference | Principal contributors | Event |
|---|---|---|---|
| 1806 | * | J. Alibert | Clinical description of mycosis fungoides |
| 1828 | * | R. Carswell | “Cancer cerebriformis of the lymphatic glands and spleen”; the first case of what was later recognized as “Hodgkin's disease” |
| 1832 | 46 | T. Hodgkin | “On some morbid appearances of the absorbent glands and spleen”; clinical report of what would come to be known as Hodgkin disease |
| 1865 | * | S. Wilks | Proposes the eponym “Hodgkin's disease” |
| 1845, 1863 | * | R. Virchow | Describes both leukemia and lymphosarcoma |
| 1898, 1902 | * | C. Sternberg, D. Reed | Define the microscopic features of the neoplastic cell of Hodgkin disease, establishing an accurate micropscopic description of the disease, the first lymphoma to be defined histologically |
| 1914 | * | J. Ewing | Describe “reticulosarcomas” (reticulum cell sarcomas) of bone and lymphoid organs |
| 1928 | * | C. Oberling | Describe “reticulosarcomas” (reticulum cell sarcomas) of bone and lymphoid organs |
| 1930 | * | F. Roulet | Describe “reticulosarcomas” (reticulum cell sarcomas) of bone and lymphoid organs |
| 1916 | * | C. Sternberg | Describes “leukosarkomatose,” a process with characteristic features of precursor T-lymphoblastic lymphoma |
| 1925 | 9–11 | N. Brill | Describe “giant follicle hyperplasia” and “follicular lymphadenopathy”—processes with features of follicular lymphoma and florid follicular hyperplasia |
| 1927 | D. Symmers | ||
| 1941, 1942 | 12,13 | E. Gall, T. Mallory | Accurate description of follicular lymphoma, and propose the first modern classification system of lymphoma |
| 1947 | * | H. Jackson, F. Parker | Propose a classification of Hodgkin disease |
| 1958 | 14 | D. Burkitt | Describes clinical syndrome of Burkitt lymphoma in African children |
| 1960 | 15 | P. Nowell | Phytohemaggutinin used to “transform” lymphocytes in vitro |
| 1961 | 16 | G. O'Conor | Provides histopathologic description of Burkitt lymphoma |
| 1964 | 17 | M. A. Epstein | Description of viral particles, the Epstein-Barr virus, in cultured cells from Burkitt lymphoma |
| 1956, 1966 | 18,19 | H. Rappaport | Proposes an alternative classification for “non-Hodgkin's” lymphoma |
| 1966 | 20 | R. Lukes, J. Butler | Propose the modern classification of Hodgkin lymphoma |
| 1972 | 21 | H. Stein | Identifies high levels of IgM in “histiocytic” lymphomas |
| 1973 | K. Lennert | Lennert and colleagues (R. Gérard-Marchant, I. Hamlin, K.L., F. Rilke, A. G. Stansfeld, and J. A. M. van Unnik) meet to form the European Lymphoma Club, the predecessor of the European Association for Haematopathology | |
| 1974 | 22,60,63 | K. Lennert | Proposes the Kiel classification of lymphomas |
| 1974 | 23 | C. Taylor | Immunohistochemical detection of immunoglobulin within cells within FFPE sections |
| D. Mason | |||
| 1974 | 24 | E. Jaffe | Identification of complement receptors on cells of “nodular lymphoma” linking them to the lymphoid follicle |
| 1975 | 25 | Failed consensus meeting of proponents of lymphoma classification systems; Lennert (Kiel), Lukes and Collins, Dorfman, Bennett (BNLI); Mathe (WHO), and Rappaport, leading to Working Formulation study by NCI | |
| 1975 | 26 | E. Southern | Development of Southern blot technique to separate and analyze DNA fragments |
| 1976 | 27 | G. Klein | Identification of t(8;14)(q24;q32) as a recurrent translocation in Burkitt lymphoma |
| 1979 | 28 | S. Fukuhara, J. Rowley | Identification of t(14;18)(q32;q21) as a recurrent translocation in “lymphocytic lymphoma” (follicular lymphoma) |
| 1979 | 29 | A. McMichael | First monoclonal antibody to a human leukocyte differentiation antigen, later defined as CD1a |
| 1980-1982 | 30–33 | H. Stein, S. Poppema, R. Warnke, D. Mason | Characterization of lymphoid cells by immunohistochemistry on frozen and paraffin sections |
| 1982 | 34 | A. Bernard, L. Boumsell | First international workshop on Human Leucocyte Differentiation Antigens (HLDA) |
| 1982 | 35,36 | P. Leder, R. Dalla-Favera, C. Croce | Cloning of MYC gene, and idenfication of MYC and IGH@ as reciprocal partners in t(8;14)(q24;q32) |
| 1982 | 37 | J. Yunis | Recurrent translocations identified in follicular lymphoma, Burkitt lymphoma, and chronic lymphocytic leukemia |
| 1982 | 25 | C. Berard, R. Dorfman, V. DeVitaS. Rosenberg | Publication of “Non-Hodgkin's lymphoma pathologic classification project. National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a Working Formulation for clinical usage” |
| 1985 | 38 | K. Mullis | Development of the polymerase chain reaction (PCR) technique for amplification of specific DNA sequences |
| 1986 | 39 | T. Cremer | Development of in situ hybridization techniques for analysis of chromosome aberrations in interphase nuclei |
| 1991-1992 | 40 | P. Isaacson, H. Stein | Founding of the International Lymphoma Study Group (ILSG) and publication of consensus report on mantle cell lymphoma |
| 1994 | 41 | R. Kuppers, K. Rajewsky | Identification of IgH gene rearrangements in Reed-Sternberg cells picked from tissue sections of classical Hodgkin lymphoma |
| 1994 | 42 | N. Harris, ILSG | Publication of the Revised European-American Classification of Lymphoid Neoplasms (REAL) |
| 1997 | 43 | J. Armitage and colleagues | Validation of the REAL classification by the International Lymphoma Classification Project study |
| 2000 | 44 | L. Staudt and colleagues | Application of gene expression profiling to human lymphomas |
| 2001 | 45 | E.A.H.P., S.H. | Publication of WHO Monograph: Pathology and Genetics: Tumours of Hematopoietic and Lymphoid Tissues (3rd edition) |
| 2008 | 1 | E.A.H.P., S.H. | WHO classification of Tumours of Haematopoietic and Lymphoid Tissues (4th Edition) |
Reviewed in reference 8.
The term “reticulum cell sarcoma” is attributed to Ewing, Oberling, and Roulet, who each described tumors of large cells, thought to be related to the supporting fibrous reticulum of lymphoid tissues.8 While the origin and function of these cells were unknown, the term reticulum cell sarcoma came to be used for “large cell” neoplasms—as distinguished from “lymphosarcoma,” which was applied to those composed of smaller cells more recognizable as lymphocytes.
In 1925, Brill and colleagues described patients with enlargement of lymph nodes and spleen, characterized pathologically by a proliferation of lymphoid follicles9; additional cases were reported by Symmers,10 who also described progression to a large-cell neoplasm.11 In 1941, Edward A. Gall and Tracy B. Mallory, pathologists at Massachusetts General Hospital, proposed a morphologic classification, based on review of their own collection of 618 patients for whom clinical data were available.12 They recognized follicular lymphoma (FL) as a distinctive morphologic and clinical entity, and described instances of histologic progression over time.13 This was the first widely used classification of lymphomas in the United States; this classification included Hodgkin disease as a separate type of lymphoma, but further subdivisions of Hodgkin disease were proposed by Jackson and Parker.48
Beginning of the modern era
The Rappaport classification was initially published in 1956 in a report primarily focused on FL,18 and fully developed in the Armed Forces Institute of Pathology (AFIP) fascicle of 1966. Rappaport questioned the concept of the time that FL could arise from reactive lymphoid follicles, and to avoid this confusion, he proposed the term “nodular” to replace “follicular” when describing the pattern of the lymphoma. He further observed that most cytologic types of lymphoma could have either a nodular or diffuse pattern, including Hodgkin lymphoma. Thus, the Rappaport classification took as its primary stratification the pattern of the lymphoma—nodular or diffuse.19 The previously well-defined “FL” of Gall and Mallory was lost amid 4 categories of “nodular lymphoma”: well differentiated lymphocytic, poorly differentiated lymphocytic, mixed lymphocytic and histiocytic, and histiocytic. Within each tumor type, those with a nodular pattern in general had a better prognosis than those with a diffuse pattern. Despite the fact that by 1966, the phenomenon of lymphocyte transformation had been recognized,15 the Rappaport classification continued to regard neoplasms of large cells as derived from nonlymphoid stromal or other cell types. Thus, the term “histiocytic” replaced the terms “reticulum cell or clasmatocyte,” and “undifferentiated” replaced the “stem cell” of Gall and Mallory. This classification was widely accepted in the United States, where the high frequency of FL meant that simply recognition of a nodular pattern was highly clinically useful in predicting prognosis.
In the same era, in 1966, Lukes and Butler published a new classification of Hodgkin disease, recognizing the new categories of nodular sclerosis and mixed cellularity types, formerly lumped together as “Hodgkin granuloma.”20 They also defined what came to be known as nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), describing 2 forms rich in normal lymphocytes and histiocytes that could have either a nodular or predominantly diffuse growth pattern. This subtype contained a variant of the classical Reed-Sternberg cell, which they termed the L&H cell, often informally referred to as a “popcorn cell.” The subsequent Rye conference reduced the 6 subtypes of Lukes and Butler to 4, combining the nodular and diffuse forms of lymphocytic and histiocytic into lymphocytic predominance (LP), and the reticular and diffuse fibrosis forms of Hodgkin disease into lymphocyte depleted.49 The lymphocyte depleted group was the most problematic of the Lukes-Butler scheme, and probably included cases of classical Hodgkin lymphoma as well as pleomorphic B-cell and T-cell lymphomas.50 Nevertheless, the subsequent 50 years have seen remarkably few changes in the Lukes-Butler classification of Hodgkin lymphoma, with most of its basic principles affirmed by subsequent biological studies.51,52
In the 1960s, 2 discoveries revolutionized the understanding of the immune system and its neoplasms. These were (1) the potential of lymphocytes—which had been thought to be end-stage, terminally differentiated cells—to transform into large, proliferating cells in response to mitogens or antigens;15 and (2) the existence of several distinct lymphocyte lineages (T, B, and NK) that could not be reliably predicted by morphology, but that had different functions and physiology (Figures 2, 3).53,54 In the early 1970s, lymphoid cells were found to have surface antigens or receptors that could be exploited to identify the lineage of both normal and neoplastic cells.55,56 These observations led to the recognition that lymphomas were tumors of the immune system. One of the first lymphomas to be subjected to this analysis was Rappaport's nodular lymphoma, which was shown to be of follicular B-cell origin using these new techniques.24 Similarly, tissue lysates created from biopsy specimens of “reticulum cell sarcomas” showed high levels of IgM, providing evidence for a B-cell derivation.21 The “E rosette” phenomenon used sheep erythrocytes to identify the as-yet-undiscovered CD2 antigen on T cells; this method identified the cells of Sezary syndrome and most lymphoblastic lymphomas to be of T-cell derivation.57,58
Figure 2.
Diagrammatic representation of B-cell differentiation and relationship to major B-cell neoplasms. B-cell neoplasms correspond to stages of B-cell maturation, even though the precise cell counterparts are not known in all instances. Precursor B cells that mature in the bone marrow may undergo apoptosis or develop into mature naive B cells that, following exposure to antigen and blast transformation, may develop into short-lived plasma cells or enter the germinal center (GC), where somatic hypermutation and heavy chain class-switching occur. Centroblasts, the transformed cells of the GC, either undergo apoptosis or develop into centrocytes. Post-GC cells include both long-lived plasma cells and memory/marginal zone B cells. Most B cells are activated within the GC, but T cell–independent activation can take place outside of the GC and also probably leads to memory-type B cells. Monocytoid B cells, many of which lack somatic hypermutation, are not illustrated. AG indicates antigen; and FDC, folllicular dendritic cell. Red bar represents immunoglobulin heavy chain gene (IGH@) rearrangement; blue bar, immunoglobulin light chain gene (IGL) rearrangement; and black insertions in the red and blue bars indicate somatic hypermutation.
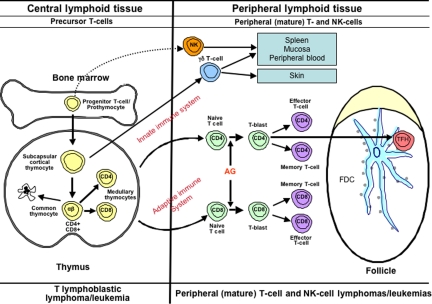
Figure 3.
Diagrammatic representation of T-cell differentiation and function. Lymphoid progenitors enter the thymus where precursor T cells develop into varied types of naive T cells. The cells of the innate immune system include NK cells, γδ T cells, and NK-like T cells. These cells constitute a primitive type of immune response that lacks both specificity and memory. In the adaptive immune system, αβ T cells leave the thymus, where, upon exposure to antigen, they may undergo blast transformation and develop further into CD4+ and CD8+ effector and memory T cells. T cells of the adaptive immune system are heterogeneous and functionally complex, and include naive, effector (regulatory and cytotoxic), and memory T cells. Another specific type of effector T cells is the follicular helper T-cell that is found in GCs (TFH). Upon antigenic stimulation, T-cell responses may occur independent of the GC, or in the context of a GC reaction. The lymphomas of the innate immune system are predominantly extranodal in presentation, mirroring the distribution of the functional components of this system.113 T-cell lymphomas of the adaptive immune system present primarily in adults, and are mainly nodal in origin.
In response to the new information, pathologists appropriately began to apply it to the classification of lymphomas. In the 1970s, several European and American groups published proposals for the classification of lymphomas.59–62 The first and most significant of these efforts was led by Karl Lennert in Kiel, Germany, who first recognized that many lymphomas resembled germinal center cells, and had used ultrastructural studies to identify follicular dendritic cells in normal and neoplastic lymph nodes, linking lymphomas with a nodular growth pattern to the lymphoid follicle.63 The Kiel classification was published in final form in 1974, fully described in a monograph in 1978,64 and later updated.65 Lymphomas were classified according to a hypothetical scheme of lymphocyte differentiation, and the nomenclature reflected the putative normal counterpart of the neoplastic cells. Although the majority of the neoplasms described were B-cell, several well-defined types of T-cell lymphomas were included. The neoplasms were grouped according to histologic features into low-grade malignancy (predominance of small cells or “-cytes”) and high-grade malignancy (predominance of “-blasts”). This classification became widely used in Europe, but never supplanted the Rappaport classification, popular among clinicians, in the U.S.
During the same era, clinicians were advancing their knowledge regarding the clinical behavior of lymphomas and patterns of spread through advances in the treatment and staging of lymphoid neoplasms.66,67 As treatment regimens improved, the heterogeneity among different types of lymphomas became more apparent.68,69 However, the use of different classification systems in clinical studies made it difficult to compare published results from different centers. Several meetings were held to try to break the deadlock, the last of which was held at Airlie House in Warrenton, Virginia, in 1975.8 The inability of the pathologists to develop consensus and agree on a common approach led to a National Cancer Institute–directed study to evaluate the 6 published schemes. None of the schemes proved clearly superior in predicting survival, and since the pathologists would not agree to using one scheme over the others, the Working Formulation (WF) for the non-Hodgkin lymphomas (NHL) was developed to translate among them.25
The WF stratified lymphomas according to clinical outcome, based on the survival of patients in clinical trials conducted in the 1970s.25 It co-opted terminology from the pathology literature related to cytologic grade (low grade, intermediate grade, and high grade) and created clinical groupings that were intended to provide a clinical guide for patient management. The categories closely followed those of the Rappaport classification,19 with updated terminology from Lukes and Collins.59 Since the scheme did not use immunophenotyping, which was felt at the time to be beyond the reach of the routine pathology laboratory, and since it attempted to cover all entities in all classifications in a few categories, the categories were of necessity heterogeneous. It is therefore not surprising that pathologists could not use these categories reproducibly.70 The WF substituted the term “large cell” for “histiocytic” and divided large cell lymphomas into 2 types: large cell and large cell immunoblastic. This separation split the diffuse large-cell lymphomas into 2 different treatment groups (intermediate and high grade), based on questionable morphologic distinctions.71 These distinctions were not reproducible, and the clinical groupings could not be validated. In many respects the WF was a step backward, since it did not recognize well-defined entities such as centrocytic lymphoma from the Kiel classification, which is now known as mantle cell lymphoma (MCL), and made no use of widely available immunologic knowledge techniques for classification or diagnosis—for example, lumping both T-cell lymphomas and many diffuse B-cell lymphomas in a single category (diffuse mixed small and large cell).
Although not intended to serve as a freestanding classification scheme, the WF was a convenient guide to therapy, quickly became popular among clinicians, and was adopted for use in many centers in the U.S. for clinical trials. Following the publication of the WF, the political situation was unchanged. The Kiel classification remained popular in Europe and in Asia; the WF largely replaced the Rappaport classification in the U.S., but many centers used the Lukes-Collins classification. This situation caused continued problems for both pathologists and clinicians in interpreting the results of published studies. In addition, in the 1980s and early 1990s, many new diseases were described that were not included in either classification, including anaplastic large cell lymphoma (ALCL), lymphomas of the mucosa-associated lymphoid tissues (MALT), and adult T-cell leukemia/lymphoma.
The immunologic and genetic revolutions
In 1975, Kohler and Milstein created the hybridoma technology that led to the development of monoclonal antibodies.72 Before this revolution, cell-surface marker and immunohistochemistry studies had been performed with rabbit or sheep polyclonal antisera, but these reagents were hampered by limited supply, lack of standardization, and the relatively few antigens that were recognized. In 1979, the first monoclonal antibody against a human lymphocyte differentiation antigen was generated against an antigen expressed on normal thymocytes.29 The antigen recognized by NA1/34 was later designated as CD1a in the human leukocyte differentiation antigen (HLDA) system.34 The CD nomenclature, which stood for “clusters of differentiation,” came into use through a series of workshops organized to bring order out of chaos in this newly emerging field. The initial workshops dealt only with antigens expressed on the cell membrane, all of which were given a CD designation, but later workshops began to look at antigens expressed in the nucleus and cytoplasm.73 By the most recent eighth meeting of what is now called Human Cell Differentiation Molecules workshop, a total of 350 CD designations had been defined.
In these early years, monoclonal antibodies were applied to cells in suspension, or somewhat later to cryostat tissue sections. However, a paradigm shift occurred when David Mason and others not only adapted the techniques to routine formalin-fixed paraffin embedded (FFPE) sections, but changed the strategy for screening monoclonals to look for those antibodies that would have optimal reactivity in FFPE sections.23,30,74 These advances had a dramatic impact on the field. First, one did not have to ensure that tissues were snap-frozen or made into cell suspension to perform immunophenotypic studies. Second, the better morphology of paraffin sections improved the pathologist's ability to characterize positive and negative cells. This technology was rapidly adopted by pathologists both at academic centers and in community hospitals. Characteristic immunophenotypes for many B-cell and T-cell malignancies were becoming recognized, and the ready accessibility of monoclonal antibodies adapted for use in immunohistochemistry in routine paraffin sections made new discoveries easily transportable and testable in different laboratories.
In parallel, there was equally dramatic progress in understanding the genetics of lymphoid malignancies. Recurrent cytogenetic abnormalities were identified, following the early observations of recurring cytogenetic alterations in myeloid leukemias.75 The first to be recognized were the t(14;18)(q32;q21) of FL, and t(8;14)(q24;q32) of Burkitt lymphoma.27,28,37 Subsequent studies led to the cloning of the genes involved in these translocations. The laboratories of Philip Leder and Carlo Croce in 1982 both identified MYC as the gene that was translocated into the immunoglobulin genes in human Burkitt lymphoma,35,36and other similar discoveries soon followed, such as the BCL2/IGH@ in FL76 and the CCND1/IGH@ in MCL.77,78 The most common paradigm for translocations involving the immunoglobulin heavy chain gene, IGH@ at 14q24, is that a cellular proto-oncogene comes under the influence of the IGH@ promoter. There are also less frequent but parallel alterations involving the T-cell receptor genes in T-cell malignancies.
The process of rearrangement of the immunoglobulin and T-cell receptor genes during normal lymphoid cell development was discovered, and the technology exploited by using rearrangement of the antigen receptor genes as markers of both lineage and clonality in lymphoid neoplasms.79 It was later shown that B cells that have transited the germinal center show evidence of somatic hypermutation of the IGH@ variable region genes.80 Thus, this knowledge could be exploited, not only to show lineage, but stage of differentiation, at least within the B-cell system.81,82
In addition, the development of polymerase chain reaction (PCR)– and fluorescence in situ hybridization (FISH)–based strategies meant that genetic testing no longer required karyotyping of viable cells or labor-intensive Southern blot analysis of fresh or snap-frozen material. The new techniques permitted analysis of antigen receptor gene and oncogene rearrangements on FFPE sections, facilitating the testing of routine biopsies for clinical diagnosis.83–86 Another critical advantage of the PCR-based strategies was the ability to investigate genetic alterations at the single-cell level. This was particularly important in the study of Hodgkin lymphoma, in which the neoplastic cells represented only a minor component of the entire tumor mass. PCR techniques coupled with single-cell microdissection led to the demonstration of IGH@ gene rearrangements in Reed-Sternberg cells, ending the long debate regarding the origin of the malignant cell.41,87
While translocations involving oncogenes or tumor suppressor genes were demonstrated to be of major importance in the pathogenesis of many lymphomas, integration of viral genomes into the neoplastic cells was demonstrated for several lymphoid malignancies. Epstein-Barr virus (EBV) was shown to be especially promiscuous and capable of transformation of B cells, T cells, and NK cells.17,88–90 HTLV1 and HHV-8/KSHV were identified as playing a role in the pathogenesis of adult T-cell leukemia/lymphoma,91 primary effusion lymphoma,92 and the lymphoma associated with multicentric Castleman disease.93–95
Identifying Hodgkin disease as a lymphoid neoplasm
From its initial description until the 1990s, the nature and lineage of the Reed-Sternberg cell and the inflammatory infiltrate that comprise Hodgkin disease was debated—was this an infectious or inflammatory process with bizarre cells infected by a virus, or a malignant process with a prominent inflammatory component—and what was the lineage of the infected or malignant cell? Hypotheses and bits of supporting evidence suggested almost every known immune cell type: reticulum cell, dendritic cell, histiocyte/macrophage, B cell, and T cell. The major problem for many years was the relative infrequency of the abnormal cells compared with reactive cells, making it difficult to apply immunophenotyping and genetic techniques to the question.
In the 1970s and 1980s, a number of cell lines were developed from patients with advanced-stage Hodgkin disease, and cytogenetic analysis of both these cells and fresh tumor specimens demonstrated abnormal karyotypes; together with the clinical aggressiveness of the disease, these observations confirmed that the disease was indeed a malignancy.96 In the early 1980s, Stein and colleagues at Kiel immunized mice with Hodgkin cell lines established by Diehl and associates, and developed the Ki-1 antibody, recognizing the CD30 antigen, which was found to be expressed by all Hodgkin and Reed-Sternberg (HRS) cells and by rare lymphoid blasts in normal lymphoid tissues.97 In vitro studies revealed that CD30 expression could be induced in B and T cells by mitogens and by EBV infection, suggesting that CD30+ HRS cells might be related to lymphoid cells.5,98 In the 1980s, the development of antibodies that detected B cell– and T cell–specific antigens in paraffin sections, providing sufficient morphologic detail to recognize HRS cells, showed expression of the B-cell antigen CD20 on some of the HRS cells in a proportion of patients with classical Hodgkin lymphoma (CHL).99,100 The detection of EBV genomes in HRS cells provided additional support for a lymphoid and possibly B-cell origin.101,102 Further evidence pointing toward a B-cell derivation came from the observation of composite lymphomas and metachronous CHL and NHLs, in which the NHL component was nearly always of B-cell origin, most commonly FL.103 Finally, in the 1990s, the PCR technique and primers specific for target sequences of the IGH@ and T-cell receptor (TCR) loci became available, and a method of isolating single HRS cells from frozen sections by microdissection was developed.80 The application of these techniques revealed that HRS cells were clonal B cells in 98% of the patients studied, thus confirming that they were neoplastic and of B-cell origin.41,82 This latter finding led to the change of the designation of Hodgkin disease into Hodgkin lymphoma in the WHO classification of lymphoid neoplasms.
With the recognition that nearly all instances of CHL show evidence of B-cell lineage, the following question arose: why do HRS cells express so few B-cell antigens, lack expression of immunoglobulin, and display markers not normally ex-pressed by B cells, such as CD15, TARC, or T cell–associated antigens?97,104–107 A number of explanations have been suggested, including the presence of crippling mutations in the immunoglobulin variable region genes,108 methylation of the immunoglobulin promotors,109 defects in B cell–associated transcription factors,110 and up-regulation of genes that suppress the B-cell gene expression program.111,112 The extinction of the B-cell gene expression program in HRS cells is a complex process, and its full elucidation will likely contribute to our understanding of the pathogenesis of CHL.
The REAL and WHO classifications
In 1991, an international group of pathologists (International Lymphoma Study Group [ILSG]) was formed by Peter Isaacson and Harald Stein to promote better understanding between European and American hematopathologists. At the first meeting held in London, the participants discussed a variety of topics, and found common ground on most issues. Shortly thereafter the group published a consensus report on the definition of MCL, and proposed that the term MCL be used to the exclusion of centrocytic, intermediate, or diffuse small cleaved cell lymphoma, which were overlapping but not precisely defined categories in the Kiel, modified Rappaport, and WF classifications, respectively.40 The ILSG defined MCL by a combination of morphology, immunophenotype, and genetic anomalies, and noted that it had a characteristic clinical profile. A similar report followed at the next meeting in 1992, defining nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) and elucidating criteria to distinguish it from CHL.51
At this meeting, the group felt it should try to define as many of the currently recognized entities as possible. At the third meeting, in Berlin in 1993, the group was able to reach consensus on a long list of lymphoid neoplasms, which formed the basis of a new classification of lymphoid neoplasms called the Revised European-American Classification of Lymphoid Neoplasms (REAL classification). The REAL classification, published in Blood in 1994,42 was among the 5 most highly cited papers in clinical medicine for the decade. The REAL classification, and its successor the WHO classification,45 represented a new paradigm in the classification of lymphoid neoplasms. The focus was on the identification of “real” diseases, rather than a global theoretical framework, such as survival (WF) or cellular differentiation (Kiel and Lukes-Collins classifications). The REAL classification was based on the building of consensus, and recognized that a comprehensive classification system was beyond the experience of any one individual. The 19 members of the ILSG contributed their diverse perspectives to achieve a unified point of view. In addition, the ILSG made the decision to base the classification exclusively on published data; in order for an entity to be included in the REAL classification, it had to be validated in more than one publication. A number of entities were listed as provisional, based on more limited published data. Recognizing that Hodgkin lymphoma and plasma cell myeloma are both lymphoid in origin, and that many lymphoid neoplasms can have both solid and circulating phases, the classification included all lymphoid neoplasms: Hodgkin lymphoma, so-called NHLs, lymphoid leukemias, and plasma cell disorders. The neoplasms were stratified according to lineage (B-cell and T/NK-cell), and further into precursor (lymphoblastic) versus mature/peripheral T- and B-cell neoplasms, and Hodgkin lymphoma.
The REAL classification defined distinct entities using a constellation of features: morphology, immunophenotype, genetic features, and clinical presentation and course. The REAL classification appreciated the importance of genetic abnormalities in defining disease entities. With advances in monoclonal antibody technology, some genetic abnormalities could be recognized by immunohistochemistry, such as cyclin D1 expression in the diagnosis of MCL, and ALK expression in ALCL. However, for many lymphoma subtypes, particularly the mature T-cell malignancies, the molecular pathogenesis was not yet known. The inclusion of clinical criteria, which played a major role in the classification of T-cell lymphomas, was one of the most novel aspects of the ILSG approach.113 The REAL classification recognized that the site of presentation is often a signpost for underlying biological distinctions, as in extranodal lymphomas of MALT,114 or primary mediastinal large B-cell lymphoma. The ILSG appreciated that accurate diagnosis cannot take place in a vacuum and requires knowledge of the clinical history since biologically distinct entities may appear cytologically similar.
The REAL classification recognized that because of limitations in current knowledge, some broad categories of disease could not be resolved with existing data, such as diffuse large B-cell lymphomas (DLBCLs) and peripheral T-cell lymphomas (PTCLs), unspecified or not otherwise specified (NOS). Although a number of morphologic variants had been described in both groups, evidence that these delineated distinct biological or clinical entities was lacking. The ILSG identified these categories as ripe for further studies. Indeed, as expected, new technologies have helped delineate separate biological entities within DLBCL and to a lesser extent PTCL-NOS. Gene expression profiling dissected 2 major categories from DLBCL, the germinal center B-cell (GCB) and activated B-cell type (ABC) types of DLBCL, with different patterns of transformation and deregulation.44 The relevance of the microenvironment was appreciated, and the various gene signatures could be correlated with clinical outcome and response to therapy.115,116 Gene expression profiling also validated the distinction of primary mediastinal large B-cell lymphoma from other DLBCL, which was a feature of the REAL classification.117,118
The REAL classification did not attempt to stratify lymphomas according to a concept of “grade,” either histologic or clinical, as had been done in the Kiel classification and the WF. We now recognize that lymphomas are a heterogeneous group of distinct diseases, most unrelated to one another, not a single disease with a spectrum of histologic grade and clinical behavior. In addition, many lymphomas have within them a spectrum of clinical behavior, which sometimes can be predicted by pathologic features (cell size, proliferation) or clinical features (age, stage, the International Prognostic Index [IPI]). Thus, the ILSG felt that it was neither practical nor helpful to attempt to sort these diverse diseases according to either histologic features or clinical aggressiveness. Both clinicians and pathologists need to learn the histologic and clinical spectrum of each distinct disease. In the REAL classification, grading was used within a disease entity, such as FL, but not across a range of different diseases. Moreover, low cytological grade does not necessarily translate into indolent clinical behavior. Both MCL and angioimmunoblastic T-cell lymphoma had been designated as cytologically low grade, but both diseases are associated with an aggressive clinical course. Conversely, ALCL, which cytologically is high grade, has a better prognosis than most other nodal T-cell lymphomas, with an excellent response to chemotherapy and prolonged disease-free survival.43
Following the publication of the REAL classification, an international study directed by James Armitage sought to determine whether the REAL classification could be readily applied by a group of independent expert pathologists and had clinical relevance.43 Other goals of the International Lymphoma Classification Project were (1) to determine the role of immunophenotyping and clinical data in the diagnosis of disease entities; (2) to determine both intraobserver and interobserver reproducibility in the diagnosis of the various entities; (3) to further investigate the clinical features and/or epidemiology of the various entities; and (4) to determine whether clinical groupings would be practical or useful for clinical trial or practice. The conclusions of that study affirmed the principles of the REAL classification.43 Virtually all cases could be classified in the published scheme. Intraobserver and interobserver rates of reproducibility were excellent. The use of precise disease definitions, as provided by the REAL scheme, enhanced diagnostic accuracy and reduced subjectivity on the part of pathologists. Immunophenotyping was found to be essential for many diagnostic categories, such as most peripheral T-cell lymphomas. Importantly, the significance of identifying individual disease entities was confirmed by survival data. The International Classification Lymphoma Classification project also elucidated the importance of clinical factors such as the IPI in determining clinical outcome and response to therapy within disease entities, confirming the concept that stratification of a diverse group of diseases by either cytologic or clinical “grade” was unrealistic.119
Shortly after publication of the REAL classification, the WHO decided to update the classification of hematopoietic and lymphoid neoplasms for its “Blue Book” series of Classifications of Tumors. The task for developing the classification was undertaken as a joint project by the Society for Hematopathology (SH) and the European Association of Hematopathology (EAHP), with a goal to achieve consensus and avoid the political divisions of the past. A steering committee was appointed by the 2 societies, which in turn established 10 individual committees to deal with different disease groups within hematopoietic and lymphoid malignancies.120 The WHO classification was developed over a period of 7 years, during which time the proposal was extensively discussed and vetted at a number of international meetings and symposia to ensure worldwide acceptance. Approximately 100 pathologists and clinicians gathered at a clinical advisory meeting in 1997 at Airlie House, where an agreement had so spectacularly failed in 1975, to discuss a number of questions of clinical relevance. A consensus was achieved on most of the questions raised.121 Importantly, the group concluded that clinical groupings for either protocol treatment or routine clinical practice were generally not feasible. In considering new therapies, each disease must be evaluated individually. Indeed, treatment approaches for one type of lymphoid malignancy are not necessarily applicable to other diseases, even of the same cell lineage. This point is exemplified by the broad spectrum of lymphoid malignancies composed of small B lymphocytes (ie, chronic lymphocytic leukemia (CLL)/small lymphocytic leukemia (SLL), hairy cell leukemia, MALT lymphoma, FL, and MCL). Each of these tumors exhibits deregulation of distinct signaling or transformation pathways, and thus there should be no expectation that the optimal therapy for all small B-cell neoplasms should be the same.
While work on the WHO classification was in progress, Paul Kleihues became Director of the International Agency for Research on Cancer (IARC), an agency of the WHO. Recognizing the growing role of genetics in the definition of cancers and the need for international consensus-based classifications, he initiated a new series of monographs focusing on the pathology and genetics of cancer, published by IARC Press.122 The approach to disease definition and consensus development that was introduced in the REAL classification and adopted in the WHO classification of hematologic and lymphoid neoplasms was a paradigm for this approach. The publication of the WHO classification in 2001 marked the beginning of a new era in which all scientists and clinicians as well as pathologists involved in lymphoma research, diagnosis, and patient care would finally speak the same language.
Discovering diseases: MALT lymphoma as a paradigm
Each lymphoma included in the WHO classification is a distinct disease entity. Defining a disease is a multistep process, but typically begins not in the basic science laboratory, but at the bedside or at the microscope. This process of disease discovery is exemplified by MALT lymphoma, but the same tale could be told for any number of diseases, such as adult T-cell leukemia/lymphoma,91,123 MCL,3,6,56,124, or ALCL.5,125,126
In 1963, Frand and Ramot drew attention to the high frequency of a unique variety of intestinal lymphoma in non-Ashkenazi Jews and Arabs in Israel.127 The histology of this condition, subsequently called Mediterranean lymphoma, was characterized as ranging from intense lymphoplasmacytic infiltration of the intestinal mucosa to frank large-cell lymphoma. Seligmann et al observed that patients with Mediterranean lymphoma had circulating alpha heavy chain with absent light chain and introduced the term “alpha chain disease (ACD).128 There were doubts whether ACD represented true lymphoma, particularly since some patients could be cured with antibiotics and, accordingly at a WHO conference in 1976, ACD was renamed yet again as an immunoproliferative small intestinal disease (IPSID). In the late 1970s and early 1980s, based on careful morphologic analysis and demonstration that ACD was monoclonal, Isaacson et al showed that IPSID was indeed a low-grade lymphoma.129
In the period between 1960 and 1980 the term “pseudolymphoma” came increasingly to be used for low-grade lymphoproliferative lesions arising at a variety of extranodal sites, including the stomach, lung, thyroid, and ocular adnexa. In 1983, Isaacson and Wright showed that gastric pseudolymphoma was a monotypic disorder (ie, a lymphoma) remarkably similar to IPSID.130 The properties of both lymphomas simulated those of MALT, giving rise to the term MALT lymphoma. It soon became clear that a number of other extranodal indolent B-cell lymphomas, many of which had previously been called pseudolymphomas, were examples of MALT lymphoma.131,132 Subsequent studies showed that the morphology and immunophenotype of MALT lymphoma recapitulated those of the Peyer patch, more specifically the Peyer patch marginal zone cells.133
MALT lymphoma usually presents as localized disease (stage 1E) but characteristically disseminates, either within the same organ or to other extranodal sites where MALT lymphomas are known to arise.134,135 MALT lymphomas typically arise from sites such as the stomach, where there is no organized lymphoid tissue. In seeking an explanation for this paradox, Isaacson hypothesized that Helicobacter pylori infection may result in accumulation of MALT from which gastric MALT lymphoma arises.136 Serial studies showed that there is indeed a close association of infection with H pylori and gastric MALT lymphoma, and that the growth of gastric MALT lymphoma cells can be stimulated by H pylori strain–specific T cells.137 Moreover, in 75% of patients, antibiotic eradication of H pylori leads to regression and effective cure of gastric MALT lymphoma.138 There is an interesting parallel with earlier observations of complete response of IPSID to antibiotics, where there is some evidence that Campylobacter jejuni may be the organism involved,139 and other bacterial and viral agents have been implicated in the pathogenesis of other forms of marginal zone lymphoma.140,141
Numeric chromosomal alterations, especially trisomies 3, 12, and 18, are common in MALT lymphomas. Chromosomal translocations associated with MALT lymphomas include t(11:18)(q21:q21), resulting in the production of a chimeric protein (API2-MALT1);142 and t(1;14)(p22;q32), t(14;18)(q32;q21), and t(3;14)(p14.1;q32), resulting respectively in transcriptional deregulation of BCL10, MALT1, and FOXP1.143–145 The frequencies at which the translocations occur vary markedly with the primary site of disease.146 This is unlike most other low-grade lymphomas, where a single translocation is characteristic. There is mounting evidence, however, that these different translocations are linked by the roles of BCL10 and MALT1 in the NFκB molecular pathway in lymphocytes. Thus, perhaps all of the genetic alternations appear to lead to deregulation of a common pathway.
Lymphoma classification in the third millennium
The WHO classification has been updated in the past 2 years, again as a joint effort of the hematopathology societies, with 2 clinical advisory committees and more than 130 authors from 22 countries (Table 3). Published in September 2008, it builds upon the advances of the past and makes some inroads into better defining heterogeneous or ambiguous categories of disease.1 Some changes include the introduction of provisional borderline categories, the recognition of small clonal lymphoid populations, and the identification of diseases characterized by involvement of specific anatomic sites or by other clinical features such as age. Unresolved issues include defining pathologic predictors of prognosis in common diseases such as FL, DLBCL, and peripheral T-cell lymphomas.
Table 3.
WHO classification of the mature B-cell, T-cell, and NK-cell neoplasms (2008)
| Mature B-cell neoplasms |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| B-cell prolymphocytic leukemia |
| Splenic marginal zone lymphoma |
| Hairy cell leukemia |
| Splenic lymphoma/leukemia, unclassifiable* |
| Splenic diffuse red pulp small B-cell lymphoma* |
| Hairy cell leukemia-variant* |
| Lymphoplasmacytic lymphoma |
| Waldenström macroglobulinemia |
| Heavy chain diseases |
| Alpha heavy chain disease |
| Gamma heavy chain disease |
| Mu heavy chain disease |
| Plasma cell myeloma |
| Solitary plasmacytoma of bone |
| Extraosseous plasmacytoma |
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue |
| (MALT lymphoma) |
| Nodal marginal zone lymphoma |
| Pediatric nodal marginal zone lymphoma* |
| Follicular lymphoma |
| Pediatric follicular lymphoma* |
| Primary cutaneous follicle center lymphoma |
| Mantle cell lymphoma |
| Diffuse large B-cell lymphoma (DLBCL), NOS |
| T-cell/histiocyte rich large B-cell lymphoma |
| Primary DLBCL of the CNS |
| Primary cutaneous DLBCL, leg type |
| EBV+ DLBCL of the elderly* |
| DLBCL associated with chronic inflammation |
| Lymphomatoid granulomatosis |
| Primary mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma |
| ALK+ large B-cell lymphoma |
| Plasmablastic lymphoma |
| Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease |
| Primary effusion lymphoma |
| Burkitt lymphoma |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse |
| large B-cell lymphoma and classical Hodgkin lymphoma |
| Mature T-cell and NK-cell neoplasms |
| T-cell prolymphocytic leukemia |
| T-cell large granular lymphocytic leukemia |
| Chronic lymphoproliferative disorder of NK cells* |
| Aggressive NK cell leukemia |
| Systemic EBV+ T-cell lymphoproliferative disease of childhood |
| Hydroa vacciniforme-like lymphoma |
| Adult T-cell leukemia/lymphoma |
| Extranodal NK/T-cell lymphoma, nasal type |
| Enteropathy-associated T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Mycosis fungoides |
| Sézary syndrome |
| Primary cutaneous CD30+ T-cell lymphoproliferative disorders |
| Lymphomatoid papulosis |
| Primary cutaneous anaplastic large cell lymphoma |
| Primary cutaneous gamma-delta T-cell lymphoma |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma* |
| Primary cutaneous CD4+ small/medium T-cell lymphoma* |
| Peripheral T-cell lymphoma, NOS |
| Angioimmunoblastic T-cell lymphoma |
| Anaplastic large cell lymphoma, ALK+ |
| Anaplastic large cell lymphoma, ALK−* |
| Hodgkin lymphoma |
| Nodular lymphocyte-predominant Hodgkin lymphoma |
| Classical Hodgkin lymphoma |
| Nodular sclerosis classical Hodgkin lymphoma |
| Lymphocyte-rich classical Hodgkin lymphoma |
| Mixed cellularity classical Hodgkin lymphoma |
| Lymphocyte-depleted classical Hodgkin lymphoma |
| Posttransplantation lymphoproliferative disorders (PTLD) |
| Early lesions |
| Plasmacytic hyperplasia |
| Infectious mononucleosis-like PTLD |
| Polymorphic PTLD |
| Monomorphic PTLD (B- and T/NK-cell types)† |
| Classical Hodgkin lymphoma type PTLD† |
Provisional entities for which the WHO Working Group felt there was insufficient evidence to recognize as distinct diseases at this time.
These lesions are classified according to the leukemia or lymphoma to which they correspond.
In the past 20 years there has been a greater appreciation of morphologic and immunophenotypic overlap between CHL and some large B-cell lymphomas—usually primary mediastinal large B-cell lymphoma (PMBL) and mediastinal nodular sclerosis subtype of classical Hodgkin lymphoma (NSCHL).103,146 In most cases, one or the other diagnosis can be made, but there may be a true biological gray zone between these entities, both of which occur in young adults and involve the mediastinum and have similarities in gene expression profiles.117,118 The 2008 WHO classification recognizes a provisional category of B-cell neoplasms with features intermediate between DLBCL and CHL.147,148 These tumors occur predominantly in young men and appear to be more aggressive than either PMBL or NSCHL; in some patients, PMBL may be followed by CHL or vice versa.
A second borderline category relates to the distinction of classical Burkitt lymphoma from diffuse large B-cell lymphomas that also carry a MYC translocation. Many of these occur in adults and morphologically resemble Burkitt lymphoma.149 The 2008 WHO classification includes a provisional category of B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma. Many lymphomas in this grouping have a translocations of both MYC and BCL2 (“double hit”), and have a very aggressive clinical course.150 While gene expression profiling may show similarities with classical Burkitt lymphoma, other data support segregation of these tumors from Burkitt lymphoma.85,151
The multistep pathway of tumorigenesis has parallels in most organ systems, best documented in the evolution of colonic adenocarcinoma.152 Histologic progression is a well-recognized feature of many lymphoid neoplasms, but the earliest events in lymphoid neoplasia are difficult to recognize. In fact, the lymphoid system has no recognized “benign neoplasms,” a fact that may be related to the propensity of lymphoid cells or to circulate or home, and not remained confined to a single anatomic site.153 The 2008 WHO classification addresses the problem of clonal expansions of B cells, or less often T cells, that appear to have limited potential for histologic or clinical progression. For example, although up to 70% of healthy adults have circulating clonal memory B cells with the t(14;18)(q32;q21), these cells presumably lack other genetic alterations necessary for development of malignant behavior.154,155 A total of 3% to 5% of healthy adults and more than 10% of those referred for evaluation of lymphocytosis have circulating monoclonal B cells with the immunophenotype of CLL (monoclonal B lymphocytosis); many of these clones also have genetic abnormalities associated with good-prognosis CLL, yet they rarely progress to overt, progressive leukemia.156,157
Among lymphomas, in situ or early lesions have been recognized for both FL and MCL.158,159 The risk for progression to clinically significant lymphoma is not yet fully known for these focal lesions, which are often incidental findings in an otherwise reactive-appearing lymph node. A related condition is localized FL presenting as small polyps in the duodenum; these duodenal FLs rarely if ever progress to nodal or systemic disease.160,161 Duodenal FL cells express intestinal homing receptors that may retain the clonal B cells within the intestinal mucosa.162 Early gastric MALT lymphomas that are dependent on the presence of H pylori–activated T cells for survival and lack genetic alternations might also be considered benign or “in situ” lymphomas.163 Cases of reactive follicular hyperplasia in children have been reported that contain clonal populations of CD10+ germinal center B cells, and yet do not progress to overt lymphoma.164 Likewise, nodal marginal zone lymphomas in children appear to have a low risk of progression.165 In the T-cell system, primary cutaneous CD30+ T-cell lymphoproliferative disorder, lymphomatoid papulosis, is a clonal T-cell proliferation that also has limited malignant potential.166,167
Through studying these early events in lymphomagenesis, we are learning more about the circulation and homing of normal and malignant B cells and gaining insight into normal B-cell physiology. One of the great attributes of pathology as a discipline is that disease is a model that provides insight into the functioning of the healthy human organism. As we better understand the abnormal or diseased tissues, we better understand normal human physiology.
The 2008 WHO classification also emphasizes the importance of anatomic site in classification. In addition to MALT lymphomas and primary mediastinal large B-cell lymphoma, both B-cell and T-cell lineages involving cutaneous or other extranodal sites such as the central nervous system are now recognized as distinctive.168 Age may also be a factor in disease definition. Pediatric marginal zone lymphomas and pediatric FLs have been described, which tend to be localized and have an excellent prognosis.165,169,170 They differ in pathogenesis from morphologically similar lymphomas in adults, as pediatric FL typically lacks BCL2 expression and the t(14;18)(q32;q21). In contrast, some diseases appear to occur most often at advanced age, such as EBV+ DLBCL in elderly persons, which likely arise because of decreased immune surveillance.171
In 2 of the most common lymphomas—FL and DLBCL—there is a need for pathologic predictors of outcome and response to treatment. FL has traditionally been graded according to the proportion of centroblasts—a continuous variable that is associated with decreasing survival duration, but that is poorly reproducible among pathologists. It has become increasingly apparent that cases graded as Grade 1 or Grade 2 have similar outcomes that are not affected by aggressive therapy, and that they do not need to be distinguished from one another. Thus, the 2008 WHO lumps cases with few centroblasts as “FL Grade 1-2 (low grade).” Stratifying FL Grade 3 according to the presence or absence of residual centrocytes (3A vs 3B) was optional in the 2001 WHO classification, but is now mandatory. Some studies have identified biological differences between these 2 subtypes, with most instances of FL Grade 3B more closely related to DLBCL at the molecular level.172,173 However, the segregation is imperfect based on current genetic and clinical studies; this stratification should provide the opportunity to collect more information. Finally, it is emphasized that areas of DLBCL in any FL should receive a separate diagnosis of DLBCL and such patients should be treated as DLBCL (ie, there is no such thing as “FL Grade 3[A or B] with diffuse areas”). After separation of specific new subtypes of DLBCL, we are still left with a large group of DLBCL for which pathologic features are lacking to further stratify them for predicting prognosis or response to therapy. Stratification according to gene expression profiles as GCB versus ABC types has prognostic value, but as yet does not direct therapy. This stratification is imperfectly replicated by morphology, immunophenotyping, or cytogenetic analysis, and thus is not officially incorporated into the classification for practical use. Development of targeted therapies and recognition of additional markers of clinical behavior will likely result in additional modifications to this category in the future.
Finally, an intriguing new development is the recent recognition of lineage plasticity in hematopoietic cells and their neoplasms. In normal B cells, down-regulation of the B-cell regulatory factor PAX-5 can result in reprogramming of both immature and mature B cells to monocytes and even T cells. Cases of lymphoblastic neoplasms of both T- and B-cell type have been reported in which clonally related histiocytic neoplasms have developed, and recently a similar phenomenon has been reported in FL.174,175 This phenomenon may complicate interpretation of the significance of antigen-receptor gene rearrangements in assigning lineage to a neoplasm.
Acknowledgments
The authors dedicate this review to David Y. Mason, Oxford University, who died on February 2, 2008. David Mason was a pivotal figure in the development of immunohistochemistry, and pioneered the development of monoclonal antibodies reactive against epitopes preserved in formalin-fixed paraffin-embedded tissues. As a leader in the establishment of the Human Leucocyte Differentiation Antigen workshops, he helped bring order to a complex and chaotic field. Through his scientific work and reagents that he generously made available on a worldwide basis he helped shape the modern classification of lymphoid malignancies and was one of the most highly cited physicians in the field of clinical oncology. His wit and satire brightened many medical symposia. Indeed he was a scriptwriter for some of the early pre-Monty Python satirical shows, especially “That Was The Week That Was,” and had a long-standing interest in the arts. His enthusiasm for international collaboration led many to visit his laboratory, and he fostered the development of new generations of translational scientists. He will be greatly missed by his students of all ages.
Biographies
Elaine S. Jaffe
Elaine S. Jaffe
My destiny to pursue a career in pathology was determined very early; as a medical student I found that the microscope opened up a whole new world. By examining cells and tissues I could see with my own eyes disruptions in normal physiology and function. Certainly one could later study these at the biochemical or molecular level, but it is careful morphologic analysis of the diseased tissues that often provides the first insight into the problem. Additionally, examination of a microscopic section can tell a whole story, revealing the patient's signs, symptoms, and expected clinical course. In some ways it is like reading tea leaves, only much more reliable as a predictive tool. The pathologist is like a detective, deciphering the morphologic clues to arrive at the correct solution, and still today, discovering and uncovering a difficult diagnosis provides me with great satisfaction. Along the way I considered renal pathology, hepatic pathology, and hematopathology. In all 3 of these subspeciality areas, the pathologist works closely with the internal medicine specialist in diagnosis and disease management. When I came to the National Cancer Institute (NCI) in 1970 the choice of hematopathology quickly became obvious. In the early 1970s Vince De Vita, George Canellos, Bob Young, and Paul Carbone were changing the face of medical oncology and curing patients with advanced stage Hodgkin disease and other lymphomas. Coincident with this revolution in therapy, understanding of the normal immune system was undergoing explosive growth. As a fellow in hematopathology in 1972 under the mentorship of Cos Berard, I began a collaboration with Drs Ira Green, Ethan Shevach, and Michael Frank, immunologists at the National Institutes of Health (NIH), and used what we would now see as primitive tools to distinguish T cells, B cells, and histiocytes. These early studies using E, EAC, and EA rosettes seemed miraculous at the time. One of my first major papers dealt with follicular lymphoma, then known as “nodular lymphoma.” In a paper published in the New England Journal of Medicine in 1974 that later became a Citation Classic, we showed that the lymphoma cells had the same receptor profile as normal follicle center cells, providing functional evidence of an origin from the lymphoid follicle, the functional heart of the B-cell system. Prior to these early studies, pathologists had limited insight into the origin and function of neoplastic hemato-lymphoid cells. The visualization of functional receptors on patient biopsy specimens opened up a new era of investigation. Most of my investigational studies have come from observations that I have made at the microscope, going from the “Bedside to the Bench.” Through astute morphologic observations, we gain insight into the basic nature of disease, and these observations can point the way toward laboratory studies to further resolve pathogenesis. In the past our work focused on the interrelationship between the Hodgkin lymphomas and non-Hodgkin lymphomas, and an elucidation of the biology and classification of peripheral T-cell lymphomas. In recent years the microscope has led me to “in situ follicular lymphoma,” exploring early events in lymphomagenesis; gray zone lymphomas, and the transition from B-cell lymphoma to classic Hodgkin lymphoma; and transdifferentiation or lineage plasticity in mature and immature lymphoid malignancies. In each of these settings, the microscope can serve as a tool not only for diagnosis but also for disease discovery.
Nancy Lee Harris
Nancy Lee Harris
In retrospect, the first clue that I would be a pathologist came in my early teens, when someone gave my sister a microscope. I was irresistibly drawn to examine bits of plants and dead insects—seeing things not visible to the naked eye. As an undergraduate at Stanford University I worked on lymphocyte transformation in the laboratory of my uncle, Dr William P. Creger, Professor of Hematology, and became intrigued by the immune system. In medical school I was attracted to hematologic oncology during rotations with Drs Saul Rosenberg and Henry Kaplan. I didn't consider a career in pathology until after an internship in Medicine and 3 years of part-time practice as a primary care physician. This experience left me desperate to reenter academic life, and a specialty in which I could focus on making diagnoses and trying to better understand diseases. The 1970s were an ideal time to train in hematopathology. The relationship of lymphoid neoplasms to the immune system was just being explored, and immunohistochemistry, flow cytometry, monoclonal antibodies, and molecular genetics made lymphomas the most exciting area of pathology. A pivotal experience was reading Karl Lennert's book, Malignant Lymphomas, in 1978: the clear descriptions and superb illustrations of diseases suddenly made sense of a bewildering field. Having traveled in Europe during college, I eagerly returned professionally, first visiting Prof Lennert at Kiel and later becoming a founding member of the European Association for Hematopathology. I was fortunate to also have as informal mentors many of the other distinguished pathologists of the time, including Henry Rappaport, Ronald Dorfman, and Costan Berard. An early colleague and ally was Sibrand Poppema, who visited Massachusetts General Hospital (MGH) and introduced me to the technique of frozen section immunohistochemistry, which we were able to exploit using monoclonal antibodies from colleagues at the Dana-Farber Cancer Institute, to further understand normal lymphoid tissues and the relationships of lymphomas to the immune system. During my residency at Boston's Beth Israel Hospital, Dr Richard Cohen had remarked on the amazing insights into the organization of human tissues obtained by staining sections of the same specimen with different reagents—the novel technique then was enzyme histochemistry. Immunohistochemistry with monoclonal antibodies exponentially increased the different views one could have of the same tissue, and was the source of endless wonder and excitement. Having trained in an age of multiple lymphoma classifications, I was painfully aware of the need for consensus in classification. A finite number of real diseases were recognized in most classifications, but with different terminology and defining features. I thought that a “real” classification could simply be a list of diseases that pa-thologists could recognize, which seemed to be clinically distinctive (Harris N. Lymphoma 1987: an interim approach to diagnosis and classification. In: Fechner R, Rosen P, eds. Pathology Annual. East Norwalk, CT: Appleton & Lange; 1987:1-67.). In 1992, I proposed that members of the International Lymphoma Study Group try to develop such a consensus list; the result was the Revised European American Classification of Lymphoid Neoplasms (REAL), which formed the basis of the World Health Organization (WHO) classifications of 2001 and 2008. The WHO classification was the first true international consensus on the classification of hematologic neoplasms and established a paradigm for collaboration among pathologists and clinicians on disease classification. Having been a clinician before becoming a pathologist, I realized in the 1980s that the Clinicopathologic Conferences, published as the Case Records of MGH in the New England Journal of Medicine, provided a unique opportunity to highlight the role of pathologists in the care of patients with lymphomas and to educate clinicians about advances in this field. Now, as Editor of the Case Records, I enjoy using the stories of real patients to bring together clinicians, radiologists, and pathologists to educate readers about new concepts in the diagnosis and management of diseases.
Harald Stein
Harald Stein
My genuine interest in the biochemistry of the human body induced me to study medicine. The excellent lectures by Karl Lennert showed me that pathology is a key discipline in medicine. After I won the Olympic sailing trials and qualified for the Olympic Games in Mexico in 1968, Lennert offered me a position in his lymph node registry with the agreement that I could spend 3 months of service time training and attending the Games. So I started my career in Lennert's institute. My initial task was to code lymphoma diagnoses. Soon I recognized irreproducibility of lymphoma diagnosis, the odd concept of lympho-histiocytic lymphoma, and doubtful macrophage origin of histiocytic lymphoma. At that time (1970) a paper was published in which a selective expression of immunoglobulin (IG) on the recently discovered B cells was reported. Then I investigated extracts from specimens of malignant lymphomas and corresponding blood sera for IG concentration. The extracts of the majority of histiocytic lymphomas contained a high concentration of IgM when compared with reactive lymph nodes and corresponding blood sera. This observation indicated that the majority of histiocytic lymphomas are not histiocytic in origin but B-cell derived (1972, 1974). The correlation of the tissue IgM data with light-microscopic and electron-microscopic morphology formed the conceptual basis for the Kiel classification in 1973. When in 1975 the monoclonal antibody (mAb) technology became available, I developed a series of mAbs in collaboration by immunizing mice with purified complement receptors and Hodgkin cell lines. We screened the reactivity of the obtained mAb clones on frozen sections with a highly sensitive immunohistologic technique. This led in 1980 to the generation of mAbs specific for B cells (later clustered as CD22) and follicular dendritic cells (later clustered as CD21), and to the detection of Ki-1 (later clustered as CD30) and Ki-67. CD30 proved to be the most specific marker for Hodgkin Reed-Sternberg cells until now, and molecular cloning performed by Horst Dürkop of my group identified the CD30 molecule as a cytokine receptor of the TNF family. Ki-67 turned out to be an ideal molecular marker for visualizing proliferating cells in reactive and neoplastic conditions. By means of the CD30 mAb I discovered a novel lymphoma category that Karl Lennert and I termed anaplastic large cell lymphoma (ALCL) in 1985. In 1991, Peter Isaacson and I founded the International Lymphoma Study Group (ILSG) by bringing 19 internationally known experts in hematopathology together with the goal of establishing a close cooperation between the New and the Old worlds in the field of lymphoid neoplasms. The activity of the ILSG proved to be essential for the development of a biology-based and clinically useful consensus classification on lymphomas (1994 and 2001). In 1997, my group and I described plasmablastic lymphoma as a new DLBCL subentity. My team and I contributed to the finding that the dysplastic cells of classic Hodgkin disease are (1) B-cell derived in 98% and T-cell derived in 2% of the cases, (2) clonal and thus neoplastic, and (3) incapable of transcribing IG and most B-cell genes due to epigenetic mechanisms associated with a constitutive expression of the inhibitor of differentiation 2 (ID2).
Peter G. Isaacson
Peter G. Isaacson
Most of my third, preclinical year of medical school in Cape Town, South Africa, was devoted to pathology, which at 592 hours was by far the longest course of the entire curriculum. Little of this remains in today's medical school curricula but the logical, evidence-based nature of the subject combined with the infectious enthusiasm of my teachers led me to chose pathology as a career. The advent of immunohistochemistry was the catalyst that caused me to embark on a career as an “academic” surgical pathologist originally focusing on gastrointestinal pathology. Throughout my career I have been fortunate to work with remarkable pathologists who were broadminded and encouraged free thinking. In joining Prof Dennis Wright's department in Southampton I encountered this same atmosphere and was given the time and resources to pursue my work on the immunoperoxidase technique and its applications to gastrointestinal pathology. In 1976 I received a small bowel resection specimen from a patient with refractory celiac disease and so-called idiopathic ulcerative jejunitis. Unwilling to accept the designation “idiopathic” for the ulcers, I doggedly sectioned ulcer after ulcer, all of which showed nonspecific inflammatory changes until, in the base of 1 ulcer I observed a minute focus of malignant lymphoma. Together with Dennis Wright, already an internationally renowned hematopathologist, I set about applying the then novel technique of immunohistochemistry and, later, molecular biology to study the association of lymphoma with celiac disease and to challenge and eventually change longheld concepts of gastrointestinal and other extranodal lymphomas. This culminated in the formulation of the MALT lymphoma concept and the discovery of the association of gastric MALT lymphoma with Helicobacter pylori infection.
Authorship
Contribution: E.J., N.L.H., H.S., and P.I. all contributed to this historical review by participating in writing the manuscript and in providing figures and graphics material.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elaine S. Jaffe, Laboratory of Pathology, National Cancer Institute, Building 10, Room 2B 42, 10 Center Drive, MSC 1500, Bethesda, MD 20892; e-mail: elainejaffe@nih.gov.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swerdlow S, Habeshaw J, Murray L, Dhaliwal H, Lister T, Stansfeld A. Centrocytic lymphoma: a distinct clinicopathologic and immunologic entity. Am J Pathol. 1983;113:181. [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffe ES, Bookman MA, Longo DL. Lymphocytic lymphoma of intermediate differentiation–mantle zone lymphoma: a distinct subtype of B-cell lymphoma. Hum Pathol. 1987;18:877–880. doi: 10.1016/s0046-8177(87)80262-9. [DOI] [PubMed] [Google Scholar]
- 5.Stein H, Mason DY, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–858. [PubMed] [Google Scholar]
- 6.Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell derived neoplasms. Blood. 1991;78:259–263. [PubMed] [Google Scholar]
- 7.Dawson PJ. The original illustrations of Hodgkin's disease. Ann Diagn Pathol. 1999;3:386–393. doi: 10.1016/s1092-9134(99)80018-5. [DOI] [PubMed] [Google Scholar]
- 8.Trumper LH, Brittinger G, Diehl V, Harris NL. Non-Hodgkin's lymphoma: a history of classification and clinical observations. In: Mauch PM, Armitage JO, Coiffier B, Dalla-Favera R, Harris NL, editors. Non-Hodgkin's Lymphomas. Philadelphia, PA: Lippincott, Williams and Wilkins; 2004. pp. 3–19. [Google Scholar]
- 9.Brill NE, Baehr G, Rosenthal N. Generalized giant lymph follicle hyperplasia of lymph nodes and spleen: a hitherto undescribed type. J Amer Med Assoc. 1925;84:668–671. doi: 10.1016/0002-9343(52)90023-5. [DOI] [PubMed] [Google Scholar]
- 10.Symmers D. Follicular lymphadenopathy with splenomegaly: a newly recognized disease of the lymphatic system. Arch Pathol. 1927;3:816–820. [Google Scholar]
- 11.Symmers D. Giant follicular lymphadenopathy with or without splenomegaly: its transformation into polymorphous cell sarcoma of the lymph follicles and its association with Hodgkin's disease, lymphatic leukemia and an apparently unique disease of the lymph nodes and spleen—a disease entity believed heretofore undescribed. Arch Pathol. 1938;26:603–647. [Google Scholar]
- 12.Gall EA, Mallory TB. Malignant lymphoma: a clinico-pathologic survey of 618 cases. Am J Pathol. 1942;18:381–429. [PMC free article] [PubMed] [Google Scholar]
- 13.Gall EA, Morrison HR, Scott AT. The follicular type of malignant lymphoma: a survey of 63 cases. Ann Intern Med. 1941;1941:2073–2090. [Google Scholar]
- 14.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–223. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 15.Nowell PC. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20:462–466. [PubMed] [Google Scholar]
- 16.O'Conor GT. Definition of Burkitt's tumor. Int J Cancer. 1968;3:411–412. [PubMed] [Google Scholar]
- 17.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 18.Rappaport H, Winter W, Hicks E. Follicular lymphoma: a re-evaluation of its position in the scheme of malignant lymphoma, based on a survey of 253 cases. Cancer. 1956;9:792–821. doi: 10.1002/1097-0142(195607/08)9:4<792::aid-cncr2820090429>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 19.Rappaport H. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology; 1966. Tumors of the hematopoietic system. Vol. Section III (Series I ed) [Google Scholar]
- 20.Lukes R, Butler J, Hicks E. Natural history of Hodgkin's disease as related to its pathological picture. Cancer. 1966;19:317–344. [Google Scholar]
- 21.Stein H, Lennert K, Parwaresch MR. Malignant lymphomas of B-cell type. Lancet. 1972;2:855–857. doi: 10.1016/s0140-6736(72)92215-5. [DOI] [PubMed] [Google Scholar]
- 22.Lennert K, Mohri N. Histological classification and occurrence of Hodgkin's disease. Internist. 1974;15:57–65. [PubMed] [Google Scholar]
- 23.Taylor CR, Mason DY. The immunohistological detection of intracellular immunoglobulin in formalin-paraffin sections from multiple myeloma and related conditions using the immunoperoxidase technique. Clin Exp Immunol. 1974;18:417–429. [PMC free article] [PubMed] [Google Scholar]
- 24.Jaffe ES, Shevach EM, Frank MM, Berard CW, Green I. Nodular lymphoma: evidence for origin from follicular B lymphocytes. N Engl J Med. 1974;290:813–819. doi: 10.1056/NEJM197404112901501. [DOI] [PubMed] [Google Scholar]
- 25.Non-Hodgkin's Lymphoma Pathologic Classification Project. National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a Working Formulation for clinical usage. Cancer. 1982;49:2112–2135. doi: 10.1002/1097-0142(19820515)49:10<2112::aid-cncr2820491024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Southern EM. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 27.Zech L, Haglund U, Nilsson K, Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976;17:47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]
- 28.Fukuhara S, Rowley JD, Variakojis D, Golomb HM. Chromosome abnormalities in poorly differentiated lymphocytic lymphoma. Cancer Res. 1979;39:3119–3128. [PubMed] [Google Scholar]
- 29.McMichael AJ, Pilch JR, Galfre G, Mason DY, Fabre JW, Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979;9:205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- 30.Stein H, Gerdes J, Mason DY. The normal and malignant germinal centre. Clin Haematol. 1982;11:531–559. [PubMed] [Google Scholar]
- 31.Stein H, Bonk A, Tolksdorf G, Lennert K, Rodt H, Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980;28:746–760. doi: 10.1177/28.8.7003001. [DOI] [PubMed] [Google Scholar]
- 32.Poppema S, Bhan A, Reinherz E, McCluskey R, Schlossman S. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981;153:30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poppema S, Bhan A, Reinherz E, Posner M, Schlossman S. In situ immunologic characterization of cellular constituents in lymph nodes and spleens involved by Hodgkin's disease. Blood. 1982;59:226–232. [PubMed] [Google Scholar]
- 34.Bernard A, Boumsell L. The clusters of differentiation (CD) defined by the First International Workshop on Human Leucocyte Differentiation Antigens. Hum Immunol. 1984;11:1–10. doi: 10.1016/0198-8859(84)90051-x. [DOI] [PubMed] [Google Scholar]
- 35.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taub R, Kirsch I, Morton C, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A. 1982;79:7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yunis JJ, Oken MM, Kaplan ME, Ensrud KM, Howe RR, Theologides A. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin's lymphoma. N Engl J Med. 1982;307:1231–1236. doi: 10.1056/NEJM198211113072002. [DOI] [PubMed] [Google Scholar]
- 38.Saiki RK, Scharf S, Faloona F, et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 39.Cremer T, Landegent J, Bruckner A, et al. Detection of chromosome aberrations in the human interphase nucleus by visualization of specific target DNAs with radioactive and non-radioactive in situ hybridization techniques: diagnosis of trisomy 18 with probe L1.84. Hum Genet. 1986;74:346–352. doi: 10.1007/BF00280484. [DOI] [PubMed] [Google Scholar]
- 40.Banks P, Chan J, Cleary M, et al. Mantle cell lymphoma: a proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol. 1992;16:637–640. doi: 10.1097/00000478-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Kuppers R, Rajewsky K, Zhao M, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci U S A. 1994;91:10962–10966. doi: 10.1073/pnas.91.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 43.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 44.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 45.Jaffe ES, Harris NL, Stein H, Vardiman J. Lyon, France: IARC Press; 2001. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 46.Hodgkin T. On some morbid experiences of the absorbent glands and spleen. Medico-Chirurgical Trans. 1832;17:68–97. doi: 10.1177/095952873201700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dawson PJ. Whatever happened to Dorothy Reed? Ann Diagn Pathol. 2003;7:195–203. doi: 10.1016/s1092-9134(03)00020-0. [DOI] [PubMed] [Google Scholar]
- 48.Jackson H, Parker F. Hodgkin's disease, II: pathology. N Engl J Med. 1944;231:35–44. [Google Scholar]
- 49.Lukes RJ, Craver LF, Hall TC, Rappaport H, Rubin P. Report of the nomenclature committee. Cancer Res. 1966:26. [Google Scholar]
- 50.Kant JA, Hubbard SM, Longo DL, Simon RM, DeVita VT, Jaffe ES. The pathologic and clinical heterogeneity of lymphocyte-depleted Hodgkin's disease. J Clin Oncol. 1986;4:284–294. doi: 10.1200/JCO.1986.4.3.284. [DOI] [PubMed] [Google Scholar]
- 51.Mason D, Banks P, Chan J, et al. Nodular lymphocyte predominance Hodgkin's disease: a distinct clinico-pathological entity. Am J Surg Pathol. 1994;18:528–530. doi: 10.1097/00000478-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Harris NL. Hodgkin's lymphomas: classification, diagnosis, and grading. Semin Hematol. 1999;36:220–232. [PubMed] [Google Scholar]
- 53.Papermaster BW, Good RA. Relative contributions of the thymus and the bursa of Fabricius to the maturation of the lymphoreticur system and immunological potential in the chicken. Nature. 1962;196:838–840. doi: 10.1038/196838a0. [DOI] [PubMed] [Google Scholar]
- 54.Cooper MD, Peterson RD, Good RA. Delineation of the thymic and bursal lymphoid systems in the chicken. Nature. 1965;205:143–146. doi: 10.1038/205143a0. [DOI] [PubMed] [Google Scholar]
- 55.Shevach EM, Jaffe ES, Green I. Receptors for complement and immunoglobulin on human and animal lymphoid cells. Transplant Rev. 1973;16:3–28. doi: 10.1111/j.1600-065x.1973.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 56.Jaffe ES, Braylan RC, Nanba K, Frank MM, Berard CW. Functional markers: a new perspective on malignant lymphomas. Cancer Treat Rep. 1977;61:953–962. [PubMed] [Google Scholar]
- 57.Jaffe ES, Braylan RC, Frank MM, Green I, Berard CW. Heterogeneity of immunologic markers and surface morphology in childhood lymphoblastic lymphoma. Blood. 1976;48:213–222. [PubMed] [Google Scholar]
- 58.Tsukimoto I, Wong KY, Lampkin BC. Surface markers and prognostic factors in acute lymphoblastic leukemia. N Engl J Med. 1976;294:245–248. doi: 10.1056/NEJM197601292940503. [DOI] [PubMed] [Google Scholar]
- 59.Lukes R, Collins R. Immunologic characterization of human malignant lymphomas. Cancer. 1974;34:1488–1503. doi: 10.1002/1097-0142(197410)34:8+<1488::aid-cncr2820340822>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 60.Gerard-Marchant R, Hamlin I, Lennert K, Rilke F, Stansfeld A, van Unnik J. Classification of non-Hodgkin's lymphomas. Lancet. 1974;ii:406–408. [Google Scholar]
- 61.Bennett MH, Farrer-Brown G, Henry K, Jeliffe AM. Classification of non-Hodgkin's lymphomas. Lancet. 1974;2:405–406. [Google Scholar]
- 62.Dorfman RF. Classification of non-Hodgkin's lymphomas [letter]. Lancet. 1974;2:961–962. doi: 10.1016/s0140-6736(74)91177-5. [DOI] [PubMed] [Google Scholar]
- 63.Lennert K, Stein H, Kaiserling E. Cytological and functional criteria for the classification of malignant lymphomata. Br J Cancer Suppl. 1975;2:29–43. [PMC free article] [PubMed] [Google Scholar]
- 64.Lennert K. New York: Springer-Verlag; 1978. Malignant Lymphomas Other than Hodgkin's Disease. [Google Scholar]
- 65.Lennert K, Feller A. Histopathology of Non-Hodgkin's Lymphomas. 2nd ed. New York: Springer-Verlag; 1992. [Google Scholar]
- 66.Dorfman RF, Kim H. Relationship of histology to site in the non-Hodgkin's lymphomata: a study based on surgical staging procedures. Br J Cancer Suppl. 1975;2:217–220. [PMC free article] [PubMed] [Google Scholar]
- 67.Chabner BA, Johnson RE, Young RC, et al. Sequential nonsurgical and surgical staging of non-Hodgkin's lymphoma. Cancer. 1978;42:922–925. doi: 10.1002/1097-0142(197808)42:2+<922::aid-cncr2820420714>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 68.Van Unnik JA, Breur K, Burgers JM, et al. Non-Hodgkin's lymphomata: clinical features in relation to histology. Br J Cancer Suppl. 1975;2:201–207. [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenberg SA, Kaplan HS. Clinical trials in the non-Hodgkin's lymphomata at Stanford University experimental design and preliminary results. Br J Cancer Suppl. 1975;2:456–464. [PMC free article] [PubMed] [Google Scholar]
- 70.NCI non-Hodgkin's Classification Project Writing Committee. Classification of non-Hodgkin's lymphomas: reproducibility of major classification systems. Cancer. 1985;55:91–95. doi: 10.1002/1097-0142(19850101)55:1<91::aid-cncr2820550115>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 71.Strauchen JA, Young RC, DeVita VT, Jr, Anderson T, Fantone JC, Berard CW. Clinical relevance of the histopathological subclassification of diffuse “histiocytic” lymphoma. N Engl J Med. 1978;299:1382–1387. doi: 10.1056/NEJM197812212992503. [DOI] [PubMed] [Google Scholar]
- 72.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 73.Zola H, Swart B, Banham A, et al. CD molecules 2006: human cell differentiation molecules. J Immunol Methods. 2007;319:1–5. doi: 10.1016/j.jim.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 74.Mason DY, Cordell JL, Abdulaziz Z, Naiem M, Bordenave G. Preparation of peroxidase: antiperoxidase (PAP) complexes for immunohistological labeling of monoclonal antibodies. J Histochem Cytochem. 1982;30:1114–1122. doi: 10.1177/30.11.6183312. [DOI] [PubMed] [Google Scholar]
- 75.Nowell PC, Hungerford DA. Chromosome studies in human leukemia, II: chronic granulocytic leukemia. J Natl Cancer Inst. 1961;27:1013–1035. [PubMed] [Google Scholar]
- 76.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 77.Tsujimoto Y, Yunis J, Onorato-Showe L, Erikson J, Nowell P, Croce CM. Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translation. Science. 1984;224:1403–1406. doi: 10.1126/science.6610211. [DOI] [PubMed] [Google Scholar]
- 78.Arnold A, Kim HG, Gaz RD, et al. Molecular cloning and chromosomal mapping of DNA rearranged with the parathyroid hormone gene in a parathyroid adenoma. J Clin Invest. 1989;83:2034–2040. doi: 10.1172/JCI114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arnold A, Cossman J, Bakhshi A, Jaffe ES, Waldmann TA, Korsmeyer SJ. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983;309:1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- 80.Kuppers R, Zhao M, Hansmann ML, Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:1520–1529. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- 82.Tamaru J, Hummel M, Zemlin M, Kalvelage B, Stein H. Hodgkin's disease with a B-cell phenotype often shows a VDJ rearrangement and somatic mutations in the VH genes. Blood. 1994;84:708–715. [PubMed] [Google Scholar]
- 83.Deane M, Norton JD. Detection of immunoglobulin gene rearrangement in B lymphoid malignancies by polymerase chain reaction gene amplification. Br J Haematol. 1990;74:251–256. doi: 10.1111/j.1365-2141.1990.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 84.Ngan BY, Nourse J, Cleary ML. Detection of chromosomal translocation t(14;18) within the minor cluster region of bcl-2 by polymerase chain reaction and direct genomic sequencing of the enzymatically amplified DNA in follicular lymphomas. Blood. 1989;73:1759–1762. [PubMed] [Google Scholar]
- 85.Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt's lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354:2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 86.Wlodarska I, Mecucci C, Stul M, et al. Fluorescence in situ hybridization identifies new chromosomal changes involving 3q27 in non-Hodgkin's lymphomas with BCL6/LAZ3 rearrangement. Genes Chromosomes Cancer. 1995;14:1–7. doi: 10.1002/gcc.2870140102. [DOI] [PubMed] [Google Scholar]
- 87.Marafioti T, Hummel M, Foss HD, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95:1443–1450. [PubMed] [Google Scholar]
- 88.Jaffe ES, Chan JKC, Su IJ, et al. Report of the workshop on nasal and related extranodal angiocentric T/NK cell lymphomas: definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996;20:103–111. doi: 10.1097/00000478-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 89.Delecluse HJ, Anagnostopoulos I, Dallenbach F, et al. Plasmablastic lymphomas of the oral cavity: a new entity associated with the human immunodeficiency virus infection. Blood. 1997;89:1413–1420. [PubMed] [Google Scholar]
- 90.Quintanilla-Martinez L, Kumar S, Fend F, et al. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443–451. [PubMed] [Google Scholar]
- 91.Poiesz B, Ruscetti F, Gazdar A. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 93.Oksenhendler E, Boulanger E, Galicier L, et al. High incidence of Kaposi sarcoma-associated herpesvirus-related non-Hodgkin lymphoma in patients with HIV infection and multicentric Castleman disease. Blood. 2002;99:2331–2336. doi: 10.1182/blood.v99.7.2331. [DOI] [PubMed] [Google Scholar]
- 94.Dupin N, Fisher C, Kellam P, et al. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999;96:4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du MQ, Diss TC, Liu H, et al. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–3418. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 96.Diehl V, Kirchner HH, Schaadt M, et al. Hodgkin's disease: establishment and characterization of four in vitro cell lies. J Cancer Res Clin Oncol. 1981;101:111–124. doi: 10.1007/BF00405072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stein H, Gerdes J, Schwab U, et al. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer. 1982;30:445–459. doi: 10.1002/ijc.2910300411. [DOI] [PubMed] [Google Scholar]
- 98.Andreesen R, Osterholz J, Lohr GW, Bross KJ. A Hodgkin cell-specific antigen is expressed on a subset of auto- and alloactivated T (helper) lymphoblasts. Blood. 1984;63:1299–1302. [PubMed] [Google Scholar]
- 99.Pinkus G, Said J. Hodgkin's disease, lymphocyte predominance type, nodular: a distinct entity? Unique staining profile of L&H variants of Reed-Sternberg cells defined by monoclonal antibodies to leukocyte common antigen, granulocyte specific antigen, and B-cell specific antigen. Am J Pathol. 1985;116:1–6. [PMC free article] [PubMed] [Google Scholar]
- 100.Schmid C, Pan L, Diss T, Isaacson PG. Expression of B-cell antigens by Hodgkin's and Reed-Sternberg cells. Am J Pathol. 1991;139:701–707. [PMC free article] [PubMed] [Google Scholar]
- 101.Weiss LM, Movahed LA, Warnke RA, Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin's disease. N Engl J Med. 1989;320:502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- 102.Jaffe ES. The elusive Reed-Sternberg cell. N Engl J Med. 1989;320:529–531. doi: 10.1056/NEJM198902233200813. [DOI] [PubMed] [Google Scholar]
- 103.Jaffe ES, Zarate OA, Medeiros LJ. The interrelationship of Hodgkin's disease and non-Hodgkin's lymphomas: lessons learned from composite and sequential malignancies. Semin Diagn Pathol. 1992;9:297–303. [PubMed] [Google Scholar]
- 104.Hsu SM, Yang K, Jaffe ES. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985;118:209–217. [PMC free article] [PubMed] [Google Scholar]
- 105.Falini B, Stein H, Pileri S, et al. Expression of lymphoid-associated antigens on Hodgkin's and Reed-Sternberg cells of Hodgkin's disease: an immunocytochemical study on lymph node cytospins using monoclonal antibodies. Histopathology. 1987;11:1229–1242. doi: 10.1111/j.1365-2559.1987.tb01869.x. [DOI] [PubMed] [Google Scholar]
- 106.Dallenbach FE, Stein H. Expression of T-cell-receptor beta chain in Reed-Sternberg cells. Lancet. 1989;2:828–830. doi: 10.1016/s0140-6736(89)92997-8. [DOI] [PubMed] [Google Scholar]
- 107.Tzankov A, Bourgau C, Kaiser A, et al. Rare expression of T-cell markers in classical Hodgkin's lymphoma. Mod Pathol. 2005;18:1542–1549. doi: 10.1038/modpathol.3800473. [DOI] [PubMed] [Google Scholar]
- 108.Kanzler H, Kuppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin's disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med. 1996;184:1495–1505. doi: 10.1084/jem.184.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ushmorov A, Ritz O, Hummel M, et al. Epigenetic silencing of the immunoglobulin heavy-chain gene in classical Hodgkin lymphoma-derived cell lines contributes to the loss of immunoglobulin expression. Blood. 2004;104:3326–3334. doi: 10.1182/blood-2003-04-1197. [DOI] [PubMed] [Google Scholar]
- 110.Stein H, Marafioti T, Foss HD, et al. Down-regulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood. 2001;97:496–501. doi: 10.1182/blood.v97.2.496. [DOI] [PubMed] [Google Scholar]
- 111.Ehlers A, Oker E, Bentink S, Lenze D, Stein H, Hummel M. Histone acetylation and DNA demethylation of B cells result in a Hodgkin-like phenotype. Leukemia. 2008;22:835–841. doi: 10.1038/leu.2008.12. [DOI] [PubMed] [Google Scholar]
- 112.Mathas S, Janz M, Hummel F, et al. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat Immunol. 2006;7:207–215. doi: 10.1038/ni1285. [DOI] [PubMed] [Google Scholar]
- 113.Jaffe ES. Pathobiology of peripheral T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2006:317–322. doi: 10.1182/asheducation-2006.1.317. [DOI] [PubMed] [Google Scholar]
- 114.Isaacson P, Spencer J. Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathol. 1987;11:445–462. doi: 10.1111/j.1365-2559.1987.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 115.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- 116.Shipp MA, Ross KN, Tamayo P, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 117.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 118.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 120.Jaffe ES, Harris NL, Diebold J, Muller-Hermelink HK. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: A progress report. Am J Clin Pathol. 1999;111:S8–S12. [PubMed] [Google Scholar]
- 121.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 122.Kleihues P, Cavenee WK. Lyon, France: IARC Press; 2000. Pathology and Genetics: Tumours of the Nervous System. [Google Scholar]
- 123.Takatsuki K. Adult T-cell leukemia. Intern Med. 1995;34:947–952. doi: 10.2169/internalmedicine.34.947. [DOI] [PubMed] [Google Scholar]
- 124.Yang WI, Zukerberg LR, Motokura T, Arnold A, Harris NL. Cyclin D1 (Bcl-1, PRAD1) protein expression in low-grade B-cell lymphomas and reactive hyperplasia. Am J Pathol. 1994;145:86–96. [PMC free article] [PubMed] [Google Scholar]
- 125.Stein H, Foss HD, Durkop H, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–3695. [PubMed] [Google Scholar]
- 126.Jaffe ES. Anaplastic large cell lymphoma: the shifting sands of diagnostic hematopathology. Mod Pathol. 2001;14:219–228. doi: 10.1038/modpathol.3880289. [DOI] [PubMed] [Google Scholar]
- 127.Frand UI, Ramot B. [Malignant lymphomata in Israel: an epidemiological study based on 399 cases]. Harefuah. 1963;65:83–86. [PubMed] [Google Scholar]
- 128.Seligmann M, Danon F, Hurez D, Mihaesco E, Preud'homme JL. Alpha-chain disease: a new immunoglobulin abnormality. Science. 1968;162:1396–1397. doi: 10.1126/science.162.3860.1396. [DOI] [PubMed] [Google Scholar]
- 129.Isaacson P. Middle East lymphoma and alpha-chain disease: an immunohistochemical study. Am J Surg Pathol. 1979;3:431–441. doi: 10.1097/00000478-197910000-00005. [DOI] [PubMed] [Google Scholar]
- 130.Isaacson P, Wright D. Malignant lymphoma of mucosa associated lymphoid tissue: a distinctive B cell lymphoma. Cancer. 1983;52:1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 131.Harris NL, Pilch BZ, Bhan AK, Harmon DC, Goodman ML. Immunohistologic diagnosis of orbital lymphoid infiltrates. Am J Surg Pathol. 1984;8:83–91. doi: 10.1097/00000478-198402000-00001. [DOI] [PubMed] [Google Scholar]
- 132.Isaacson PG. Gastric MALT lymphoma: from concept to cure. Ann Oncol. 1999;10:637–645. doi: 10.1023/a:1008396618983. [DOI] [PubMed] [Google Scholar]
- 133.Spencer J, Finn T, Pulford K, Mason D, Isaacson P. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells. Clin Exp Immunol. 1985;62:607–612. [PMC free article] [PubMed] [Google Scholar]
- 134.Wotherspoon AC, Diss TC, Pan LX, et al. Primary low-grade B-cell lymphoma of the conjunctiva: a mucosa-associated lymphoid tissue type lymphoma Epstein-Barr virus (EBV) in enteropathy-associated T-cell lymphoma (EATL). Histopathology. 1993;23:417–424. doi: 10.1111/j.1365-2559.1993.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 135.Isaacson PG. Mucosa-associated lymphoid tissue lymphoma. Semin Hematol. 1999;36:139–147. [PubMed] [Google Scholar]
- 136.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 137.Hussell T, Isaacson PG, Crabtree JE, Spencer J. The response of cells from low-grade B-cell gastric lymphomas of mucosa-associated lymphoid tissue to Helicobacter pylori. Lancet. 1993;342:571–574. doi: 10.1016/0140-6736(93)91408-e. [DOI] [PubMed] [Google Scholar]
- 138.Wotherspoon A, Doglioni C, Diss T, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 139.Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med. 2004;350:239–248. doi: 10.1056/NEJMoa031887. [DOI] [PubMed] [Google Scholar]
- 140.Roulland S, Suarez F, Hermine O, Nadel B. Pathophysiological aspects of memory B-cell development. Trends Immunol. 2008;29:25–33. doi: 10.1016/j.it.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 141.Rowe M, Peng-Pilon M, Huen DS, et al. Upregulation of bcl-2 by the Epstein-Barr virus latent membrane protein LMP1: a B-cell-specific response that is delayed relative to NF-kappa B activation and to induction of cell surface markers. J Virol. 1994;68:5602–5612. doi: 10.1128/jvi.68.9.5602-5612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dierlamm J, Baens M, Wlodarska I, et al. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood. 1999;93:3601–3609. [PubMed] [Google Scholar]
- 143.Willis TG, Jadayel DM, Du MQ, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:35–45. doi: 10.1016/s0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 144.Streubel B, Lamprecht A, Dierlamm J, et al. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood. 2003;101:2335–2339. doi: 10.1182/blood-2002-09-2963. [DOI] [PubMed] [Google Scholar]
- 145.Streubel B, Simonitsch-Klupp I, Mullauer L, et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722–1726. doi: 10.1038/sj.leu.2403501. [DOI] [PubMed] [Google Scholar]
- 146.Jaffe ES, Wilson WH. Gray zone, synchronous, and metachronous lymphomas: Diseases at the interface of non-Hodgkin's lymphomas and Hodgkin's Lymphoma. In: Mauch PM, Armitage JO, Coiffier B, Dalla-Favera R, Harris NL, editors. Non-Hodgkin's Lymphoma. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2004. pp. 69–80. [Google Scholar]
- 147.Rudiger T, Jaffe ES, Delsol G, et al. Workshop report on Hodgkin's disease and related diseases (“grey zone” lymphoma). Ann Oncol. 1998;9:S31–S38. doi: 10.1093/annonc/9.suppl_5.s31. [DOI] [PubMed] [Google Scholar]
- 148.Traverse-Glehen A, Pittaluga S, Gaulard P, et al. Mediastinal gray zone lymphoma: the missing link between classic Hodgkin's lymphoma and mediastinal large B-cell lymphoma. Am J Surg Pathol. 2005;29:1411–1421. doi: 10.1097/01.pas.0000180856.74572.73. [DOI] [PubMed] [Google Scholar]
- 149.Haralambieva E, Boerma EJ, van Imhoff GW, et al. Clinical, immunophenotypic, and genetic ana-lysis of adult lymphomas with morphologic features of Burkitt lymphoma. Am J Surg Pathol. 2005;29:1086–1094. [PubMed] [Google Scholar]
- 150.Karsan A, Gascoyne RD, Coupland RW, Shepherd JD, Phillips GL, Horsman DE. Combination of t(14;18) and a Burkitt's type translocation in B-cell malignancies. Leuk Lymphoma. 1993;10:433–441. doi: 10.3109/10428199309148200. [DOI] [PubMed] [Google Scholar]
- 151.Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt's lymphoma. N Engl J Med. 2006;354:2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 152.Smith D, Ballal M, Hodder R, Selvachandran SN, Cade D. The adenoma carcinoma sequence: an indoctrinated model for tumorigenesis, but is it always a clinical reality? Colorectal Dis. 2006;8:296–301. doi: 10.1111/j.1463-1318.2005.00936.x. [DOI] [PubMed] [Google Scholar]
- 153.Jaffe ES. Follicular lymphomas: possibility that they are benign tumors of the lymphoid system. J Natl Cancer Inst. 1983;70:401–403. [PubMed] [Google Scholar]
- 154.Limpens J, de Jong D, van Krieken J, et al. Bcl-2 in benign lymphoid tissue with follicular hyperplasia. Oncogene. 1991;6:2271–2276. [PubMed] [Google Scholar]
- 155.Roulland S, Navarro JM, Grenot P, et al. Follicular lymphoma-like B cells in healthy individuals: a novel intermediate step in early lymphomagenesis. J Exp Med. 2006;203:2425–2431. doi: 10.1084/jem.20061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marti GE, Rawstron AC, Ghia P, et al. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130:325–332. doi: 10.1111/j.1365-2141.2005.05550.x. [DOI] [PubMed] [Google Scholar]
- 157.Rawstron AC, Bennett FL, O'Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359:575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 158.Cong P, Raffeld M, Teruya-Feldstein J, Sorbara L, Pittaluga S, Jaffe ES. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood. 2002;99:3376–3382. doi: 10.1182/blood.v99.9.3376. [DOI] [PubMed] [Google Scholar]
- 159.Aqel N, Barker F, Patel K, Naresh KN. In-situ mantle cell lymphoma: a report of two cases. Histopathology. 2008;52:256–260. doi: 10.1111/j.1365-2559.2007.02906.x. [DOI] [PubMed] [Google Scholar]
- 160.Yoshino T, Miyake K, Ichimura K, et al. Increased incidence of follicular lymphoma in the duodenum. Am J Surg Pathol. 2000;24:688–693. doi: 10.1097/00000478-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 161.Poggi MM, Cong PJ, Coleman CN, Jaffe ES. Low-grade follicular lymphoma of the small intestine. J Clin Gastroenterol. 2002;34:155–159. doi: 10.1097/00004836-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 162.Bende RJ, Smit LA, Bossenbroek JG, et al. Primary follicular lymphoma of the small intestine: alpha4beta7 expression and immunoglobulin configuration suggest an origin from local antigen-experienced B cells. Am J Pathol. 2003;162:105–113. doi: 10.1016/s0002-9440(10)63802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. 2001;357:39–40. doi: 10.1016/S0140-6736(00)03571-6. [DOI] [PubMed] [Google Scholar]
- 164.Kussick SJ, Kalnoski M, Braziel RM, Wood BL. Prominent clonal B-cell populations identified by flow cytometry in histologically reactive lymphoid proliferations. Am J Clin Pathol. 2004;121:464–472. doi: 10.1309/4EJ8-T3R2-ERKQ-61WH. [DOI] [PubMed] [Google Scholar]
- 165.Taddesse-Heath L, Pittaluga S, Sorbara L, Bussey M, Raffeld M, Jaffe ES. Marginal zone B-cell lymphoma in children and young adults. Am J Surg Pathol. 2003;27:522–531. doi: 10.1097/00000478-200304000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Weiss LM, Wood GS, Trela M, Warnke RA, Sklar J. Clonal T-cell populations in lymphomatoid papulosis: evidence of a lymphoproliferative origin for a clinically benign disease. N Engl J Med. 1986;315:475–479. doi: 10.1056/NEJM198608213150802. [DOI] [PubMed] [Google Scholar]
- 167.Willemze R, Beljaards RC. Spectrum of primary cutaneous CD30 (Ki-1)-positive lymphoproliferative disorders: a proposal for classification and guidelines for management and treatment. J Am Acad Dermatol. 1993;28:973–980. doi: 10.1016/0190-9622(93)70140-o. [DOI] [PubMed] [Google Scholar]
- 168.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 169.Lorsbach RB, Shay-Seymore D, Moore J, et al. Clinicopathologic analysis of follicular lymphoma occurring in children. Blood. 2002;99:1959–1964. doi: 10.1182/blood.v99.6.1959. [DOI] [PubMed] [Google Scholar]
- 170.Finn LS, Viswanatha DS, Belasco JB, et al. Primary follicular lymphoma of the testis in childhood. Cancer. 1999;85:1626–1635. doi: 10.1002/(sici)1097-0142(19990401)85:7<1626::aid-cncr27>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 171.Oyama T, Ichimura K, Suzuki R, et al. Senile EBV+ B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol. 2003;27:16–26. doi: 10.1097/00000478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 172.Ott G, Katzenberger T, Lohr A, et al. Cytomorphologic, immunohistochemical, and cytogenetic profiles of follicular lymphoma: 2 types of follicular lymphoma grade 3. Blood. 2002;99:3806–3812. doi: 10.1182/blood.v99.10.3806. [DOI] [PubMed] [Google Scholar]
- 173.Bosga-Bouwer AG, van Imhoff GW, Boonstra R, et al. Follicular lymphoma grade 3B includes 3 cytogenetically defined subgroups with primary t(14;18), 3q27, or other translocations: t(14;18) and 3q27 are mutually exclusive. Blood. 2003;101:1149–1154. doi: 10.1182/blood.V101.3.1149. [DOI] [PubMed] [Google Scholar]
- 174.Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008;111:5433–5439. doi: 10.1182/blood-2007-11-124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Feldman AL, Berthold F, Arceci R, et al. Clonal relationship between precursor T-lymphoblastic leukaemia/lymphoma and Langerhans-cell histiocytosis. Lancet Oncology. 2005;6:435–437. doi: 10.1016/S1470-2045(05)70211-4. [DOI] [PubMed] [Google Scholar]