Abstract
Study Objectives:
To examine the effects of socially enriched versus socially impoverished environments on performance and alertness decline during sleep deprivation in extraverts versus introverts.
Design:
Participants (n = 29 men, n = 19 women) were assigned to socially enriched (n = 24; 13 introverts, 11 extraverts) or socially impoverished (n = 24; 12 introverts, 12 extraverts) conditions (activities matched) for 12 hours (1000–2200) on Day 1 followed by 22 hours of sleep deprivation (2200-2000; 36 h awake total), monitored by actigraphy. The median split of volunteers' Eysenck Extraversion scores was used for extravert/introvert categorization. The Psychomotor Vigilance Task (PVT), modified Maintenance of Wakefulness Test (MWT), and Stanford Sleepiness Scale (SSS) were administered every 2 hours throughout. PVT speed, transformed lapses, modified MWT sleep-onset latency, and SSS were analyzed using mixed-model analyses of variance, with covariates of age and total actigraphic activity during enrichment or impoverishment.
Setting:
Residential sleep/performance testing facility.
Participants:
Forty-eight healthy adults (aged 18–39).
Interventions:
Twelve hours of socially enriched or isolated environments in extraverts and introverts prior to sleep deprivation.
Results
Social experience interacted with personality type to affect alertness and vigilance. Social enrichment, as compared with social impoverishment, was associated with more PVT lapses at 04:00 overall. Similarly, following social enrichment, PVT speed was significantly slower among extraverts than among introverts during sleep deprivation, but no personality-group differences emerged following social impoverishment. MWT sleep latency and SSS subjective sleepiness did not show significant personality or social-condition effects during sleep deprivation.
Conclusions:
The effect of social exposure on vulnerability or resiliency to sleep deprivation was modulated by introversion and extraversion. Extraverts exposed to social environments were more vulnerable to subsequent sleep deprivation than were introverts.
Citation:
Rupp TL; Killgore WDS; Balkin TJ. Socializing by day may affect performance by night: vulnerability to sleep deprivation is differentially mediated by social exposure in extraverts vs introverts. SLEEP 2010;33(11):1475-1485.
Keywords: Sleep deprivation, personality, extraversion, waking experience
WAKING EXPERIENCE HAS SIGNIFICANT EFFECTS ON SUBSEQUENT SLEEP NEED AND SLEEP ARCHITECTURE. ANIMAL RESEARCH HAS SHOWN THAT SLOW wave sleep (SWS) activity is affected by the quality of waking experience.1 For example, rats exposed to social defeat during waking show subsequent increases in SWS.2 Emerging evidence suggests that changes in sleep that occur following waking experiences are likely related to cortical plasticity and consolidation of memories.3 For many species, particularly humans, social interactions are among the most frequently occurring and cognitively complex experiences during normal waking. In humans, social experiences are associated with recruitment of prefrontal brain regions.4 According to Krueger and colleagues, enriched experiences during waking are associated with greater localized fatigue within the specific neuronal assemblies involved in navigating such experiences,5–7 leading to waking activity-dependent sleep regulation. Recent research with Drosophila has shown that sleep need is significantly increased as a function of social experience.8 Indeed, fruit flies housed in socially isolated conditions show significantly lower sleep requirements than do those living together in groups, despite similar levels of physical activity.8 Moreover, the sleep need of the flies in that study increased monotonically in conjunction with the size of the social group to which they were exposed. Recent findings in Drosophila have also shown that, in addition to increasing sleep need, greater levels of social experience lead to increased numbers of synaptic terminals within clock neurons in the brain.9 The number of these synaptic terminals is sustained during sleep deprivation but is decreased with sleep, suggesting that the process of sleep itself may downscale superfluous synaptic connections formed as a function of waking experience.9,10 Despite a growing literature on the effects of social experience on sleep propensity in animals, to our knowledge no study has yet examined the effect of social stimulation on sleep functions in humans. If the findings with Drosophila generalize to humans, it would be expected that high levels of social stimulation during waking would be associated with a more rapid increase in the need for sleep, manifested as greater difficulty sustaining alertness beyond the normal waking period.
However, even if waking social experience is found to have an effect on subsequent sleep need, it is likely that this effect would interact with trait-like differences in the biologic need for sleep, the individual vulnerability to sleep deprivation, or both.11–14 Considerable evidence suggests that some individuals have great difficulty resisting the effects of sleep deprivation, whereas others seem to have a trait-like resistance to sleep loss that permits them to sustain alertness and performance (on specific tasks) under conditions of considerably reduced sleep.13 Some of this trait-like vulnerability may be rooted in genetic differences.15 Recent findings suggest that differences in a variable-number tandem-repeat polymorphism of the Period3 (PER3) clock gene may contribute to differences in vulnerability to sleep deprivation on cognitive tasks.16 Individuals who are homozygous for the 5-repeat allele (PER35/5) show greater declines in executive functioning during early morning hours following sleep deprivation than do individuals with the PER34/4 allele.17 Furthermore, individuals with the vulnerable 5-repeat genotype show significant declines in activation of prefrontal cortical regions (which mediate executive functions), whereas those with a resistant genotype sustain activation in these regions.18 These findings are consistent with an earlier functional neuroimaging study showing that individuals with greater baseline activation of the prefrontal cortex are more resistant to sleep loss.11 Thus, prefrontal activation appears to be higher at rested baseline in individuals who are resistant to sleep loss and appears to show sustained activation in more resistant individuals during periods of extended wakefulness.
Recent work from our laboratory has been focused on determining the extent to which stable behavioral indicators of baseline cortical arousal and prefrontal functioning are associated with resistance to sleep loss.19–21 In particular, we have examined whether stable personality traits previously shown to be associated with cortical arousal are predictive of declines in vigilance during sleep deprivation.21 A longstanding theory of personality suggests that the trait of introversion-extraversion is related to cortical arousal.22 Eysenck's theory posits that the relatively greater social gregariousness and sensation-seeking behaviors common among extraverts are due, at least in part, to lower levels of tonic arousal of the reticulo-thalamic-cortical activation system.23 Because of their generally lower levels of basal cortical arousal, extraverts therefore seek out social contact and stimulation in their environment to increase their brain arousal to optimal levels. Introverts, on the other hand, are believed to have relatively higher tonic cortical arousal and, therefore, tend to avoid social stimulation to prevent their level of arousal from exceeding optimal levels. Some support for Eysenck's theory has come from functional neuroimaging studies showing that higher introversion scores correlate with greater activation of the prefrontal cortex and anterior thalamus in rested individuals.24 Based on Eysenck's theory, we previously demonstrated that higher scores on a test of introversion are correlated with greater resistance to sleep deprivation.21 Previous research has also shown that extraverts typically show greater cognitive and psychomotor impairment than do introverts during sleep deprivation.25
In our previous study on the relationship between personality traits and resistance to sleep loss, we hypothesized that our findings were due to relatively higher levels of basal cortical arousal among introverts. However, it was also possible that the previous findings were simply a function of the higher level of social stimulation and sensational experiences of extraverts during sleep deprivation. It was hypothesized that a higher level of social stimulation may have led to more rapid fatiguing of brain regions—particularly those regions known to mediate high-level social, attention, and executive processes.5–7 Presently, it is not known how social exposure affects the ability to resist the adverse effects of sleep loss on alertness in humans, nor is it known whether the effects of social exposure during sleep deprivation differ between introverts and extraverts.
In the present study, the effects of high versus low social experience during 36 hours of continuous wakefulness was compared in introverts and extraverts, with particular emphasis on measures reflecting behavioral resilience to sleep loss. Based on prior animal work on the effects of social exposure on sleep need and our own work on the effects of introversion-extraversion on resistance to sleep loss, the following was hypothesized (see Figure 1 also): (1) individuals exposed to a period of social impoverishment prior to total sleep deprivation would be more effective at sustaining performance and alertness relative to volunteers exposed to an equal period of social enrichment, (2) individuals high in introversion would be more effective at sustaining performance and alertness during total sleep deprivation than would individuals high in extraversion, and (3) the trait of introversion-extraversion would interact with the enrichment of the social environment such that extraverts would be more adversely affected by the enrichment level than would be introverts. Specifically, extraverts would show greater deficits in the ability to resist sleep loss when exposed to socially enriched environments than would introverts.
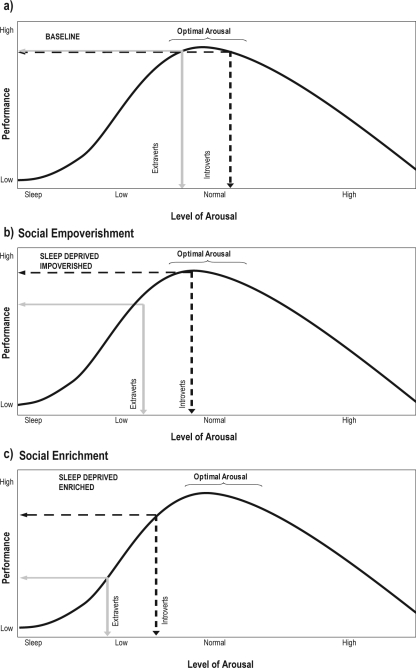
Figure 1.
Theoretical predictions of the effects of sleep deprivation following a period of either Social Impoverishment or Social Enrichment for introverts (black dashed lines) and extraverts (gray solid lines), based on the theory of optimal levels of stimulation and arousal by Eysenck (1967, 1981). The theory suggests that cortical arousal is related to performance in the form of an inverted U-shaped function, with optimal levels of performance associated with moderate levels of arousal. Eysenck also proposed that at resting baseline, Introverts tend to be more cortically aroused than Extraverts. Based on this model we hypothesize that a) at rested baseline, Introverts and Extraverts will show similar levels of objective performance, despite hypothesized lower levels of arousal in Extraverts. Further, it is expected that sleep deprivation will lead to reductions in general cortical arousal, which will be observed as greater declines in objective performance among Extraverts than Introverts. However, we further hypothesize that the level of social exposure and the personality of the individual will modulate the effect of sleep deprivation on performance. Social Enrichment is expected to produce greater cognitive demands on subjects such that differences between Introverts and Extraverts will be b) negligible under conditions of Social Impoverishment, but will be c) highly significant under conditions of Social Enrichment.
METHODS
Participants
Civilian and active-duty military men and women 18 to 39 years of age were recruited via flyers posted at local colleges, universities, and military installations. After providing informed consent, participants completed questionnaires to determine eligibility based on physical state, psychological state, sleep habits, and chronotype. To reduce intersubject variability in nighttime sleep, participants were excluded if they reported any of the following for the preceding month: (1) habitual daytime napping (> 1 nap per week) in conjunction with otherwise normal nightly sleep amounts (8 or more h), (2) average nighttime lights-out times earlier than 21:00 Sunday through Thursday, (3) average morning wake-up times later than 09:00 Monday through Friday, or (4) travel across more than 3 time zones within the last month. Additional exclusion criteria included cardiovascular disease; hypertension or high blood pressure; resting pulse greater than 95 bpm; past or present neurologic, psychiatric, or sleep disorder; present or past use of over-the-counter substances with purported psychoactive properties (e.g., ginko, St. John's wort); asthma or other reactive airways diseases; prior history of cancer; allergies; regular nicotine use (or addiction) within the last 1 year; current heavy alcohol use; current use of other illicit drugs (to include but not limited to benzodiazepines, amphetamines, cocaine, and marijuana); known liver disease or liver abnormalities; self-reported history of caffeine use of more than 400 mg (8 caffeinated sodas or 3–4 cups of coffee) per day on average; score of 41 or more on either scale of the State Trait Anxiety Inventory26; score of 13 or more on the Beck Depression Inventory27,28; score less than 31 or greater than 69 on the Horne-Östberg Morningness-Eveningness Questionnaire29; and pregnancy.
To ensure adequate sampling of introverts and extraverts, a 2-step screening and assignment procedure was conducted. First, all participants were prescreened using the NEO Personality Inventory Revised (NEO-PI-R)30 at their initial screening visit. To be eligible for the study, participants had to score below 45 (i.e., introverted) or above 55 (i.e., extraverted) on the Introversion/Extraversion scale of the NEO-PI-R. These values represent cutoffs of 0.5 standard deviations below or above the mean normative scores for each individual's age group. Thus, to be initially included in the study, participants had to score in the top or bottom 31% of the normative distribution of introversion or extraversion.
Though the NEO-PI-R was used for screening purposes (because it has standardized norms and cutoffs for Extravert and Introvert categorization), final assignment to the Introverted or Extraverted group was made on the day of arrival for the study. We used the Eysenck Personality Questionnaire-Revised31 for this classification to be consistent with the Eysenck personality theory on which the study hypotheses were based.22,23 Studies have shown substantial to high correlations between instruments for extraversion.32,33 Participants were administered the EPQ-R at approximately 19:00 on the arrival night. A median split was performed on the Extraversion scale scores to classify volunteers as introverts (score < 16; n = 25) or extraverts (score > 15, n = 23).
Upon arrival, volunteers were randomly assigned to either the Socially Enriched (SE) or Socially Impoverished (SI) waking condition (described below) (n = 24 per group). Thirteen introverts (mean age [SD] = 25.2 [6.9], 9 men) and 11 extraverts (mean age [SD] = 24.4 [6.2], 5 men) were assigned to the SE group; 12 introverts (mean age [SD] = 24.1 [6.0], 7 men) and 12 extraverts (mean age [SD] = 25.0 [5.3], 8 men) were assigned to the SI group. Waking conditions were heterogeneous for personality type (i.e., groups consisted of extraverts and introverts combined). Two introverts assigned to the SI condition dropped out during the impoverishment (i.e., social isolation) phase due to anxiety and headache, respectively, and were replaced by 2 subsequently recruited volunteers. Data from the withdrawn subjects were not included in any analyses.
Testing Facilities
During testing and sleep periods, each subject was housed individually in a private, sound-attenuated, 8′ × 10′ room that included a bed and computer workstation. Ambient temperature was approximately 23°C, and lighting was approximately 500 lux (with lights off during sleep periods). Background white noise was 60 dB at all times. Outside of the 12-hour SI period, when not engaged in testing or sleep, participants remained in a common living area to play games, eat, read, or watch television and movies. Participants were monitored continuously by at least 1 laboratory technician; during the 12-hour SE period, 4 technicians were present at all times. All volunteers were instructed by the principal investigator at the beginning of the study that discussion of how they were feeling (i.e., “sleepy”) or of testing or study procedures was not allowed and would be grounds for dismissal from the study.
Study Design and Procedure
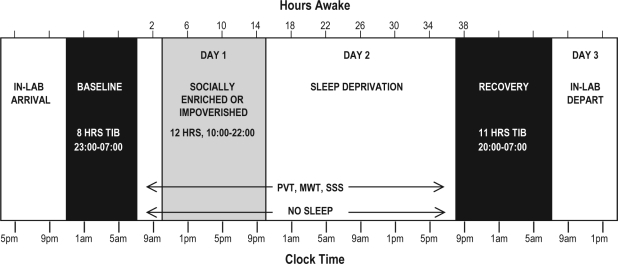
A schematic of the study design and procedures is shown in Figure 2. As shown in the figure, following 1 night of baseline sleep of 8 hours time in bed from 23:00 to 07:00, volunteers began hourly testing (alternating Psychomotor Vigilance Test [PVT] and Maintenance of Wakefulness Test [MWT] each hour, so that each was administered every 2 h) on Day 1. Social exposure was manipulated on Day 1 between 10:00 and 22:00 (SE or SI), and volunteers remained awake for a total of 36 hours ending on Day 2. (All volunteers were kept in the sleep-suite lounge area and monitored by a technician at all times during the sleep-deprivation period to ensure that they stayed awake.) Volunteers were given 11 hours of time in bed for recovery sleep. Testing occurred following the recovery night, but these test results are not included in the present paper. Further protocol details follow below. Study participants' conditions during testing were identical between conditions (i.e., at their desk alone in their bedrooms) across all study phases.
Figure 2.
Schematic of the general study design and procedures. Hours awake is on the top x-axis and clock time is on the bottom x-axis. Allocated time in bed is indicated by black shading on Baseline and Recovery nights. Gray shading indicates the social exposure period from 10:00 to 22:00 on Day 1. PVT, modified MWT and SSS testing occurred hourly throughout (PVT and SSS alternated every 2 hrs with MWT administration).
Social-Experience Conditions
Prior to arriving for the in-laboratory portion of the study, all volunteers participating in the same session were randomized or “blocked” together (i.e., assigned the same social-exposure condition). Members of the SE group were exposed to a 12-hour block (10:00–22:00) of controlled and structured group social activities beginning at 10:00 after awakening at 07:00 following a baseline night of 8 hours of time in bed. These activities included a preestablished series of interactive card and board games, group discussions, movies, and group projects, such as puzzles, along with hourly alertness and cognitive performance testing. SE volunteers were run in groups of no fewer than 2 and no more than 4 at a time and interacted socially with an additional 4 research technicians who kept them socially and interpersonally engaged for the entire 12-hour period. Technicians were instructed to continuously engage volunteers in social interaction, including casual and dyadic conversation, social banter, and eye contact throughout the 12-hour period. Volunteers in the SI group were exposed to a 12-hour block (10:00–22:00) of nearly identical activities matched for general physical activity level and cognitive load, but these activities were completed in relative isolation. SI volunteers were run in groups of 1 to 4 at a time, but, during the 12-hour experimental block, volunteers remained generally isolated in their private rooms and completed the same activities as the SE group (card games, movies, puzzle and game books, computerized tests) in the absence of other people. Instructions were presented to them verbally; requests for bathroom breaks, meals, or other necessary requests were made by pressing a call button, and only minimal interpersonal contact, as necessary, occurred during these times. No contact was permitted among the volunteers during the 12-hour block, and technicians were instructed to interact minimally with the volunteers. Except for the social-experience randomization, both groups were otherwise treated identically throughout the study. Technicians were blind to the specific hypotheses of the study.
MEASURES
The EPQ-R
This self-report questionnaire measures 3 dimensions of personality, including extraversion.31 The scale includes 100 items, and administration time is approximately 25 minutes.
Actigraphy
Wrist movements were recorded using wrist actigraphy (Actiwatch; Mini-Mitter, a Phillips Respironics Co., Bend, OR). Data were scored for total activity (sum of activity counts) during the social-experience period (SE or SI, 10:00–22:00).
Polysomnography
Polysomnographic measurements included electroencephalogram (C3 and C4), electrooculogram (outer canthus of each eye), and electromyogram (mental/submental). Contralateral mastoid leads served as references for all unipolar measurements (electroencephalography and electrooculography). Polysomnography data were scored by a trained research technician in accordance with Rechtschaffen and Kales criteria34 using Alice 4 Sleepware software (Respironics, Inc., Murraysville, PA). Dependent measures for nighttime sleep periods (defined as lights out to lights on) included minutes of individual stages (wake, 1, 2, SWS, and rapid eye movement) and total sleep time (sum of minutes spent in all sleep stages).
The PVT
A 5-minute version of the PVT was administered on a hand-held device.35 Participants performed the PVT beginning at 08:00 on Day 1 and every 2 hours thereafter during all waking periods (practice on the PVT occurred on the previous evening). PVT was analyzed for speed (1/reaction time ×1000) and transformed lapses (reaction times ≥ 500 msec, square root transform [SQR(Lapses)+SQR(Lapses+1)]).
Modified MWT
For the modified MWT, participants were escorted to their individual darkened sound-attenuated bedrooms and allowed to lie down on their beds. They were instructed to close their eyes and to try to remain awake. Polysomnography was monitored online. Participants were awakened after 3 consecutive epochs of stage 1 sleep or at the onset of stage 2 sleep. If participants did not fall asleep after 20 minutes, the test was terminated. The dependent measure for the MWT was latency to the first 30-second epoch of sleep. The modified MWT was administered every 2 hours during waking beginning at 09:20 on Day 1.
The SSS
Participants selected which of 7 statements best described their current state of alertness, ranging from “1–feeling active and vital; alert; wide awake” to “7–almost in reverie; sleep onset soon; losing struggle to remain awake” for the SSS. The dependent variable was the self-rated sleepiness score on a scale of 1 to 7.36 The SSS was administered every 2 hours during waking beginning at 08:00 on Day 1 (immediately prior to the PVT).
Analyses
Nighttime sleep
Nighttime polysomnography data was analyzed using a mixed-model analysis of variance (ANOVA) in SPSS Version 12.0 for PC (SPSS Inc., Chicago, IL). The model included fixed effects for Personality (Extraverts or Introverts), Social Condition (SE or SI), and Night (2 levels: Baseline and Recovery). Significant interactions were followed by posthoc t tests (Bonferroni correction). Greenhouse-Geisser corrections were applied to repeated-measures effects. Statistical significance was P < 0.05.
Waking activity; actigraphy
Waking total actigraphic activity during the SE or SI conditions was examined using mixed-model ANOVA with fixed effects for Personality (Introvert vs Extravert) and Social Condition (SE vs SI) from 10:00 to 22:00 during the social exposure. Total actigraphic activity was used as a covariate in all statistical analyses.
Performance and sleepiness
Social exposure:
Two-way mixed-model ANOVA was performed with EPQ Personality type (2 levels; median split, Introvert vs Extravert) × Social-Exposure condition (SE vs SI) as between-subjects factor and Social Exposure testing sessions (6 sessions over 12 hours during Social Exposure) as within-subjects factors for PVT, MWT, and SSS values occurring between 10:00 and 22:00 during the social exposure, with total actigraphic activity during this period and age as covariates. Significant main effects and interactions were assessed using posthoc t tests (Bonferroni corrected) to determine which sessions and/or personality types were significantly different. This analysis was done to determine if there were any differences between Personality (Extraverts/Introverts) and Social Conditions (SE or SI) prior to the sleep-deprivation period and during the social-exposure manipulation.
Sleep deprivation:
To examine if individuals exposed to SI environments across subsequent sleep deprivation were more effective at sustaining performance and alertness relative to volunteers exposed to SE environments and if the trait of introversion-extraversion interacted with social environment, a 2-way mixed-model ANOVA was performed with EPQ Personality type (2 levels; median split, Introvert vs Extravert) x Social-Exposure condition (SE vs SI), with mean PVT transformed lapses and speed, modified MWT sleep latency, and SSS as dependent variables. Total actigraphic activity during the SE and SI conditions and age were included as covariates for all analyses. Significant main effects and interactions were assessed using posthoc t tests (Bonferroni corrected) to determine which sessions and/or personality types were significantly different.
RESULTS
Nighttime Sleep, Polysomnography
Table 1 lists mean minutes of each polysomnography sleep variable as a function of Personality, Social Condition, and Day (Baseline or Recovery). The number of minutes of TST, Stage 2 sleep, and rapid eye movement sleep significantly increased from Baseline to Recovery (TST, F1,29 = 2383.85, P < 0.001, mean ± SEM Baseline = 439 ± 3, Recovery = 631 ± 3; Stage 2 sleep, F1,12 = 359.09, P < 0.001, mean ± SEM Baseline = 230 ± 6, Recovery = 333 ± 6; rapid eye movement sleep, F1,35 = 50.83, P < 0.001, mean ± SEM Baseline = 91 ± 5, Recovery = 137 ± 5) and the minutes of Wake significantly decreased from Baseline to Recovery (F1,29 = 4.82, P = 0.036, mean ± SEM Baseline = 33 ± 3, Recovery = 25 ± 3). When collapsed across Baseline and Recovery nights, Stage 1 sleep was greater overall for volunteers in the SE condition than for volunteers in the SI condition (F1,42 = 4.56, P = 0.039, mean ± SEM SE = 39 ± 17, SI = 29 ± 18) but did not differ as a function of the experimental manipulation. SWS was significantly greater in the SI versus the SE conditions on the Recovery night (condition x night, F1,36 = 4.6, P = 0.038, mean ± SEM SE = 120 ± 15, SI = 181 ± 14). No other sleep or wake variable showed any main effects or interactions with social condition or personality.
Table 1.
Minutes of sleep during each sleep stage, TST, and wake for socially enriched extraverts, socially enriched introverts, socially impoverished extraverts, and socially impoverished introverts during baseline and recovery nights
| Variable | Socially Enriched |
Socially Impoverished |
||||||
|---|---|---|---|---|---|---|---|---|
| Extravert |
Introvert |
Extravert |
Introvert |
|||||
| Baseline | Recovery | Baseline | Recovery | Baseline | Recovery | Baseline | Recovery | |
| TST | 438 ± 7 | 623 ± 6 | 434 ± 6 | 630 ± 7 | 436 ± 7 | 636 ± 6 | 448 ± 6 | 635 ± 6 |
| Sleep stage | ||||||||
| 1 | 40 ± 6 (9.1) | 40 ± 5 (6.4) | 37 ± 5 (8.5) | 41 ± 6 (6.5) | 34 ± 6 (7.8) | 26 ± 5 (4.1) | 31 ± 5 (6.9) | 28 ± 5 (4.4) |
| 2 | 236 ± 13 (53.9) | 321 ± 12 (51.5) | 224 ± 11 (51.6) | 349 ± 12 (55.4) | 234 ± 12 (53.7) | 337 ± 12 (53.0) | 226 ± 11 (50.5) | 328 ± 11 (51.7) |
| SWS | 86 ± 24 (19.6) | 127 ± 20 (20.4) | 91 ± 20 (21.0) | 112 ± 22 (17.8) | 86 ± 21 (19.7) | 172 ± 20 (27.0) | 88 ± 20 (19.6) | 190 ± 20 (29.9) |
| REM | 84 ± 12 (19.2) | 137 ± 10 (22.0) | 89 ± 10 (20.5) | 127 ± 11 (20.2) | 88 ± 11 (20.2) | 134 ± 10 (21.1) | 104 ± 10 (23.2) | 151 ± 10 (23.8) |
| Wake | 33 ± 7 (7.5) | 32 ± 6 (5.1) | 41 ± 5 (9.5) | 24 ± 6 (3.8) | 27 ± 6 (6.2) | 21 ± 6 (3.3) | 29 ± 6 (6.5) | 21 ± 5 (3.3) |
Data are presented as mean ± SEM with sleep-stage values as percentages of total sleep time (TST) in parentheses; SWS refers to slow-wave sleep; REM, rapid eye movement sleep.
Waking Activity, Actigraphy
Total actigraphic activity (sum of activity counts) during the SE and SI period (10:00–22:00) significantly differed between Social Conditions (F1,47 = 10.73, P = 0.002), with volunteers in the SE condition showing greater total activity (mean ± SD 113,568 ± 43,922), compared with the SI condition (mean ± SD = 81,646 ± 19,011. Total actigraphic activity did not differ between introverts and extraverts (P > 0.05).
Performance and Sleepiness
Social exposure
Results of mixed-model ANOVAs for PVT, modified MWT, and SSS testing sessions occurring between 10:00 and 22:00 are presented in Table 2. As reported in Table 2 and shown in Figure 3, there were no PVT performance differences between introverts and extraverts or between SE and SI conditions during social exposure. During the social-exposure period overall (with personality groups and conditions combined), PVT speed was slower at 14:00 and 18:00 than at 10:00 (main effect of session, P = 0.002).
Table 2.
Mixed-model ANOVA results for PVT speed, PVT lapses (transformed), modified MWT sleep latency, and SSS scores during social exposure
| Psychomotor Vigilance Test, speed | Num df | Den df | F value | P value |
|---|---|---|---|---|
| Intercept | 1 | 41 | 116.61 | 0.000 |
| Personality | 1 | 41 | 0.37 | 0.547 |
| Social Condition | 1 | 41 | 0.09 | 0.767 |
| Sessiona | 5 | 148 | 3.97 | 0.002 |
| Personality × Social Condition | 1 | 41 | 1.97 | 0.168 |
| Personality × Session | 5 | 148 | 0.79 | 0.556 |
| Social Condition × Session | 5 | 148 | 0.62 | 0.685 |
| Personality × Social Condition × Session | 5 | 148 | 1.38 | 0.235 |
| Total Activity | 1 | 41 | 1.02 | 0.319 |
| Age | 1 | 41 | 0.00 | 0.978 |
| Psychomotor Vigilance Test, lapses (transformed) | ||||
| Intercept | 1 | 41 | 12.45 | 0.001 |
| Personality | 1 | 41 | 0.11 | 0.741 |
| Social Condition | 1 | 41 | 0.76 | 0.389 |
| Session | 5 | 153 | 1.17 | 0.325 |
| Personality × Social Condition | 1 | 41 | 0.13 | 0.725 |
| Personality × Session | 5 | 153 | 2.08 | 0.071 |
| Social Condition × Session | 5 | 153 | 0.90 | 0.485 |
| Personality × Social Condition × Session | 5 | 153 | 1.40 | 0.227 |
| Total Activity | 1 | 41 | 1.19 | 0.282 |
| Age | 1 | 41 | 0.74 | 0.395 |
| Sleep latency on the modified Maintenance of Wakefulness Test | ||||
| Intercept | 1 | 41 | 9.51 | 0.004 |
| Personalitya | 1 | 42 | 8.80 | 0.005 |
| Social Condition | 1 | 42 | 0.00 | 0.983 |
| Sessiona | 5 | 168 | 2.92 | 0.015 |
| Personality × Social Condition | 1 | 42 | 0.01 | 0.929 |
| Personality × Session | 5 | 168 | 0.85 | 0.517 |
| Social Condition × Sessiona | 5 | 168 | 2.62 | 0.026 |
| Personality × Social Condition × Session | 5 | 168 | 0.97 | 0.440 |
| Total Activity | 1 | 41 | 2.66 | 0.111 |
| Age | 1 | 42 | 0.19 | 0.666 |
| Sleepiness according to the Stanford Sleepiness Scale | ||||
| Intercept | 1 | 50 | 21.81 | 0.000 |
| Personality | 1 | 50 | 0.12 | 0.729 |
| Social Condition | 1 | 50 | 1.12 | 0.294 |
| Session | 5 | 111 | 1.39 | 0.233 |
| Personality × Social Condition | 1 | 50 | 1.41 | 0.239 |
| Personality × Sessiona | 5 | 111 | 2.87 | 0.018 |
| Social Condition × Sessiona | 5 | 111 | 4.02 | 0.002 |
| Personality × Social Condition × Session | 5 | 111 | 0.84 | 0.522 |
| Total Activity | 1 | 50 | 0.24 | 0.625 |
| Age | 1 | 50 | 0.77 | 0.383 |
ANOVA refers to analysis of variance; PVT, Psychomotor Vigilance Test; MWT, Maintenance of Wakefulness Test; SSS, Stanford Sleepiness Scale; den df, degrees of freedom of the denominator; nom df, degrees of freedom of the numerator.
Figure 3.
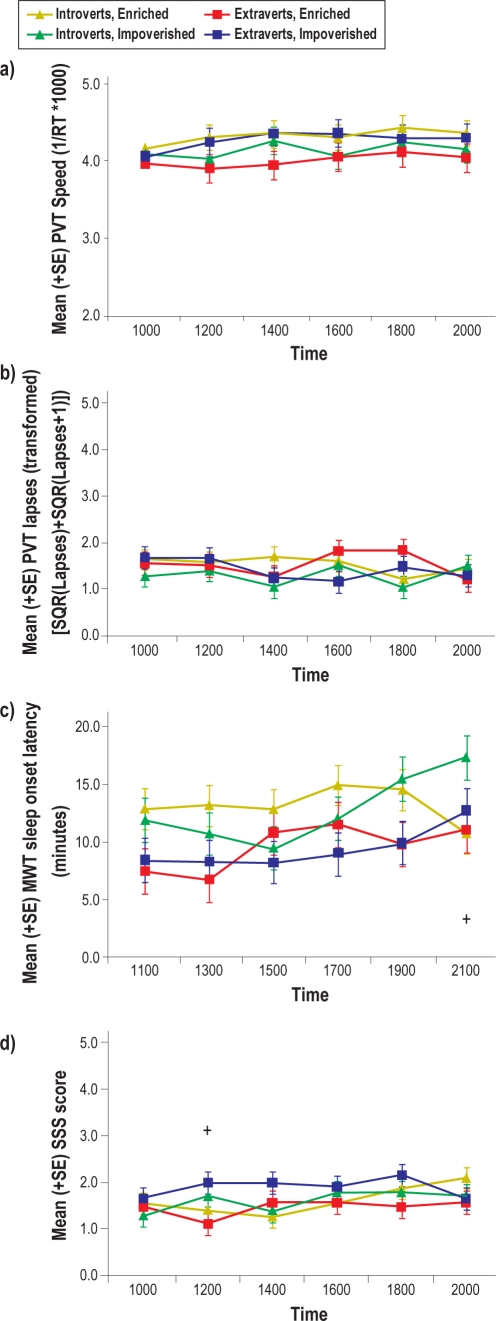
Mean (SE) scores for a) PVT speed, b) PVT lapses (transformed), c) MWT sleep latency, and d) SSS during social exposure. Plus signs indicate significant differences between Enriched versus Impoverished conditions.
During social exposure, introverts showed greater alertness than did extraverts on the modified MWT (main effect of Personality, P = 0.003). In addition, as revealed by the significant Social Condition × Session interaction (P = 0.005), and illustrated in Figure 3c, volunteers in the SI condition were more alert (i.e., longer sleep latency) at 21:00 than were volunteers in the SE condition (P = 0.028), and volunteers in the SI condition were significantly more alert at 21:00, compared with at 11:00, 13:00, and 15:00 (P = 0.045, P = 0.010, and P = 0.003, respectively). Posthoc analyses for the main effect of Session did not reveal any significant differences between sessions.
Finally, there were also significant differences between Personality type and Social Condition at certain testing sessions for SSS subjective sleepiness during social exposure (significant (P < 0.05) interactions of Social Condition × Session and Personality × Session), with extraverts reporting that they felt sleepier than did introverts at 14:00 (nonsignificant trend, P = 0.052). Also, volunteers in the SI condition reported feeling sleepier at 12:00 (P = 0.021), illustrated in Figure 3d.
Sleep deprivation
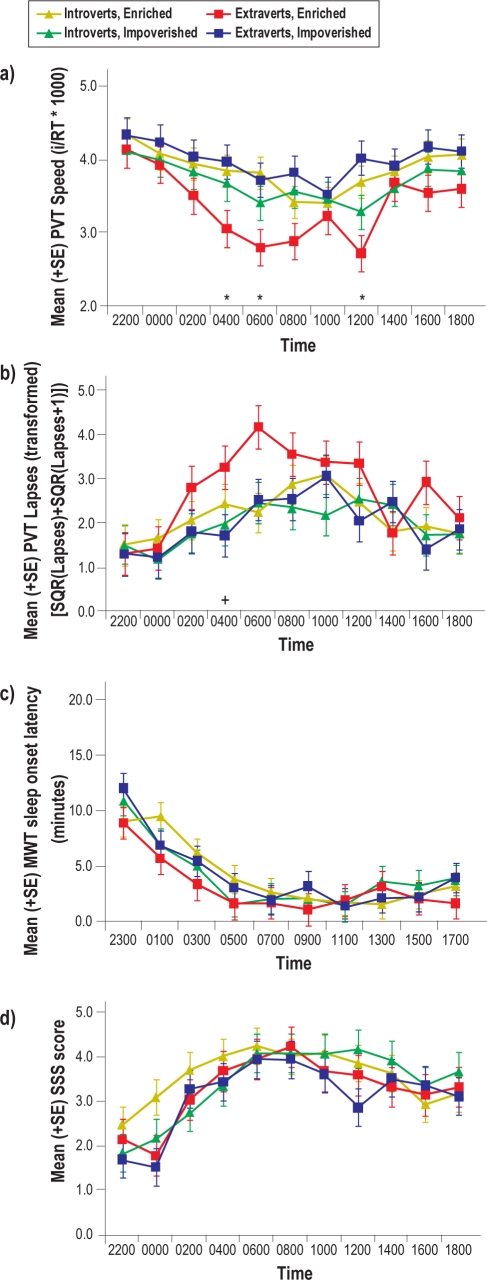
Mixed-model ANOVA results with Personality (extraversion/introversion) and Social Condition as between-subjects factors and with PVT lapses (transformed), PVT speed, modified MWT sleep latency, and SSS as the dependent variables are presented in Table 3. As shown in Figure 4a, PVT speed deteriorated across the night of sleep deprivation and was worse for extraverts in the SE condition than for extraverts in the SI condition, specifically at 04:00, 06:00, and 12:00. Those in the SE condition showed more PVT lapses (transformed), compared to those in the SI condition at 04:00 (Figure 4b). There were no significant main effects of Social Condition or Personality Type, nor were there significant interactions (P values > 0.05) involving these variables on MWT sleep latency (Figure 4c) and subjective sleepiness (SSS) (Figure 4d). There was a main effect of Session, revealing that sleep latency decreased and subjective sleepiness increased as time awake accrued (with some reversal following the 08:00 testing session for SSS).
Table 3.
Mixed-model ANOVA results for PVT speed, PVT lapses (transformed), modified MWT sleep latency, and SSS scores during sleep deprivation
| Psychomotor Vigilance Test, speed | Num df | Den df | F value | P value |
|---|---|---|---|---|
| Intercept | 1 | 41 | 72.00 | 0.000 |
| Personality | 1 | 42 | 0.28 | 0.602 |
| Social Condition | 1 | 41 | 1.25 | 0.271 |
| Sessiona | 10 | 279 | 11.50 | 0.000 |
| Personality × Social Conditiona | 1 | 42 | 4.62 | 0.038 |
| Personality × Session | 10 | 279 | 0.64 | 0.783 |
| Social Condition × Session | 10 | 279 | 1.50 | 0.139 |
| Personality × Social Condition × Sessiona | 10 | 279 | 2.53 | 0.006 |
| Total Activity | 1 | 41 | 1.52 | 0.224 |
| Age | 1 | 41 | 0.07 | 0.791 |
| Psychomotor Vigilance Test, lapses (transformed) | ||||
| Intercept | 1 | 42 | 7.00 | 0.012 |
| Personality | 1 | 42 | 0.79 | 0.380 |
| Social Condition | 1 | 42 | 1.66 | 0.205 |
| Sessiona | 10 | 285 | 8.18 | 0.000 |
| Personality × Social Condition | 1 | 42 | 0.75 | 0.391 |
| Personality × Session | 10 | 285 | 0.87 | 0.566 |
| Social Condition × Sessiona | 10 | 285 | 2.02 | 0.032 |
| Personality × Social Condition × Session | 10 | 285 | 1.34 | 0.208 |
| Total Activity | 1 | 42 | 0.98 | 0.328 |
| Age | 1 | 42 | 0.40 | 0.532 |
| Sleep latency on the modified Maintenance of Wakefulness Test | ||||
| Intercept | 1 | 41 | 11.12 | 0.002 |
| Personality | 1 | 41 | 0.89 | 0.350 |
| Social Condition | 1 | 41 | 0.63 | 0.433 |
| Sessiona | 10 | 247 | 21.67 | 0.000 |
| Personality × Social Condition | 1 | 41 | 0.45 | 0.506 |
| Personality × Session | 10 | 247 | 0.56 | 0.849 |
| Social Condition × Session | 10 | 247 | 0.74 | 0.687 |
| Personality × Social Condition × Session | 10 | 247 | 0.69 | 0.731 |
| Total Activity | 1 | 41 | 5.82 | 0.994 |
| Age | 1 | 41 | 1.33 | 0.256 |
| Sleepiness according to the Stanford Sleepiness Scale | ||||
| Intercept | 1 | 42 | 28.70 | 0.000 |
| Personality | 1 | 43 | 0.95 | 0.335 |
| Social Condition | 1 | 42 | 0.21 | 0.650 |
| Sessiona | 10 | 204 | 11.88 | 0.000 |
| Personality × Social Condition | 1 | 43 | 0.00 | 0.985 |
| Personality × Session | 10 | 204 | 1.48 | 0.149 |
| Social Condition × Session | 10 | 204 | 0.64 | 0.783 |
| Personality × Social Condition×Session | 10 | 204 | 0.82 | 0.615 |
| Total Activity | 1 | 42 | 0.07 | 0.790 |
| Age | 1 | 42 | 2.06 | 0.159 |
ANOVA refers to analysis of variance; PVT, Psychomotor Vigilance Test; MWT, Maintenance of Wakefulness Test; SSS, Stanford Sleepiness Scale; den df, degrees of freedom of the denominator; nom df, degrees of freedom of the numerator.
Figure 4.
Mean (SE) scores for a) PVT speed, b) PVT lapses (transformed), c) MWT sleep latency, and d) SSS during sleep deprivation. Asterisks indicate significant differences from post-hoc analyses between Extraverts in the Enriched versus Impoverished conditions. Plus signs indicate significant differences between Enriched versus Impoverished conditions overall.
DISCUSSION
The effects of waking social experience on vulnerability or resiliency to 1 night of sleep deprivation were mediated by individual differences in introversion and extraversion. Specifically, extraverts exposed to SE environments showed greater vulnerability to subsequent sleep deprivation (as measured by PVT speed performance) than did extraverts exposed to an identical but SI environment. The ability of introverts to resist sleep loss, on the other hand, was relatively unaffected by the social environment. Although the hypothesized main effects of social condition and personality were not confirmed, the significant interaction between these 2 factors tends to confirm our primary hypothesis that social experience does significantly affect the ability to resist subsequent sleep deprivation but that this effect is mediated by individual differences in introversion-extraversion, a personality trait that is believed to reflect general cerebral arousal level.22,23
It is possible that the greater responsiveness of extraverts to SE reflects use-dependent fatigue of local neuronal assemblies.5,7 SE experiences are hypothesized to place greater demands upon brain networks involved in social processing, self-reflection, and executive function, including the prefrontal cortex and other cerebral midline structures.25,37–41 All things being equal, SE exposure would be expected to bring about a general decline in subsequent performance relative to SI conditions. However, all things may not be equal—as it is well established that there are significant differences among individuals in their vulnerability to sleep deprivation13,14 that may be related to baseline levels of brain activation.11 According to Eysenck's Introversion-Extraversion theory,22,23 some individuals have consistently lower baseline levels of cerebral arousal than do others, a trait that tends to be associated with greater extraversion. Thus, the finding in the present study that social exposure had the greatest effect on extraverts fits well with predictions from Eysenck's Introversion-Extraversion model and theories of use-dependent fatigue on brain function.
Although differences between social-exposure conditions manifested during subsequent sleep deprivation, differences in PVT performance were not evident during the daytime social-experience manipulation, suggesting that these effects are only unmasked following prolonged wakefulness. In addition, volunteers in the SI condition reported feeling sleepier on subjective scales but showed significantly greater alertness on the MWT in the evening, compared to the SE group during the social exposure. Thus, although people may report anecdotally that talking and socializing helps them to stay awake, our data suggest that social interaction may improve subjective ratings of sleepiness but that this difference does not necessarily translate to improved performance or improved alertness on objective measures. One implication of these findings is that in occupational or “real-world” settings, individuals may underestimate their levels of sleepiness when operating in social situations, potentially placing them at risk for alertness-related accidents (e.g., gauging sleepiness level and risk while driving). Also noteworthy was the finding that introverts in this study were significantly more alert than extraverts during the daytime social-exposure period (based on modified sleep-latency scores). This greater level of alertness occurred prior to the period of extended wakefulness and, therefore, provides further support to Eysenck's Introversion-Extraversion theory22,23 and the longstanding body of work suggesting that introverts have a higher level of basal arousal, activation, and alertness than do extraverts.42,43–49 There were no significant differences in measured sleep parameters between extraverts and introverts on the baseline night, so these differences in alertness could not be attributed to sleep on the preceding night. Of note, 2 volunteers assigned to the SI condition dropped out of the study due to anxiety; it is possible that anxiety (as a condition-related mild stress) could have led to higher arousal states and sustained performance. However, measures of salivary cortisol were taken throughout the study (as markers of stress), and no differences were found between social conditions or personality typesInterestingly, subjects exposed to the SI condition demonstrated more SWS on the recovery night than did subjects exposed to the SE condition. This finding of less SWS during the recovery night after the SE condition was unexpected, as SWS (reflecting sleep homeostasis and thus sleep need) would be expected to be increased under the circumstances. It is unclear why this was so, but 1 possibility could involve the role of SWS in learning and memory.1,50 Specifically, it is possible that the SE condition may have provided greater levels of distraction that reduced initial encoding and depth of processing of the tasks in which subjects were engaged (i.e., multitasking). Consistent with this possibility, McCarley and colleagues found that simply engaging in conversation can significantly reduce encoding of visual-scene information.51 This and several other studies support the likelihood that dual-task processes may impair memory encoding.52 Subjects in the SI condition may have been less distracted by social stimuli and had greater opportunity to more deeply process and encode aspects of the tasks, leading to a greater need for SWS during recovery. At this point, this is purely speculation, but the question of how social exposure (or lack thereof) might interact with homeostatic sleep processes warrants further investigation.
Although we did not formulate hypotheses regarding time-of-day effects, it was noted that performance differences of extraverts following the SE versus the SI conditions were especially pronounced in the early morning hours, near the circadian trough of alertness. This suggests that circadian timing and influences on alertness may be an important factor influencing the position of individuals within the “optimal arousal” distribution (illustrated in Figure 1). The circadian dip in alertness in the early morning hours might serve as a tipping point by further exacerbating arousal degradation caused by sleep deprivation and previous social exposure. These findings are consistent with a number of studies suggesting a modest but significant relationship between evening chronotypes and the trait of extraversion.53–55 Thus, extraverts, who tend to show greater evening-type preferences, may also experience the impairing effects of social exposure more dramatically during the early morning hours than do introverts, who tend to show greater tendencies toward “morningness.”
Overall, the present results might also be interpreted more generally to suggest that waking experiences, along with their interaction with individual characteristics, influence vulnerability to subsequent sleep loss. Recent evidence suggests that waking experiences may impact subsequent sleep.56,57 Specifically, the synaptic homeostasis theory states that a function of sleep is to downscale synapses that are strengthened by waking experiences in order to maintain energy and space in the brain.56 These findings have been extended in a study of Drosophila in which the influence of daytime activities on subsequent sleep was investigated.9 Although the cited literature pertains specifically to waking experience affecting subsequent sleep, such findings might be extrapolated as follows: with sleep deprivation, there is no opportunity for synaptic downscaling. Because increased social experiences during waking promote increased synaptic strengthening,9 such experiences may result in faster fatigue in associated cortical columns, with an increased need for sleep manifested as relatively greater performance vulnerability.
In interpreting the present findings, it may be important to note that general physical activity level (as measured by actigraphy) significantly differed between SE and SI conditions (but not personality type) despite our efforts to match activities and activity level between conditions. A limitation of actigraphy, however, is that it does not reflect overall activity level, but wrist activity level only. In the SE environment containing continuous verbal exchanges, gesticulation accompanies verbal expression as part of nonverbal communication; wrist-actigraphy measurement may thus be biased, depending on social-exposure condition. Total activity was, however, included as a covariate for all statistical analyses to control for any effects of physical activity. Although total activity did not account for significant variance in any of the analyses, future studies would benefit from more rigorous controls for activity level. Another potential limitation of the study is that, although volunteers were continuously monitored by a technician to ensure wakefulness during sleep deprivation, they were not continuously electroencephalographically recorded.
Another point to consider is that the duration of continuous wakefulness in the present study was 36 hours. Our previous work showed differences between introverts and extraverts within the first 26 hours of extended wakefulness,21 but differences did not remain significant by the second or third day of sleep deprivation (perhaps due to ceiling effects). Because social experience was not manipulated, it remains unknown if the effect of social experience on vulnerability to the effects of sleep loss is modulated by personality during longer periods of total sleep deprivation (e.g., 48–72 h). Likewise, it is not known whether performance during chronic partial sleep restriction might similarly be affected by levels of extraversion, social exposure, or both. Results from our recent sleep-restriction study showed that greater extraversion was associated with greater decrements in PVT speed across 7 nights of sleep restricted to 3 hours per night.58 However, because investigation of personality was not the primary purpose of that study, the range of personality scores was limited, with the majority of participants scoring toward the extraverted end of the scale. Further studies of effects of personality on resilience during chronic sleep restriction will need to be performed.
These findings may have some direct design implications for sleep-loss studies in which volunteers are housed together and allowed to interact when not engaged in testing. In most sleep-deprivation or sleep-restriction studies, no attempt is made to control or measure the degree of social exposure of participants. Consequently, some inconsistencies among previous study outcomes may be partly explained by differences in social exposure and the interaction of social exposure with the personality traits of the volunteers. Our data suggest that housing conditions and personality differences may significantly contribute to observed performance effects during sleep loss and may warrant assessment and reporting in future sleep-deprivation studies.
In summary, results of the present study demonstrate that the effects of waking social experience interact with individual personality traits to influence vulnerability to subsequent sleep deprivation. SE impaired psychomotor vigilance performance (speed) during sleep deprivation for extraverts but not for introverts, and this effect was most prominent during the early morning (or late night) hours. These data have practical relevance for occupational shift work and military operational assignments (i.e., potential performance consequences of team vs independent work) and theoretical implications for understanding individual-difference factors influencing vulnerability or resiliency to sleep loss.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the US Army Medical Research and Materiel Command. This material has been reviewed by the Walter Reed Army Institute of Research, and there is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the position of the Department of the Army of the Department of Defense.
Footnotes
A commentary on this article appears in this issue on page 1433.
REFERENCES
- 1.Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6:553–4. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 2.Meerlo P, Pragt BJ, Daan S. Social stress induces high intensity sleep in rats. Neurosci Lett. 1997;225:41–4. doi: 10.1016/s0304-3940(97)00180-8. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro S, Shi X, Engelhard M, et al. Novel Experience Induces Persistent Sleep-Dependent Plasticity in the Cortex but not in the Hippocampus. Front Neurosci. 2007;1:43–55. doi: 10.3389/neuro.01.1.1.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33:517–29. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krueger JM, Obal F, Jr, Fang J. Why we sleep: a theoretical view of sleep function. Sleep Med Rev. 1999;3:119–29. doi: 10.1016/s1087-0792(99)90019-9. [DOI] [PubMed] [Google Scholar]
- 6.Rector DM, Schei JL, Van Dongen HP, Belenky G, Krueger JM. Physiological markers of local sleep. Eur J Neurosci. 2009;29:1771–8. doi: 10.1111/j.1460-9568.2009.06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy S, Krueger JM, Rector DM, Wan Y. A network model for activity-dependent sleep regulation. J Theor Biol. 2008;253:462–8. doi: 10.1016/j.jtbi.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–81. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 9.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324:105–8. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–12. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell JA, Mu Q, Smith JK, et al. Are individual differences in fatigue vulnerability related to baseline differences in cortical activation? Behav Neurosci. 2005;119:694–707. doi: 10.1037/0735-7044.119.3.694. [DOI] [PubMed] [Google Scholar]
- 12.Tucker AM, Dinges DF, Van Dongen HP. Trait interindividual differences in the sleep physiology of healthy young adults. J Sleep Res. 2007;16:170–80. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 13.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27(3):423–33. [PubMed] [Google Scholar]
- 14.Van Dongen HP, Vitellaro KM, Dinges DF. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28:479–96. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 15.Landolt HP. Genotype-dependent differences in sleep, vigilance, and response to stimulants. Curr Pharm Des. 2008;14:3396–407. doi: 10.2174/138161208786549344. [DOI] [PubMed] [Google Scholar]
- 16.Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2009 doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Groeger JA, Viola AU, Lo JC, von Schantz M, Archer SN, Dijk DJ. Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism. Sleep. 2008;31:1159–67. [PMC free article] [PubMed] [Google Scholar]
- 18.Vandewalle G, Archer SN, Wuillaume C, et al. Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype. J Neurosci. 2009;29:7948–56. doi: 10.1523/JNEUROSCI.0229-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killgore WD, Grugle NL, Reichardt RM, Killgore DB, Balkin TJ. Executive functions and the ability to sustain vigilance during sleep loss. Aviat Space Environ Med. 2009;80:81–7. doi: 10.3357/asem.2396.2009. [DOI] [PubMed] [Google Scholar]
- 20.Killgore WD, McBride SA, Killgore DB, Balkin TJ, Kamimori GH. Baseline odor identification ability predicts degradation of psychomotor vigilance during 77 hours of sleep deprivation. Int J Neurosci. 2008;118:1207–25. doi: 10.1080/00207450801941368. [DOI] [PubMed] [Google Scholar]
- 21.Killgore WD, Richards JM, Killgore DB, Kamimori GH, Balkin TJ. The trait of Introversion-Extraversion predicts vulnerability to sleep deprivation. J Sleep Res. 2007;16:354–63. doi: 10.1111/j.1365-2869.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- 22.Eysenck HJ. The biological basis of personality. Springfield, IL: Charles C. Thomas; 1967. [Google Scholar]
- 23.Eysenck HJ. A model for personality. Berlin: Springer; 1981. General features of the model; pp. 1–37. [Google Scholar]
- 24.Johnson DL, Wiebe JS, Gold SM, et al. Cerebral blood flow and personality: a positron emission tomography study. Am J Psychiatry. 1999;156:252–7. doi: 10.1176/ajp.156.2.252. [DOI] [PubMed] [Google Scholar]
- 25.Taylor DJ, McFatter RM. Cognitive performance after sleep deprivation: Does personality make a difference? Personality and Individual Differences. 2003;34:1179–93. [Google Scholar]
- 26.Spielberger CD, Vagg PR. Psychometric properties of the STAI: a reply to Ramanaiah, Franzen, and Schill. J Pers Assess. 1984;48:95–7. doi: 10.1207/s15327752jpa4801_16. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–671. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA. Manual for the Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- 29.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- 30.Costa PT, Jr., McCrae RR. Odessa, FL: Psychological Assessment Resources, Inc.; 1992. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) professional manual. [Google Scholar]
- 31.Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality and Individual Differences. 1985;6:21–9. [Google Scholar]
- 32.Costa PT, McCrae RR. Primary traits of Eysenck's P-E-N system: Three- and five-factor solutions. Journal of Personality and Social Psychology. 1995;69:308–17. doi: 10.1037//0022-3514.69.2.308. [DOI] [PubMed] [Google Scholar]
- 33.Barelds DPH, Luteijn F. Measuring personality: a comparison of three personality questionnaires in the Netherlands. Personality and Individual Differences. 2002;33:499–510. [Google Scholar]
- 34.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systemfor sleep stages in human subjects. US Government Printing Office; 1968. [Google Scholar]
- 35.Thorne DR, Johnson DE, Redmond DP, Sing HC, Belenky G, Shapiro JM. The Walter Reed palm-held psychomotor vigilance test. Behavior Research Methods. 2005;37:111–8. doi: 10.3758/bf03206404. [DOI] [PubMed] [Google Scholar]
- 36.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement W. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 37.Decety J, Chaminade T. Neural correlates of feeling sympathy. Neuropsychologia. 2003;41:127–38. doi: 10.1016/s0028-3932(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 38.Jenkins AC, Mitchell JP. Mentalizing under uncertainty: dissociated neural responses to ambiguous and unambiguous mental stateinferences. Cereb Cortex. 2010;20:404–10. doi: 10.1093/cercor/bhp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak AK, Hu ZG, Zhang JX, Xiao ZW, Lee TM. Neural correlates of regulation of positive and negative emotions: an fmri study. Neurosci Lett. 2009;457:101–6. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- 40.Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc Neurosci. 2009;4:443–54. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- 41.Wolf I, Dziobek I, Heekeren HR. Neural correlates of social cognition in naturalistic settings: a model-free analysis approach. Neuroimage. 2010;49:894–904. doi: 10.1016/j.neuroimage.2009.08.060. [DOI] [PubMed] [Google Scholar]
- 42.Beauducel A, Brocke B, Leue A. Energetical bases of extraversion: effort, arousal, EEG, and performance. Int J Psychophysiol. 2006;62:212–23. doi: 10.1016/j.ijpsycho.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Fink A. Event-related desynchronization in the EEG during emotional and cognitive information processing: differential effects of extraversion. Biol Psychol. 2005;70:152–60. doi: 10.1016/j.biopsycho.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Kumari V, ffytche DH, Williams SC, Gray JA. Personality predicts brain responses to cognitive demands. J Neurosci. 2004;24:10636–41. doi: 10.1523/JNEUROSCI.3206-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieberman MD, Rosenthal R. Why introverts can't always tell who likes them: multitasking and nonverbal decoding. J Pers Soc Psychol. 2001;80:294–310. doi: 10.1037/0022-3514.80.2.294. [DOI] [PubMed] [Google Scholar]
- 46.Pearson GL, Freeman FG. Effects of extraversion and mental arithmetic on heart-rate reactivity. Percept Mot Skills. 1991;72:1239–48. doi: 10.2466/pms.1991.72.3c.1239. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt A, Beauducel A, Brocke B, Strobel A. Vigilance performance and extraversion reconsidered: some performance differences can indeed be induced. Personality and Individual Differences. 2004;36:1343–51. [Google Scholar]
- 48.Smith BD, Kline R, Lindgren K, Ferro M, Smith DA, Nespor A. The lateralized processing of affect in emotionally labile extraverts and introverts: central and autonomic effects. Biol Psychol. 1995;39:143–57. doi: 10.1016/0301-0511(94)00968-4. [DOI] [PubMed] [Google Scholar]
- 49.Smulders FTY, Meijer EH. Extraversion and performance: a cognitive-energetical approach. Personality and Individual Differences. 2008;44:475–86. [Google Scholar]
- 50.Ramadan W, Eschenko O, Sara SJ. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PLoS One. 2009;4:e6697. doi: 10.1371/journal.pone.0006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCarley JS, Vais MJ, Pringle H, Kramer AF, Irwin DE, Strayer DL. Conversation disrupts change detection in complex traffic scenes. Hum Factors. 2004;46:424–36. doi: 10.1518/hfes.46.3.424.50394. [DOI] [PubMed] [Google Scholar]
- 52.Gold DA, Park NW. The effects of dividing attention on the encoding and performance of novel naturalistic actions. Psychol Res. 2009;73:336–49. doi: 10.1007/s00426-008-0148-4. [DOI] [PubMed] [Google Scholar]
- 53.Adan A, Almirall H. The influence of age, work schedule and personality on morningness dimension. Int J Psychophysiol. 1992;12:95–9. doi: 10.1016/0167-8760(92)90001-r. [DOI] [PubMed] [Google Scholar]
- 54.Mecacci L, Zani A, Rocchetti G, Lucioli R. The relationship between morningness-eveningness, ageing and personality. Personality and Individual Differences. 1986;7:911–3. [Google Scholar]
- 55.Mitchell PJ, Redman JR. The relationship between morningness-eveningness, personality and habitual caffeine consumption. Personality and Individual Differences. 1993;15:105–8. [Google Scholar]
- 56.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6(8):e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Smith K, Reid C, Killgore WD, Rupp TL, Balkin TJ. Personality factors associated with performance and sleepiness during sleep restriction and recovery. Sleep. 2008;31:A376. [Google Scholar]