Abstract
The International Prognostic Index and the Follicular Lymphoma International Prognostic Index are widely used for the risk assessment of follicular lymphoma (FL). Although molecular studies have provided insight into the biology of FL, no molecular marker has impacted on treatment stratification. Because TP53 mutations are associated with poor prognosis in hematologic malignancies, we investigated the prognostic value of TP53 mutation at diagnosis in FL. Heterozygous TP53 mutation was detected in 12 of 185 (6%) analyzed cases. Mutation was associated with older age (P = .02) and higher International Prognostic Index score (P = .04). On multivariate analysis, TP53 mutation correlated with shorter progression-free survival (P < .001) and overall survival (P = .009). TP53 mutation was associated with low expression of the immune-response 1 gene expression signature (P = .016) and with an unfavorable gene expression-based survival predictor score (P < .001), demonstrating for the first time that molecular features of the malignant cell may correlate with the nature of the immune response in FL.
Introduction
Follicular lymphoma (FL) is characterized by episodes of progression alternating with periods of remission and is associated with a median survival of 8 to 10 years.1,2 However, a proportion of patients die within the first 2 years; furthermore, histologic transformation may occur, dramatically reducing overall survival (OS).3,4 Despite the introduction of immunochemotherapy, which has improved outcome,5–7 the management of high-risk patients remains challenging. The International Prognostic Index (IPI) and the Follicular Lymphoma International Prognostic Index are widely used for risk assessment in FL,8 the latter retaining its predictive capacity with the current use of upfront immunochemotherapy.9 Newer molecular studies have provided insight into the biology of FL, and as yet no molecular markers have impacted on treatment stratification. Gene expression profiling studies have identified 2 prognostic signatures, immune-response 1 (IR1) and immune-response 2 (IR2), both based on nonmalignant tumor-infiltrating cells.10 The IR1 signature is a molecular correlate of a T cell–rich tumor microenvironment, whereas the IR2 signature reflects a microenvironment enriched in myeloid-lineage cells. A survival predictor score was formed from these expression signatures, high values of which indicated enrichment for the IR2 signature and unfavorable OS, which has been confirmed recently.11
Mutations in TP53 are frequent in cancer12 and hematologic malignancies where they correlate with unfavorable prognosis and chemotherapy resistance.13–15 TP53 mutation has been reported in 10% to 20% of various histologic subtypes of non-Hodgkin lymphoma.16 In FL TP53 mutation occurs infrequently at diagnosis and usually in association with transformation.17–20 We therefore set out to clarify the role of TP53 mutation in a large series of previously untreated FL patients and assess its impact on patient prognosis and clinical outcome.
Methods
Patient information
DNA from 191 untreated patients with FL presenting between 1974 and 2001 was obtained through the Lymphoma/Leukemia Molecular Profiling Project; 185 cases were analyzed for TP53 mutation. These samples were chosen because they were fully characterized molecularly.10 Clinical data were available in 172 cases. Approval to use clinical material for mutation analysis was obtained from the London Research Ethics Committee and East London and the City London Research Ethics Committee, and their stipulations regarding patient consent, confidentiality, and data protection were followed. The ethics submission covering this project is 06/Q0605/69. This study was conducted in accordance with the Declaration of Helsinki.
TP53 mutation detection
Because our previous data20 showed poor correlation between TP53 protein status by immunohistochemistry and mutation status, and in keeping with the recommendation from the International Agency for Research on Cancer (IARC) TP53 database,21 we screened genomic DNA samples for DNA sequence variants using high-resolution melting curve analysis followed by bidirectional sequencing. Primers were designed to amplify the coding sequence and flanking 3′ and 5′ splice sites of exons 5 to 8 of TP53 using Primer322 (primers and conditions are available on request). Analysis was restricted to these exons as they harbor 94.2% of all somatic mutations in the most recent IARC database.23 Melting profiles of the polymerase chain reaction products were determined using DHPLC Melt program (Genome Technology Center, Stanford University, Stanford, CA; http://insertion.stanford.edu/melt.html). Melting curve analysis was carried out using a HR96 LightScanner and data collected and normalized for fluorescence and temperature shift using LightScanner software (Idaho Technology, Salt Lake City, UT). All samples with melting profiles different from wild-type control samples were bidirectionally sequenced, using the primers (earlier in same paragraph) and the Big Dye Terminator kit on the Applied Biosystems 3730 Genetic Analyser (Applied Biosystems, Foster City, CA). Data were analyzed by visual inspection of electropherograms and Mutation Surveyor software (SoftGenetics, State College, PA). Single nucleotide polymorphism array analysis and conventional comparative genomic hybridization were used to assess the frequency of del 17p in this cohort.
Statistical analysis
Association with clinical characteristics was investigated by Fisher exact test for (not ordered) categorical data, t test for normally distributed continuous data, and Mann-Whitney U test for ordered data. OS was defined as the time from diagnosis to death, or for patients remaining alive, the time from diagnosis to last contact. Progression-free survival (PFS) was defined as the time from diagnosis to first progression, transformation or death from any cause, or for patients remaining alive and disease free, the time from diagnosis to last contact. Transformation was defined histologically (n = 27) or clinically (n = 12) with signs including: rapid nodal or extranodal growth, unusual extranodal sites, or sudden rise in lactic dehydrogenase. Kaplan-Meier survival estimates were obtained and the log-rank test used to compare differences between the mutated and wild-type subgroups. Multivariate Cox regression was used to determine whether mutation remained independently predictive of PFS and OS after adjusting for clinical variables. Significance was set at P less than .05. Gene expression correlation was performed as previously described.10
Results and discussion
TP53 mutation was detected in 12 of 185 (6%) cases of diagnostic FL. All were heterozygous. Eleven mutations were missense (exon 5 (1), exon 7 (5), and exon 8 (5)), and a single mutation arose in the splice site of intron 7 (Table 1). All mutations were previously reported in the IARC TP53 mutation database.12 Single nucleotide polymorphism and comparative genomic hybridization analysis showed 5 cases with loss of heterozygosity involving the TP53 locus (data not shown), suggesting that del 17p is a rare event at diagnosis.
Table 1.
Analysis of TP53 mutations in FL and correlation with tumor stage and clinical outcome
| Patient no. | Age at diagnosis, y | Genomic no.* | Predicted amino acid no.† | Location | Predicted effect‡ | Predicted TP53 function‡ | Disease stage at presentation | Initial treatment | PFS, mo | OS, y |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | g.13216 T>A | p.H179Q | Exon 5 | Missense | Nonfunctional | IIe | Single-agent chemotherapy | 10 | 7.7 |
| 2 | Not known | g.14028 A>G | p.Y234C | Exon 7 | Missense | Nonfunctional | Not known | Multiagent chemotherapy | 53 | 4.5 |
| 3 | 47 | g.14057 G>A | p.G244S | Exon 7 | Missense | Nonfunctional | III | Multiagent chemotherapy radiotherapy | 11 | 5.0 |
| 4 | 71 | g.14060 G>A | p.G245S | Exon 7 | Missense | Nonfunctional | II | Radiotherapy | 21 | 11.9 |
| 5 | 64 | g.14060 G>T | p.G245C | Exon 7 | Missense | Nonfunctional | IV | Expectant management | 24 | 9.8 |
| 6 | 59 | g.14061 G>T | p.G245V | Exon 7 | Missense | Nonfunctional | IV | Multiagent chemotherapy | 4 | 0.5 |
| 7 | 65 | g.14111 T>G | NA | Intron 7 | Splice site | Splice | III | Multiagent chemotherapy | 11 | 0.9 |
| 8 | 78 | g.14486 C>T | p.R273C | Exon 8 | Missense | Nonfunctional | III | Multiagent chemotherapy | 12 | 7.7 |
| 9 | 53 | g.14487 G>A | p.R273H | Exon 8 | Missense | Nonfunctional | III | Radiotherapy | 25 | 4.4 |
| 10 | 63 | g.14487 G>A | p.R273H | Exon 8 | Missense | Nonfunctional | II | Multiagent chemotherapy | 16 | 2.3 |
| 11 | 63 | g.14513 C>T | p.R282W | Exon 8 | Missense | Nonfunctional | IV | Multiagent chemotherapy | 15 | 2.0 |
| 12 | 75 | g.14517 G>A | p.R283H | Exon 8 | Missense | Functional | IV | Multiagent chemotherapy | 15 | 1.3 |
PFS indicates progression-free survival; OS, overall survival; and NA, not applicable.
Genomic numbers refer to reference sequence U94788.
Predicted amino acid numbers refer to a translation of NM000546.
Predicted structural and functional characteristics of the mutations were obtained from the mutation validation tool of the IARC TP53 Mutation Database.
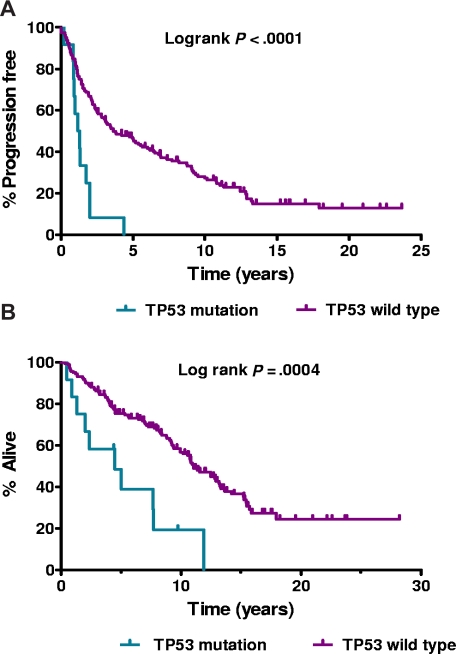
The major clinical characteristics of the 172 patients are summarized in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). TP53 mutation occurred more frequently in older patients (P = .02) and those with higher IPI (P = .04). Mutated patients were treated heterogeneously (Table 1); and although most responded well, responses were frequently of short duration (median, 15 months; range, 4-53 months). This resulted in inferior PFS (log-rank, P < .001) and OS (log-rank, P < .001) for patients with mutation (Figure 1A and 1B, respectively).
Figure 1.
Survival. (A) Progression-free survival by TP53 mutational status. (B) Overall survival by TP53 mutational status.
Multivariate analyses confirmed that TP53 mutation was predictive for shorter PFS when adjusted for IPI (hazard ratio [HR] = 3.6; 95% confidence interval [CI], 1.8-7.2, P < .001), or for age and stage (HR = 3.4; 95% CI, 1.7-6.7, P < .001). However, the effect of TP53 mutation on OS was significant only after adjustment for IPI (HR = 2.7; 95% CI, 1.3-5.6, P = .009) but not when adjusted for age and stage. Transformation data were available on 142 cases. There was no association seen between TP53 mutation and transformation in this group; 3 of 11 patients with TP53 mutation transformed in contrast to 36 of 131 with normal TP53 status (Table S1; P = 1.0).
TP53 mutation was associated with low expression of the IR1 gene expression signature (P = .016), but not with the IR2 signature (P = .53). The survival predictor score formed from these 2 signatures, higher values of which are associated with an unfavorable prognosis, was associated with TP53 mutation (P = .001); 11 of 12 mutated cases were within the upper 51% of the survival predictor scores. Incorporating TP53 status into the IR1/IR2-based survival model did not, however, lead to an improved predictor (P = .21), perhaps reflecting the strong association of TP53 mutation status with the survival predictor score. These findings provide the first suggestion that molecular features of the malignant cell may correlate with the host immune response, as reflected by the IR1 and IR2 signatures in keeping with the complex interaction between the tumor and its microenvironment in FL.10
This group of patients was treated heterogeneously; because TP53 mutation is associated with high-risk disease, this raises the issue of whether these patients should receive more aggressive therapy. Because none of the mutated cases received upfront chemo-immunotherapy, it remains to be determined whether rituximab will improve survival in this group. Patients with chronic lymphocytic leukemia and TP53 abnormalities have not seen improvements in survival with rituximab.24 Therapeutic strategies could include alemtuzumab or lenalidomide, which have demonstrated activity in chronic lymphocytic leukemia patients with TP53 dysfunction. Although TP53 mutation is more frequent in older patients, 4 of 12 mutated cases here were younger than 60 years, and nonmyeloablative allogeneic stem cell transplantation may be an option. Radiotherapy would not be predicted to prolong responses,25 as the majority of mutations observed here were nonfunctional (Table 1).
These results suggest that TP53 screening could be incorporated into the design of clinical trials in FL. If mutation is validated as a high-risk genetic marker, investigational treatments could be considered in this group of patients.
Acknowledgments
The work was supported by grants from Cancer Research UK, the Medical Research Council, and NIH SPEC grant 5U01CA114778. D.O. is supported by a Medical Research Council Clinical Research Fellow grant. C.O. is a Cancer Research UK Barts-Cambridge Molecular Pathology Clinical Research Fellow.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.O. designed the study, analyzed data, and wrote the paper; D.O., C.O., and C.T. performed research; R.W., F.M., E.C., J.G., and G.W. analyzed data; A.R., G.O., L.M.R., E.B.S., N.J., E.C., T.C.G., W.C.C., and R.D.G. collected data: and L.M.S., T.A.L., and J.F. designed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Derville O'Shea, Centre for Medical Oncology, Barts and the London School of Medicine, Charterhouse Square, London EC1M6BQ, United Kingdom; e-mail: derville.oshea@cancer.org.uk.
References
- 1.Gallagher CJ, Gregory WM, Jones AE, et al. Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol. 1986;4:1470–1480. doi: 10.1200/JCO.1986.4.10.1470. [DOI] [PubMed] [Google Scholar]
- 2.Horning SJ. Natural history of and therapy for the indolent non-Hodgkin's lymphomas. Semin Oncol. 1993;20:75–88. [PubMed] [Google Scholar]
- 3.Acker B, Hoppe RT, Colby TV, Cox RS, Kaplan HS, Rosenberg SA. Histologic conversion in the non-Hodgkin's lymphomas. J Clin Oncol. 1983;1:11–16. doi: 10.1200/JCO.1983.1.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Bastion Y, Sebban C, Berger F, et al. Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J Clin Oncol. 1997;15:1587–1594. doi: 10.1200/JCO.1997.15.4.1587. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Fayad L, Cabanillas F, et al. Improvement of overall and failure-free survival in stage IV follicular lymphoma: 25 years of treatment experience at the University of Texas M.D. Anderson Cancer Center. J Clin Oncol. 2006;24:1582–1589. doi: 10.1200/JCO.2005.03.3696. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 7.Sacchi S, Pozzi S, Marcheselli L, et al. Introduction of rituximab in front-line and salvage therapies has improved outcome of advanced-stage follicular lymphoma patients. Cancer. 2007;109:2077–2082. doi: 10.1002/cncr.22649. [DOI] [PubMed] [Google Scholar]
- 8.Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 9.Buske C, Hoster E, Dreyling M, Hasford J, Unterhalt M, Hiddemann W. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood. 2006;108:1504–1508. doi: 10.1182/blood-2006-01-013367. [DOI] [PubMed] [Google Scholar]
- 10.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 11.Byers RJ, Sakhinia E, Joseph P, et al. Clinical quantitation of immune signature in follicular lymphoma by RT-PCR-based gene expression profiling. Blood. 2008;111:4764–4770. doi: 10.1182/blood-2007-10-115915. [DOI] [PubMed] [Google Scholar]
- 12.Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 13.Prokocimer M, Rotter V. Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood. 1994;84:2391–2411. [PubMed] [Google Scholar]
- 14.Preudhomme C, Fenaux P. The clinical significance of mutations of the P53 tumour suppressor gene in haematological malignancies. Br J Haematol. 1997;98:502–511. doi: 10.1046/j.1365-2141.1997.2403057.x. [DOI] [PubMed] [Google Scholar]
- 15.Krug U, Ganser A, Koeffler HP. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene. 2002;21:3475–3495. doi: 10.1038/sj.onc.1205322. [DOI] [PubMed] [Google Scholar]
- 16.Koduru PR, Raju K, Vadmal V, et al. Correlation between mutation in P53, p53 expression, cytogenetics, histologic type, and survival in patients with B-cell non-Hodgkin's lymphoma. Blood. 1997;90:4078–4091. [PubMed] [Google Scholar]
- 17.Sander CA, Yano T, Clark HM, et al. p53 mutation is associated with progression in follicular lymphomas. Blood. 1993;82:1994–2004. [PubMed] [Google Scholar]
- 18.Lo Coco F, Gaidano G, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. p53 mutations are associated with histologic transformation of follicular lymphoma. Blood. 1993;82:2289–2295. [PubMed] [Google Scholar]
- 19.Symmans WF, Katz RL, Ordonez NG, Dalton H, Romaguera JE, Cabanillas F. Transformation of follicular lymphoma: expression of p53 and bcl-2 oncoprotein, apoptosis and cell proliferation. Acta Cytol. 1995;39:673–682. [PubMed] [Google Scholar]
- 20.Davies AJ, Lee AM, Taylor C, et al. A limited role for TP53 mutation in the transformation of follicular lymphoma to diffuse large B-cell lymphoma. Leukemia. 2005;19:1459–1465. doi: 10.1038/sj.leu.2403802. [DOI] [PubMed] [Google Scholar]
- 21.Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001;1:233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- 22.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 23.Petitjean A, Mathe E, Kato S, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 24.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24:437–443. doi: 10.1200/JCO.2005.03.1021. [DOI] [PubMed] [Google Scholar]
- 25.Knoops L, Haas R, de Kemp S, et al. In vivo p53 response and immune reaction underlie highly effective low-dose radiotherapy in follicular lymphoma. Blood. 2007;110:1116–1122. doi: 10.1182/blood-2007-01-067579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.