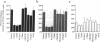Abstract
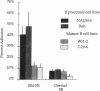
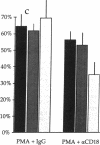
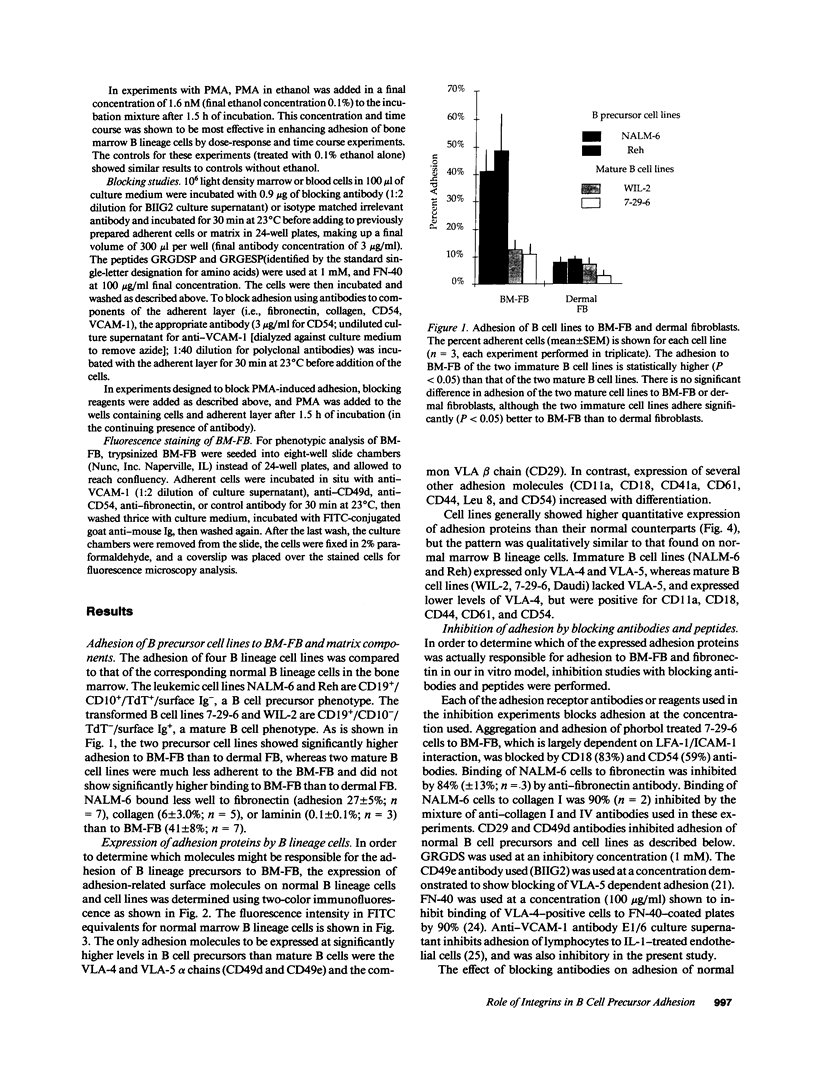
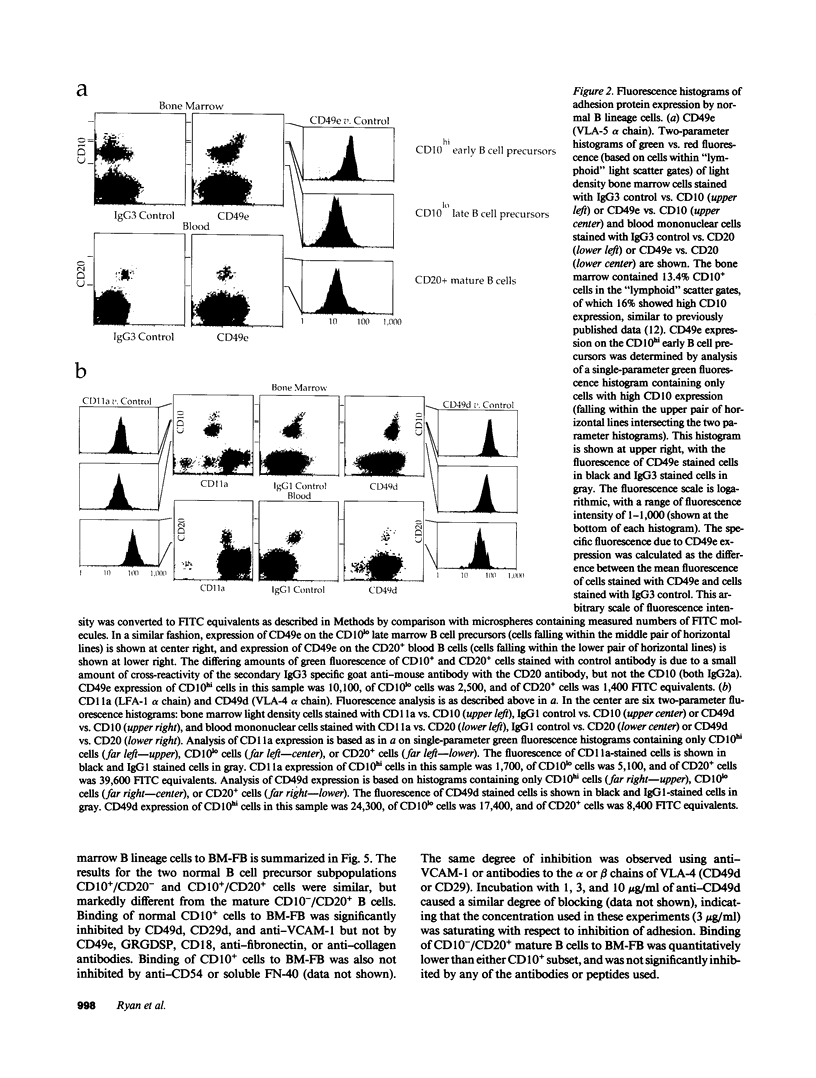
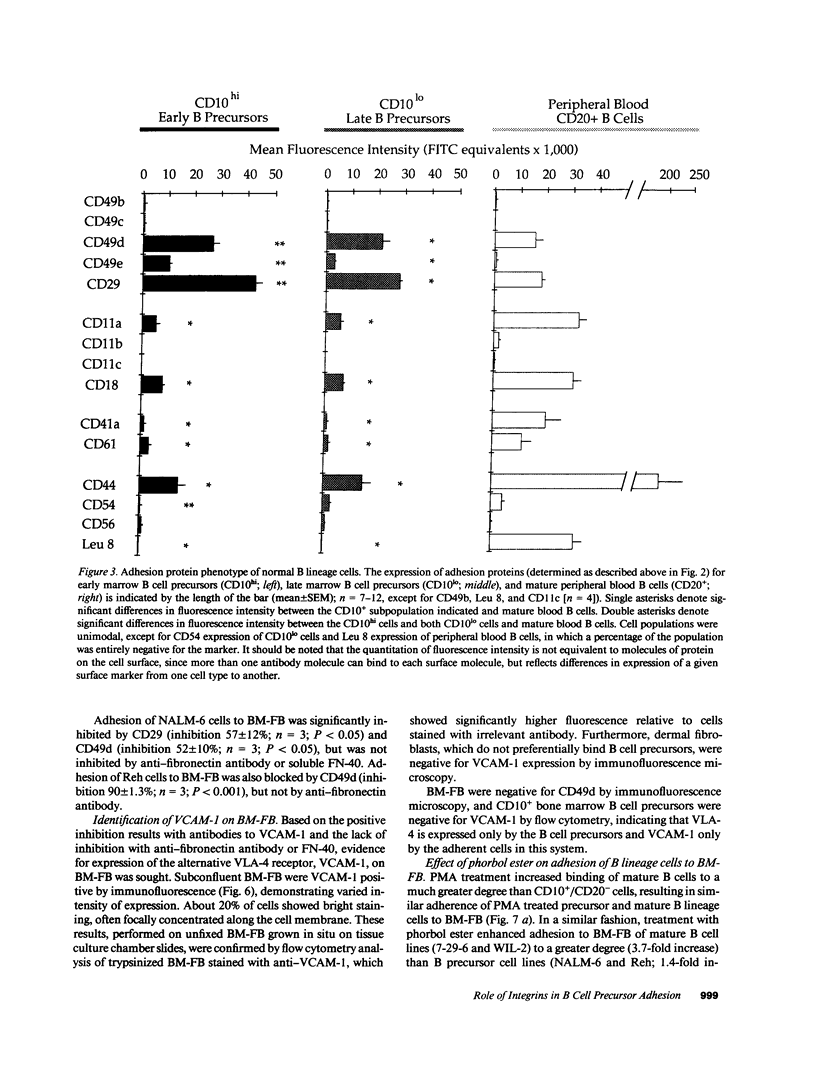
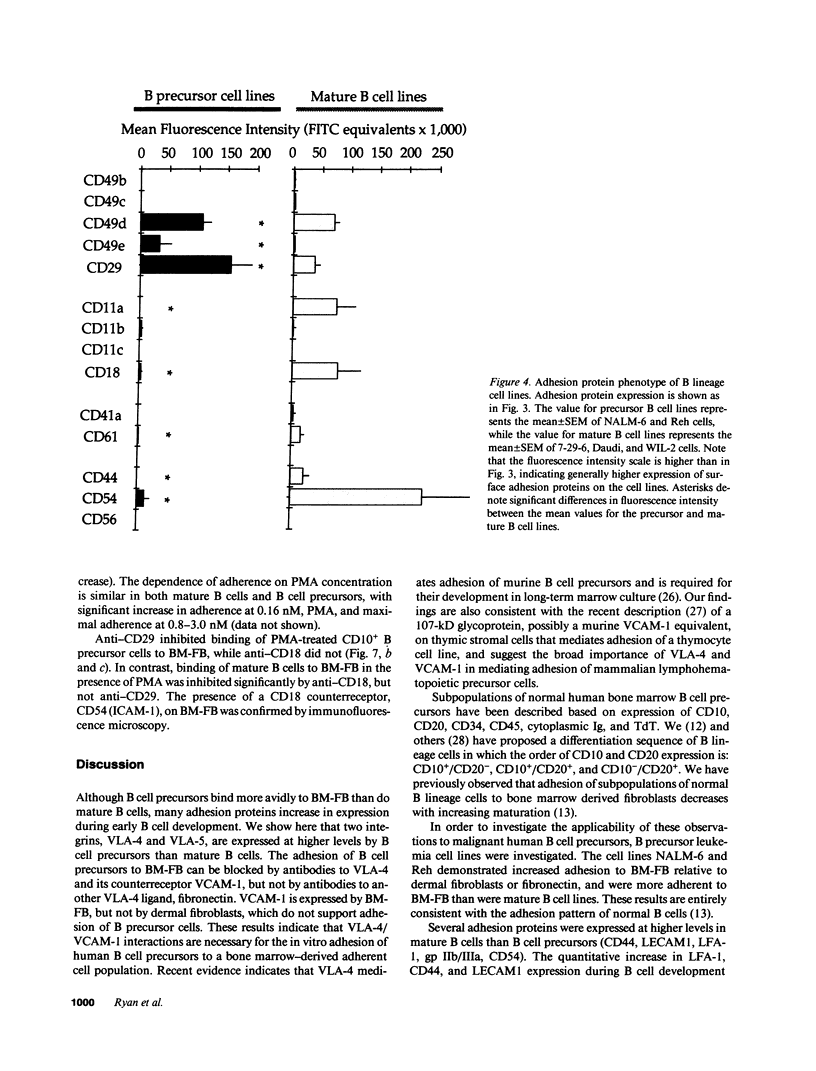
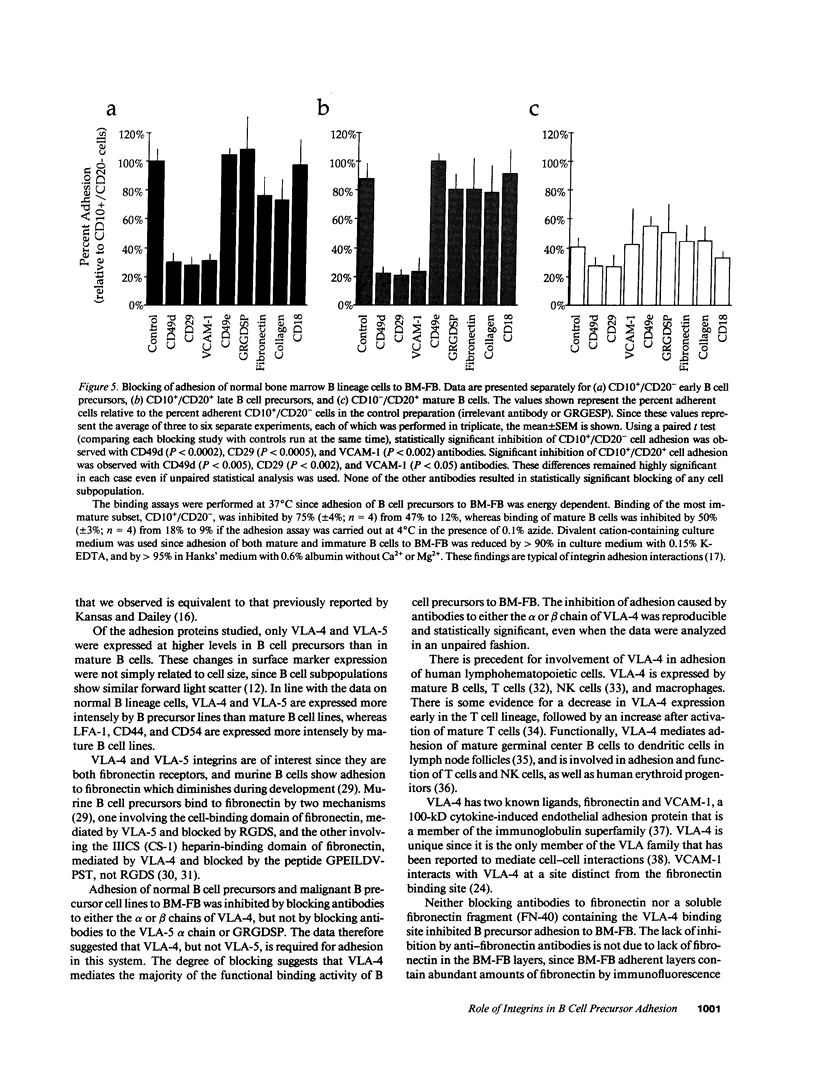
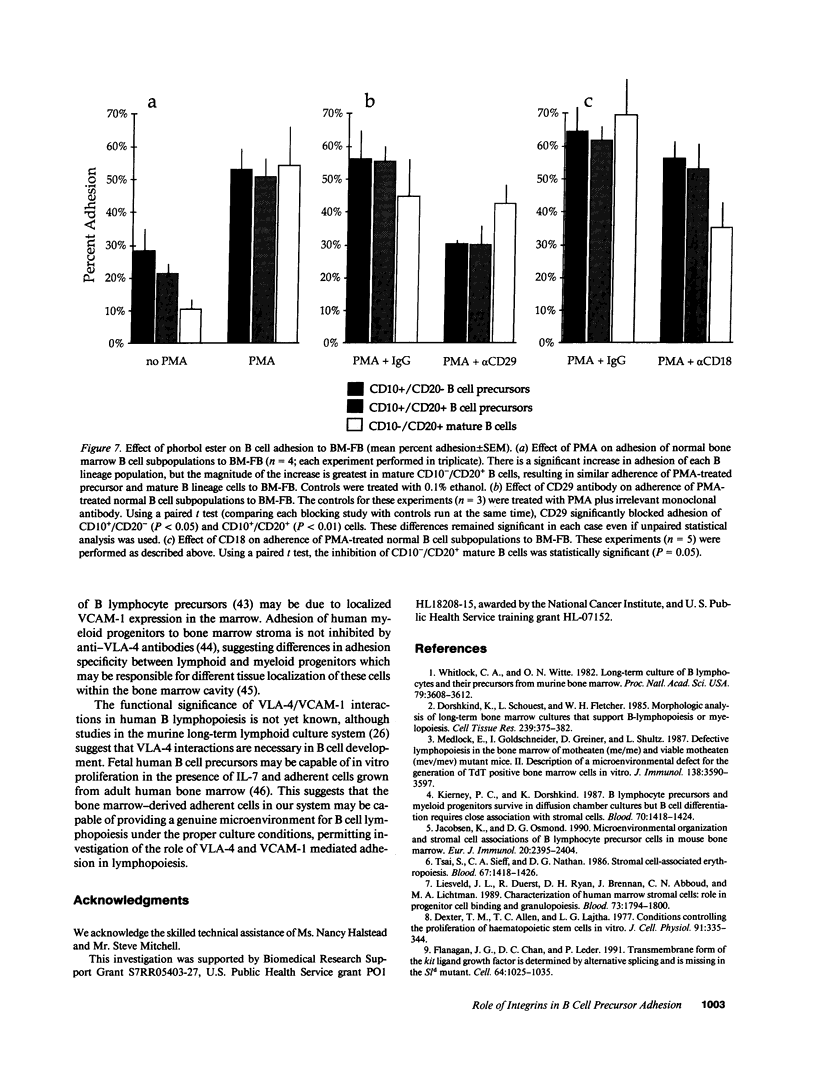
Adhesion of B cell precursors to accessory cells in the bone marrow microenvironment may be required for normal early B cell development. Human bone marrow B cell precursors adhere more avidly than mature B cells to bone marrow-derived fibroblasts. To determine the mechanism of this adhesion, expression of adhesion proteins on human B precursor cells and cell lines was measured by flow cytometry. The very late antigen (VLA) integrins VLA-4 and VLA-5 were the only adhesion proteins expressed at higher levels in B cell precursors than mature B cells. Antibodies to the alpha and beta chains of VLA-4, but not VLA-5, significantly blocked binding to bone marrow-derived fibroblasts of immature B cells and cell lines. Although fibronectin is a ligand for VLA-4, anti-fibronectin antibody and a soluble fibronectin fragment containing the VLA-4 binding domain did not block adhesion, suggesting that VLA-4 is involved in adhesion of B cell precursors, but not as a fibronectin receptor. Vascular cell adhesion molecule-1 (VCAM-1), the other known counterreceptor for VLA-4, was identified on bone marrow-derived fibroblasts, and anti-VCAM-1 significantly blocked adhesion of normal B cell precursors to bone marrow-derived fibroblasts, indicating that VLA-4/VCAM-1 interactions are important in adhesion of B cell precursors to the bone marrow microenvironment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi P., Patel V. P., Lodish H. F. Lymphoid precursor cells adhere to two different sites on fibronectin. J Cell Biol. 1987 Jul;105(1):489–498. doi: 10.1083/jcb.105.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P., Patel V. P., Lodish H. F. Lymphoid precursor cells adhere to two different sites on fibronectin. J Cell Biol. 1987 Jul;105(1):489–498. doi: 10.1083/jcb.105.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandley B. K., Swiedler S. J., Robbins P. W. Carbohydrate ligands of the LEC cell adhesion molecules. Cell. 1990 Nov 30;63(5):861–863. doi: 10.1016/0092-8674(90)90487-y. [DOI] [PubMed] [Google Scholar]
- Brown D. L., Phillips D. R., Damsky C. H., Charo I. F. Synthesis and expression of the fibroblast fibronectin receptor in human monocytes. J Clin Invest. 1989 Jul;84(1):366–370. doi: 10.1172/JCI114166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanero M. R., Pulido R., Ursa M. A., Rodríguez-Moya M., de Landázuri M. O., Sánchez-Madrid F. An alternative leukocyte homotypic adhesion mechanism, LFA-1/ICAM-1-independent, triggered through the human VLA-4 integrin. J Cell Biol. 1990 Jun;110(6):2157–2165. doi: 10.1083/jcb.110.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Schouest L., Fletcher W. H. Morphologic analysis of long-term bone marrow cultures that support B-lymphopoiesis or myelopoiesis. Cell Tissue Res. 1985;239(2):375–382. doi: 10.1007/BF00218018. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Chan D. C., Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991 Mar 8;64(5):1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Garcia-Pardo A., Wayner E. A., Carter W. G., Ferreira O. C., Jr Human B lymphocytes define an alternative mechanism of adhesion to fibronectin. The interaction of the alpha 4 beta 1 integrin with the LHGPEILDVPST sequence of the type III connecting segment is sufficient to promote cell attachment. J Immunol. 1990 May 1;144(9):3361–3366. [PubMed] [Google Scholar]
- Gismondi A., Morrone S., Humphries M. J., Piccoli M., Frati L., Santoni A. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol. 1991 Jan 1;146(1):384–392. [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Humphries M. J. The molecular basis and specificity of integrin-ligand interactions. J Cell Sci. 1990 Dec;97(Pt 4):585–592. doi: 10.1242/jcs.97.4.585. [DOI] [PubMed] [Google Scholar]
- Jacobsen K., Osmond D. G. Microenvironmental organization and stromal cell associations of B lymphocyte precursor cells in mouse bone marrow. Eur J Immunol. 1990 Nov;20(11):2395–2404. doi: 10.1002/eji.1830201106. [DOI] [PubMed] [Google Scholar]
- Jacobsen K., Tepper J., Osmond D. G. Early B-lymphocyte precursor cells in mouse bone marrow: subosteal localization of B220+ cells during postirradiation regeneration. Exp Hematol. 1990 May;18(4):304–310. [PubMed] [Google Scholar]
- Kansas G. S., Dailey M. O. Expression of adhesion structures during B cell development in man. J Immunol. 1989 May 1;142(9):3058–3062. [PubMed] [Google Scholar]
- Kierney P. C., Dorshkind K. B lymphocyte precursors and myeloid progenitors survive in diffusion chamber cultures but B cell differentiation requires close association with stromal cells. Blood. 1987 Nov;70(5):1418–1424. [PubMed] [Google Scholar]
- Kina T., Majumdar A. S., Heimfeld S., Kaneshima H., Holzmann B., Katsura Y., Weissman I. L. Identification of a 107-kD glycoprotein that mediates adhesion between stromal cells and hematolymphoid cells. J Exp Med. 1991 Feb 1;173(2):373–381. doi: 10.1084/jem.173.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G., van Kooyk Y., de Graaff M., Meyer C. J., Figdor C. G., Pals S. T. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol. 1990 Dec 1;145(11):3589–3593. [PubMed] [Google Scholar]
- LeBien T. W. Growing human B-cell precursors in vitro: the continuing challenge. Immunol Today. 1989 Sep;10(9):296–298. doi: 10.1016/0167-5699(89)90084-4. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Virolainen M., Defendi V. Human lymphoblastoid lines from lymph node and spleen. Cancer. 1968 Sep;22(3):517–524. doi: 10.1002/1097-0142(196809)22:3<517::aid-cncr2820220305>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Liesveld J. L., Abboud C. N., Duerst R. E., Ryan D. H., Brennan J. K., Lichtman M. A. Characterization of human marrow stromal cells: role in progenitor cell binding and granulopoiesis. Blood. 1989 May 15;73(7):1794–1800. [PubMed] [Google Scholar]
- Loken M. R., Shah V. O., Dattilio K. L., Civin C. I. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood. 1987 Nov;70(5):1316–1324. [PubMed] [Google Scholar]
- Lord B. I. The architecture of bone marrow cell populations. Int J Cell Cloning. 1990 Sep;8(5):317–331. doi: 10.1002/stem.5530080501. [DOI] [PubMed] [Google Scholar]
- Medlock E. S., Goldschneider I., Greiner D. L., Shultz L. Defective lymphopoiesis in the bone marrow of motheaten (me/me) and viable motheaten (mev/mev) mutant mice. II. Description of a microenvironmental defect for the generation of terminal deoxynucleotidyltransferase-positive bone marrow cells in vitro. J Immunol. 1987 Jun 1;138(11):3590–3597. [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Terstappen L. W., Rott L. S., Streeter P. R., Stein H., Butcher E. C. Differential expression of homing-associated adhesion molecules by T cell subsets in man. J Immunol. 1990 Nov 15;145(10):3247–3255. [PubMed] [Google Scholar]
- Rice G. E., Bevilacqua M. P. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989 Dec 8;246(4935):1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Corless C., Bevilacqua M. P. Vascular and nonvascular expression of INCAM-110. A target for mononuclear leukocyte adhesion in normal and inflamed human tissues. Am J Pathol. 1991 Feb;138(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Rosemblatt M., Vuillet-Gaugler M. H., Leroy C., Coulombel L. Coexpression of two fibronectin receptors, VLA-4 and VLA-5, by immature human erythroblastic precursor cells. J Clin Invest. 1991 Jan;87(1):6–11. doi: 10.1172/JCI115002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D. H., Nuccie B. L., Abboud C. N., Liesveld J. L. Maturation-dependent adhesion of human B cell precursors to the bone marrow microenvironment. J Immunol. 1990 Jul 15;145(2):477–484. [PubMed] [Google Scholar]
- Ryan D., Kossover S., Mitchell S., Frantz C., Hennessy L., Cohen H. Subpopulations of common acute lymphoblastic leukemia antigen-positive lymphoid cells in normal bone marrow identified by hematopoietic differentiation antigens. Blood. 1986 Aug;68(2):417–425. [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Horgan K. J., Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990 May 17;345(6272):250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tedder T. F., Cooper M. D., Clement L. T. Human lymphocyte differentiation antigens HB-10 and HB-11. II. Differential production of B cell growth and differentiation factors by distinct helper T cell subpopulations. J Immunol. 1985 May;134(5):2989–2994. [PubMed] [Google Scholar]
- Tsai S., Sieff C. A., Nathan D. G. Stromal cell-associated erythropoiesis. Blood. 1986 May;67(5):1418–1426. [PubMed] [Google Scholar]
- Uckun F. M. Regulation of human B-cell ontogeny. Blood. 1990 Nov 15;76(10):1908–1923. [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]