Abstract
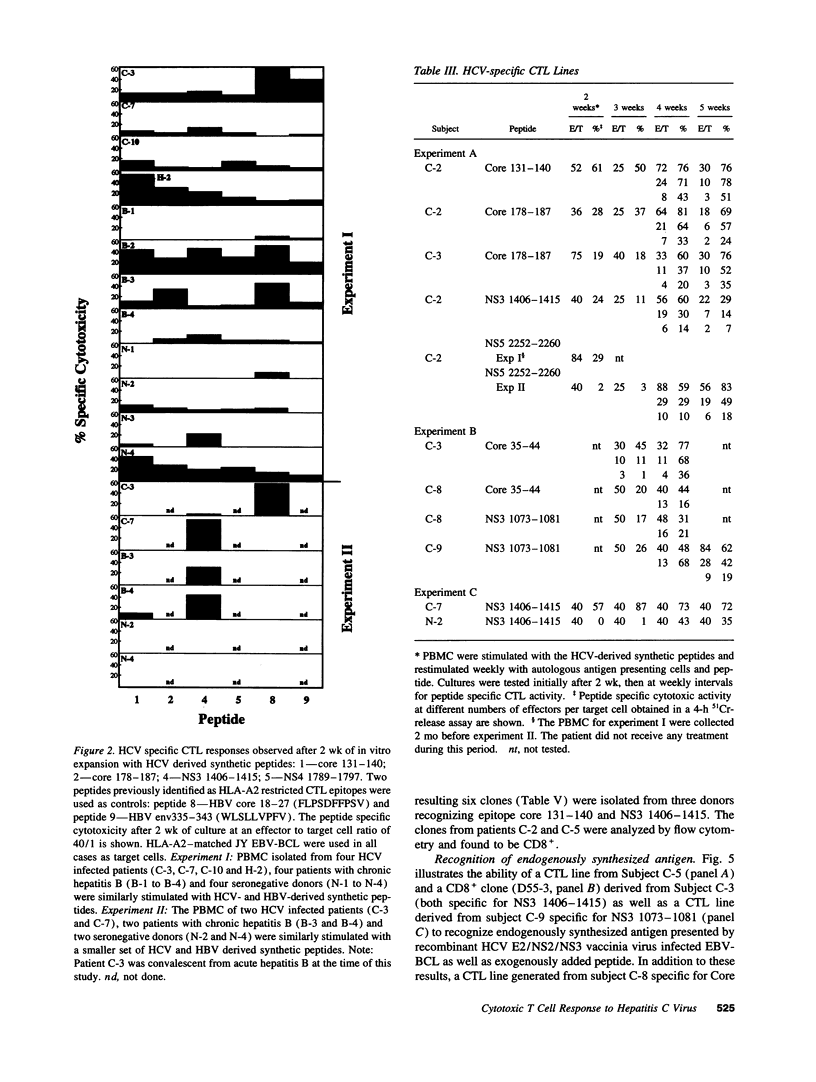
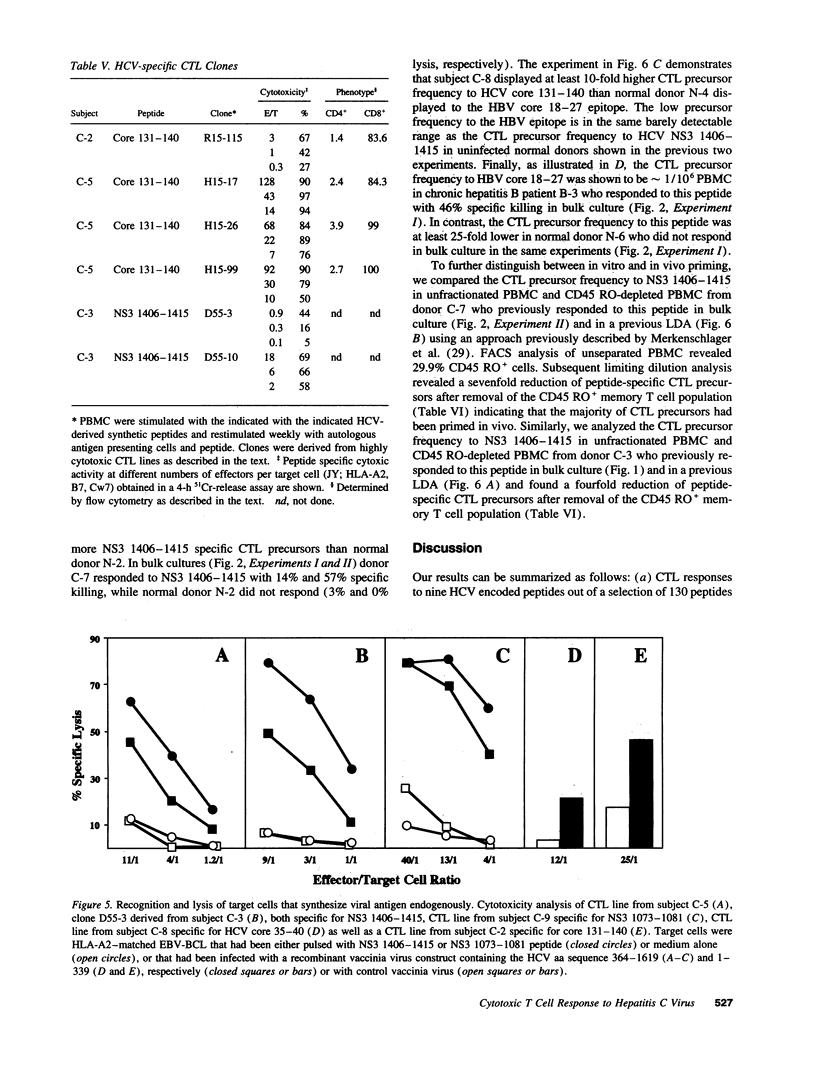
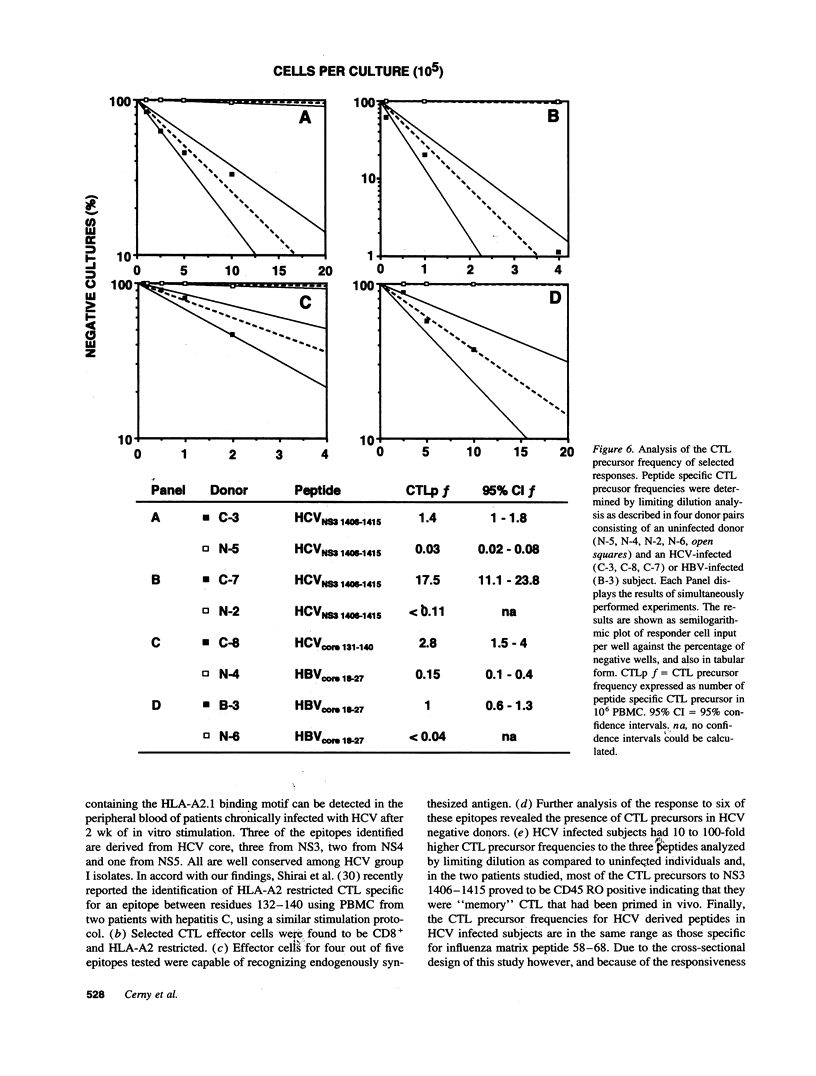
The HLA class I-restricted cytotoxic T lymphocyte (CTL) response is a major defense mechanism in viral infections. It has been suggested that the CTL response may contribute to viral clearance and liver cell injury during hepatitis C virus (HCV) infection. To test this hypothesis requires an understanding of the characteristics of HCV-specific cytotoxic effector cells and identification of the target antigens to which they respond. To begin this process we stimulated peripheral blood mononuclear cells (PBMC) from a group of HLA-A2 positive patients with chronic hepatitis C with a panel of 130 HCV-derived peptides containing the HLA-A2 binding motif. Effector cells were tested for their capacity to lyse HLA-A2-matched target cells that were either sensitized with peptide or infected with a vaccinia virus construct containing HCV sequences. Using this approach we have identified nine immunogenic peptides in HCV, three of which are derived from the putative core protein, three from the nonstructural (NS) 3 domain, two from NS4 and one from NS5. Selected responses were shown to be HLA-A2 restricted, mediated by CD8+ T cells and to recognize endogenously synthesized viral antigen. Unexpectedly, peptide-specific CTL responses could also be induced in sero-negative individuals, suggesting in vitro activation of naive CTL precursors. The precursor frequency of peptide-specific CTL was 10 to 100-fold higher in infected patients compared to uninfected controls, and the responses were greatly diminished by removal of CD45 RO+ (memory) T cells. Further quantitative studies are clearly required to establish whether a correlation exists between the HCV-specific CTL response and the clinical course of this disease. Definition of the molecular targets of the human CTL response to HCV creates this opportunity, and may also contribute to the development of a T cell-based HCV vaccine.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnaba V., Franco A., Alberti A., Balsano C., Benvenuto R., Balsano F. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989 Oct 15;143(8):2650–2655. [PubMed] [Google Scholar]
- Bertoletti A., Ferrari C., Fiaccadori F., Penna A., Margolskee R., Schlicht H. J., Fowler P., Guilhot S., Chisari F. V. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10445–10449. doi: 10.1073/pnas.88.23.10445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):187–191. doi: 10.1073/pnas.89.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. C., Smith G. L., Moss B. Hepatitis B virus large surface protein is not secreted but is immunogenic when selectively expressed by recombinant vaccinia virus. J Virol. 1986 Nov;60(2):337–344. doi: 10.1128/jvi.60.2.337-344.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Grahovac B., Schendel D., Stevanović S., Gnau V., Jung G., Strominger J. L., Rammensee H. G. Allele-specific peptide ligand motifs of HLA-C molecules. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12005–12009. doi: 10.1073/pnas.90.24.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Gotch F. M., Nixon D. F., Alp N., McMichael A. J., Borysiewicz L. K. High frequency of memory and effector gag specific cytotoxic T lymphocytes in HIV seropositive individuals. Int Immunol. 1990;2(8):707–712. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- Gotch F., Rothbard J., Howland K., Townsend A., McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. 1987 Apr 30-May 6Nature. 326(6116):881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- Guilhot S., Fowler P., Portillo G., Margolskee R. F., Ferrari C., Bertoletti A., Chisari F. V. Hepatitis B virus (HBV)-specific cytotoxic T-cell response in humans: production of target cells by stable expression of HBV-encoded proteins in immortalized human B-cell lines. J Virol. 1992 May;66(5):2670–2678. doi: 10.1128/jvi.66.5.2670-2678.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H. C., Jardetzky T. S., Garrett T. P., Lane W. S., Strominger J. L., Wiley D. C. Different length peptides bind to HLA-Aw68 similarly at their ends but bulge out in the middle. Nature. 1992 Nov 26;360(6402):364–366. doi: 10.1038/360364a0. [DOI] [PubMed] [Google Scholar]
- Hadida F., Parrot A., Kieny M. P., Sadat-Sowti B., Mayaud C., Debre P., Autran B. Carboxyl-terminal and central regions of human immunodeficiency virus-1 NEF recognized by cytotoxic T lymphocytes from lymphoid organs. An in vitro limiting dilution analysis. J Clin Invest. 1992 Jan;89(1):53–60. doi: 10.1172/JCI115585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M., Weiner A., Han J., Kuo G., Choo Q. L. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology. 1991 Aug;14(2):381–388. [PubMed] [Google Scholar]
- Huczko E. L., Bodnar W. M., Benjamin D., Sakaguchi K., Zhu N. Z., Shabanowitz J., Henderson R. A., Appella E., Hunt D. F., Engelhard V. H. Characteristics of endogenous peptides eluted from the class I MHC molecule HLA-B7 determined by mass spectrometry and computer modeling. J Immunol. 1993 Sep 1;151(5):2572–2587. [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Koziel M. J., Dudley D., Wong J. T., Dienstag J., Houghton M., Ralston R., Walker B. D. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J Immunol. 1992 Nov 15;149(10):3339–3344. [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Matsumura M., Fremont D. H., Peterson P. A., Wilson I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992 Aug 14;257(5072):927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M., Terry L., Edwards R., Beverley P. C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988 Nov;18(11):1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- Missale G., Redeker A., Person J., Fowler P., Guilhot S., Schlicht H. J., Ferrari C., Chisari F. V. HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J Exp Med. 1993 Mar 1;177(3):751–762. doi: 10.1084/jem.177.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nayersina R., Fowler P., Guilhot S., Missale G., Cerny A., Schlicht H. J., Vitiello A., Chesnut R., Person J. L., Redeker A. G. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993 May 15;150(10):4659–4671. [PubMed] [Google Scholar]
- Okamoto H., Sugiyama Y., Okada S., Kurai K., Akahane Y., Sugai Y., Tanaka T., Sato K., Tsuda F., Miyakawa Y. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J Gen Virol. 1992 Mar;73(Pt 3):673–679. doi: 10.1099/0022-1317-73-3-673. [DOI] [PubMed] [Google Scholar]
- Penna A., Chisari F. V., Bertoletti A., Missale G., Fowler P., Giuberti T., Fiaccadori F., Ferrari C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991 Dec 1;174(6):1565–1570. doi: 10.1084/jem.174.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Shirai M., Akatsuka T., Pendleton C. D., Houghten R., Wychowski C., Mihalik K., Feinstone S., Berzofsky J. A. Induction of cytotoxic T cells to a cross-reactive epitope in the hepatitis C virus nonstructural RNA polymerase-like protein. J Virol. 1992 Jul;66(7):4098–4106. doi: 10.1128/jvi.66.7.4098-4106.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P. Variability of hepatitis C virus genome. Curr Stud Hematol Blood Transfus. 1994;(61):12–35. doi: 10.1159/000423266. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Alexander D., Rugroden M. E., Choo Q. L., Berger K., Crawford K., Kuo C., Leng S., Lee C., Ralston R. Characterization of the hepatitis C virus E2/NS1 gene product expressed in mammalian cells. Virology. 1992 Jun;188(2):819–830. doi: 10.1016/0042-6822(92)90537-y. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Haenseler E., Leist T., Cerny A., Hengartner H., Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med. 1986 Oct 1;164(4):1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]


