Abstract
The calcium-sensing receptor (CaR) is the major sensor and regulator of extracellular Ca2+, whose activity is allosterically regulated by amino acids and pH. Recently, CaR has been identified in the stomach and intestinal tract, where it has been proposed to function in a non-Ca2+ homeostatic capacity. Luminal nutrients, such as Ca2+ and amino acids, have been recognized for decades as potent stimulants for gastrin and acid secretion, although the molecular basis for their recognition remains unknown. The expression of CaR on gastrin-secreting G cells in the stomach and their shared activation by Ca2+, amino acids, and elevated pH suggest that CaR may function as the elusive physiologic sensor regulating gastrin and acid secretion. The genetic and pharmacologic studies presented here comparing CaR-null mice and wild-type littermates support this hypothesis. Gavage of Ca2+, peptone, phenylalanine, Hepes buffer (pH 7.4), and CaR-specific calcimimetic, cinacalcet, stimulated gastrin and acid secretion, whereas the calcilytic, NPS 2143, inhibited secretion only in the wild-type mouse. Consistent with known growth and developmental functions of CaR, G-cell number was progressively reduced between 30 and 90 d of age by more than 65% in CaR-null mice. These studies of nutrient-regulated G-cell gastrin secretion and growth provide definitive evidence that CaR functions as a physiologically relevant multimodal sensor. Medicinals targeting diseases of Ca2+ homeostasis should be reviewed for effects outside traditional Ca2+-regulating tissues in view of the broader distribution and function of CaR.
Keywords: chemosensation, multimodal sensor, peptide hormone, calcilytic, calcimimetic
Meal-related nutrients are potent stimulants of gastrointestinal hormones, whose coordinated release initiates and regulates digestion, absorption, and metabolism through their actions on digestive enzyme release, hormone secretion, and GI motility. These nutrients are sensed by enteroendocrine cells scattered along the mucosal epithelium lining the GI tract and by mucosal neurons within the enteric nervous system. The molecular basis for the GI recognition of luminal nutrients other than glucose remains elusive, including in the stomach, where peptone and amino acids have long been recognized as potent stimulants of gastric acid (1, 2).
Gastrin, a 17-aa carboxy-amidated peptide produced by G cells located at the base of gastric antral glands, acts in the endocrine stimulation of gastric body enterochromaffin-like (ECL) cell histamine release, with subsequent paracrine stimulation of parietal cell acid secretion. Gastric G cells are exposed to the lumen and potentially sense luminal contents. In fact, luminal vs. i.v. amino acids are more potent stimulants for gastrin secretion (3).
Pepsin, activated by gastric acid, digests nutrient protein into hydrophobic peptides and free aromatic amino acids, which are the major stimulants of gastrin secretion (2). Meal neutralization of gastric pH (4) and intraluminal Ca2+ each potently release gastrin (5).
The sensing of extracellular Ca2+ and the regulation of Ca2+ homestasis has been largely attributed to the calcium-sensing receptor (CaR), a member of the C family of G protein-coupled receptors (GPCR). Consistent with this function, CaR is expressed on extracellular Ca2+-regulating cells such as parathyroid, thyroid parafollicular, renal tubular, and bone cells (6).
The CaR has a large NH2 terminal extracellular domain (ECD) composed of multiple binding sites for positively charged multivalent cations. Similar to other C class GPCRs that bind amino acids, CaR ECD has a homologous amino acid binding Venous Fly Trap domain with preference for aromatic amino acids. The seven transmembrane domains of CaR can bind phenylalkamines, which, similar to amino acids interacting with the ECD, are capable of allosterically regulating receptor activation in the presence of ionized Ca2+ (7). Thus, it is not surprising that the CaR has subsequently been recognized as a multimodal sensor of multivalent cations, polyamines, l-amino acids, and pH in addition to Ca2+ and Mg2+ (7).
In addition to classical Ca2+-regulating tissues, CaR is expressed in many other tissues, including those within the GI tract (7); it has been proposed to regulate a diverse set of noncalcium homeostatic functions such as acid regulation by gastric G (8) and parietal (9) cells, GI epithelial cell mucus secretion and proliferation, motility by enteric nerve plexuses, and colonocyte fluid transport and differentiation (10).
The presence of CaR on antral G cells and the common sensitivity of both CaR and G-cell gastrin secretion to amino acids, pH, and Ca2+ raise the possibility that the gastric phase of meal-stimulated gastrin is regulated by CaR in addition to its direct activation by intraluminal Ca2+ (10).
Although there have been several in vitro-based studies in either a variety of heterologously transfected cell systems (11–13) or acutely isolated primary cell cultures (14) that support the role of CaR as a multimodal sensor regulating both Ca2+ and non- Ca2+ homeostatic cell systems, there has not been any in vivo physiologic evidence confirming this proposed role.
We report a genetic and pharmacologic study using CaR-null mice and CaR-specific calcimimetic, cinacalcet, and calcilytic NPS 2143, to assess the role of CaR in nutrient chemosensation and regulation of gastrin and acid secretion. We show that CaR on gastric antral G cells acts as the physiologic multimodal sensor that regulates both gastrin secretion in response to a variety of luminal nutrients and maintenance of G-cell number.
Results and Discussion
Mouse Antral G Cells Express Cell Surface CaR.
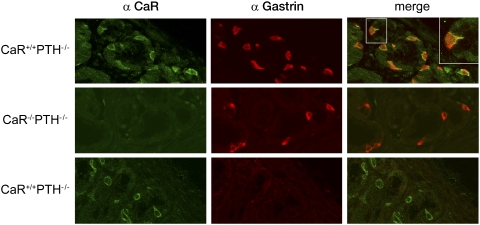
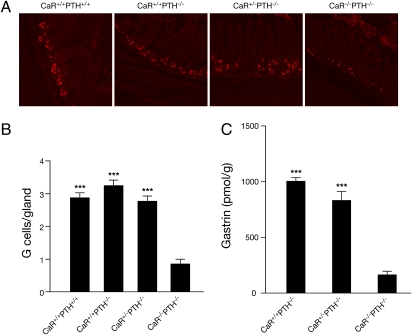
Although human antral G cells express functional CaR (8), CaR expression in rodent antral G cells has only been inferred. Therefore, CaR wild type (WT) and null littermates were examined for CaR expression by immunofluorescence to an extracellular domain epitope of CaR common to mouse and human (15). CaR was detected at the base of the antral glands in the distribution and frequency expected for G cells (Fig. 1, green). The cell surface pattern of immunofluorescence was consistent with the expected membranous distribution of cell surface receptors. The presence of the receptor at both the apical and basolateral cell surfaces agrees with previous reports (16) and supports the potential for sensing ligands from both the gastric lumen as well as the serum. The G-cell specificity for CaR immunofluorescence was confirmed by specific colocalized gastrin immunofluorescence and the lack of CaR immunofluorescence in CaR-null littermates and in the absence of primary antibody (Fig. 1).
Fig. 1.
Mouse antral gastrin-producing cells express CaR protein. Dual immunohistochemistry for CaR and gastrin in CaR WT (CaR+/+PTH−/−; Top) and CaR-null (CaR−/−PTH−/−; Middle) gastric antrum. Control (Bottom) immunostaining of CaR-null gastric antrum in the absence of primary antibody for gastrin; 5-μm sections of formalin fixed paraffin embedded gastric antrum from fasted mice were double immunostained for gastrin and CaR using monoclonal anti-human CaR and rabbit polyclonal anti-human gastrin. CaR and gastrin expression were detected with highly absorbed goat anti-mouse Alexa 488 and goat anti-rabbit Alexa 594 secondary antibodies, respectively. Immunofluoresence of representative images chosen from multiple sections was obtained using a Zeiss LSM510 confocal microscope.
Deletion of CaR in the Stomach Does Not Alter Oxyntic Gland Histology.
CaRs are expressed in rat and human gastric glands on parietal cells, where they regulate acid secretion (17, 18). CaRs have also been identified in both rat and human gastric mucus epithelial cells and rat chief cells in primary culture, and this suggests that CaR may regulate mucus and pepsinogen secretion. CaR has been increasingly recognized for mediating proliferation, differentiation, and survival in a variety of tissues, including the GI tract (10). In addition, gastrin is a growth factor for ECL and parietal cells of the oxyntic gland (19). Despite the absence of CaR and lower gastrin secretion in CaR-null mice (Fig. 2), H&E staining of oxyntic mucosa (Fig. S1 Upper) showed no significant changes in gland length, architecture, or distribution of cell types. Parietal cell mass was also unchanged (Fig. S1 Lower), despite parietal cell CaR expression (17, 18) and gastrin growth dependence (19).
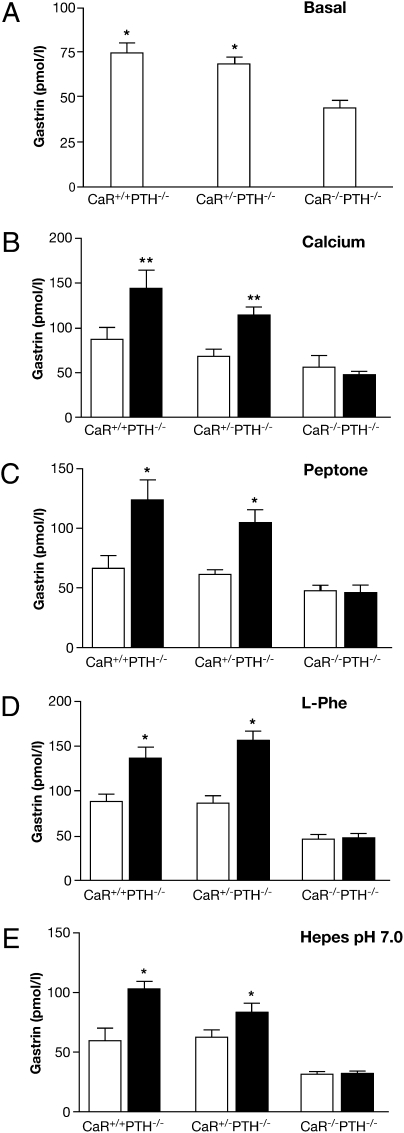
Fig. 2.
Loss of gastrin response to gavaged secretagogues in CaR-null mice. Basal (open bars) and stimulated (filled bars) plasma gastrin measured by RIA in CaR WT (CaR+/+ PTH−/−), heterozygous (CaR+/− PTH−/−), and null (CaR−/− PTH−/−) littermates after an overnight fast (0 min; A) and after gavage (30 min) of either (B) Ca2+ gluconate (100 mM), (C) peptone (8%), (D) l-phenylalanine (100 mM), or (E) Hepes buffer (150 mM, pH 7.0). (A) *P < 0.05 vs. CaR−/− PTH−/− (n = 5 mice). (B) **P < 0.01 vs. basal (n = 6 mice). (C) *P < 0.05 vs. basal (n = 5 mice). (D) *P < 0.05 vs. basal (n = 7 mice).
Low Basal Gastrin and Absent Gastrin Response to Luminal Nutrients in CaR Gene-Deleted Mice.
The basal plasma gastrin levels in CaR-null mice were lower than their CaR WT or heterozygous littermates (Fig. 2A). Although the trend was lower, CaR heterozygous mice were not significantly different from CaR WT littermates. The low basal gastrin exhibited by CaR-null mice is impressive considering that it occurs in the setting of an elevated gastric pH (Fig. S4B), a major stimulus for gastrin secretion.
In humans, oral Ca2+ causes a marked secretion of gastrin (20). This can be counterproductive for Ca2+ carbonate antacids, such as Tums, where the weak buffer is compromised by Ca2+-induced gastrin release and subsequent acid secretion (20). Similar to humans, gavage of 100 mM Ca2+ gluconate caused an increase in plasma gastrin in both CaR WT and heterozygous mice (Fig. 2B). However, there was no significant response to Ca2+ in CaR-null mice. Consistent with studies in patients with hypocalciuric hypercalcemia caused by heterozygous mutations in CaR (21), there was only a trend to a diminished response in heterozygous littermates. This CaR-dependent gastrin response to Ca2+ forms the basis for the diagnostic Ca2+ stimulation test in patients with Zollinger-Ellison (ZES) syndrome, whose gastrinomas express CaR, and this also explains the observed exacerbation of acid hypersecretion in multiple endocrine neoplasia type 1 patients with hyperparathyroidism and ZES (15). Because acid promotes Ca2+ absorption, the CaR-expressing G cell behaves like a Ca2+ regulatory cell, as evidenced by the fact that gastrin receptor null mice become hypocalcemic (22).
Although protein has long been recognized as the most potent meal-related stimulant of gastrin secretion (3), the basis for its molecular recognition remains unknown. Studies in isolated parathyroid cells show that CaR can be allosterically activated by most l-amino acids in the presence of extracellular Ca2+ (14). Mice were gavaged with peptone to assess the role of CaR in protein-stimulated gastrin secretion. Unlike CaR WT and heterozygous littermates, there was no increase in plasma gastrin in CaR-null mice (Fig. 2C). Although CaR WT and heterozygous littermates were not significantly different, the heterozygote response trended lower. These results in mice are consistent with the reduced gastrin response to oral Ca2+ and peptone observed in patients with hypocalciuric hypercalcemia harboring heterozygous mutations in CaR (21).
Early physiologic studies have shown that aromatic amino acids are the most potent amino acids stimulating gastrin release (2). CaR, similar to other class 3 family GPCRs that bind amino acids such as the metabotropic glutamate (mGluR1) (23) and heterodimeric GABABR (24) receptors, shares a functional N-terminal Venus Fly Trap domain. Unlike the mGluR1 and GABABR but similar to the taste receptor type 1 members 1 and 3 amino acid sensing taste receptor heterodimer (25) and the less well-characterized nutrient-sensing receptor, GPRC6A (26), CaR recognizes a broad array of l-amino acids interacting within the Venus Fly Trap domain (27). However, CaR has a preference for aromatic amino acids, such as Phe and Trp, in vitro in transfected HEK293 cells (11) and acutely isolated human parathyroid cells (14). Consistent with these studies, l-Phe, gavaged at a concentration expected during a meal, elicited a gastrin response that could account for most of the stimulus observed with peptone in WT and heterozygous littermates. Similar to peptone, l-Phe did not stimulate gastrin secretion in CaR-null mice (Fig. 2 C and D). These results provide a molecular basis for protein meal stimulation of gastrin and by logical extension, may account for amino acid stimulation of other CaR-expressing cells, such as acid-secreting parietal cells (17), cholecystokinin-secreting enteroendocrine I cells (28), and insulin-secreting β cells (29).
Acid is a well-established inhibitor of gastrin secretion thought to act directly on the G cell (30) and indirectly through the paracrine inhibitory peptide, somatostatin (Sst), from antral D cells (31). Similarly, neutralization of gastric acid is a potent stimulus of gastrin secretion, partly because of a decrease in Sst secretion (31). However, Sst receptor subtype 2 null mice have basal and meal-stimulated gastrin and gastric acid levels similar to WT mice, and therefore, this suggests that other factors may compensate in the absence of Sst (32). In fact, recent studies with isolated rat G cells show decreased gastrin release after a drop in pH from 7.4 to 5.5 (30). Furthermore, the CaR response to Ca2+ is significantly enhanced with increasing pH (12) within the range of pH 5.5–9 in transfected HEK293 cells (12) and above pH 7.5 in Xenopus oocytes (33). Therefore, to determine whether the G-cell CaR could sense increasing gastric luminal pH, the acidic gastric mucosa of fasting mice was neutralized with Hepes buffer (pH 7). Plasma gastrin increased in CaR WT and heterozygous littermates, whereas there was no response in CaR-null mice (Fig. 2E). This pH-dependent gastrin response is probably not the result of putative antral D cell CaR, because elevated pH and deletion of CaR would result in changes in gastrin secretion opposite of what is observed experimentally. Thus, gastrin secretion in response to elevated intraluminal pH is not a passive release of G-cell inhibition from declining Sst but rather, an active process of CaR activation. Other examples of the pH dependence of CaR activation in the regulation of urinary excretion of Ca2+ and water, bone metabolism by acid-secreting osteoclasts, and parathyroid hormone secretion (12, 34) support a similar pH-sensing role for CaR.
Recent studies of isolated rat antral G cells did not consider CaR as a direct chemosensory regulator of G-cell secretion but did suggest that amino acids Phe and Trp may be sensed by the T1R family of receptors (30). Using T1R2/3 compound homozygous null mice that are insensitive to amino acid taste reception (35), there was no significant difference in gastrin secretion to peptone gavage compared with WT littermates (Fig. S2). These results indicate that the T1R family is not important for G-cell sensing of protein and amino acids and are consistent with the observed full loss of the gastrin response observed in CaR-null mice.
Deletion of CaR Does Not Significantly Alter the Gastrin Secretory Response to Bombesin.
Gastric enteric neurons projecting to the antral mucosa secrete the neuropeptide GRP after stimulation by either vagal preganglionic efferent neurons (36) or intraluminal nutrients such as peptone (37). GRP acts directly on antral G-cell BB1 receptors to stimulate gastrin secretion (30). The low basal secretion of gastrin along with the loss of G-cell responsiveness to diverse agonists such as peptone, Phe, alkaline pH, and Ca2+ in CaR-null mice raised the possibility that CaR plays a permissive role in G-cell responsiveness in addition to being a multimodal sensor directly interacting with a variety of ligands (38). This is unlikely, because bombesin caused a significant increase in plasma gastrin levels independent of CaR (Fig. S3A). The trend to a diminished response to bombesin in CaR-null mice compared with WT littermates was not significant when normalized for basal gastrin secretion (Fig. S3B). Therefore, the ability of CaR-null G cells to respond to a non-CaR ligand such as bombesin indicates that the deletion of CaR does not globally disable G-cell responsiveness.
Specific Activation and Inhibition of Gastric CaRs.
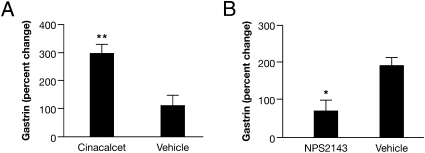
Divalent cations could have a diverse effect on the plasma membrane, resulting in the physiologic stimulation of gastrin secretion by Ca2+ independent of CaR (39). Therefore, CaR function was evaluated with the CaR-specific allosteric agonist and antagonist, cinacalcet (40), and NPS 2143 (41). Gavage of cinacalcet in CaR WT mice stimulated plasma gastrin nearly 300% compared with vehicle (Fig. 3A). NPS 2143, at a dose that stimulated PTH secretion, completely inhibited peptone-stimulated gastrin secretion (Fig. 3B). These results, along with the loss of response to peptone and Ca2+ in CaR-null mice, indicate that their secretory effect is CaR-mediated. Consistent with these results in mice, cinacalcet stimulated serum gastrin and gastric acid secretion in normal human volunteers (42). This acid stimulatory effect of cinacalcet acting on both the G (42) and parietal cell (18) may warrant acid suppressive therapy to prevent cinacalcet intolerance caused by hyperacidity in vulnerable hemodialysis patients on long-term therapy for secondary hyperparathyroidism (43). The inhibition of gastrin secretion by NPS 2143 suggests that clinical trials of calcilytics for the treatment of osteoporosis should monitor potential deleterious inhibition of CaR on nonclassical Ca2+ regulatory tissues (9, 27, 28).
Fig. 3.
CaR-specific agonist and antagonist stimulate and inhibit gastrin secretion, respectively, in CaR WT mice. (A) Gastrin stimulation by CaR-specific agonist, cinacalcet (100 mg/kg by gavage. **P < 0.01 vs. vehicle (n = 9 mice). (B) Inhibition of gavaged peptone-stimulated gastrin by the CaR-specific antagonist, NP-S2143 (1 mg/kg, i.v., 30 min before gavage) at a dose shown to significantly stimulate PTH secretion compared with vehicle. *P < 0.05 vs. vehicle (n = 9 mice). Results are expressed as the percent change (mean ± SEM) in secretion relative to basal.
CaR Expression and Not Total Plasma Ca2+ Determine Basal and Meal-Stimulated Gastrin.
As previously described (44), PTH gene deletion results in a marked reduction in total plasma Ca2+, regardless of CaR genotype (Table S1). In the absence of both CaR and PTH genes, this reduced set point for plasma Ca2+ is still tightly regulated and remains unchanged on a high Ca2+ and vitamin D diet (Table S1). Despite the marked reduction in total plasma Ca2+ in PTH null mice compared with WT (2.35 vs. 1.71 mmol/L), both basal and meal-stimulated gastrin remain unchanged (Table S1). Only the expression of CaR, not total plasma Ca2+ or chronic dietary Ca2+ and vitamin D, affects both basal and meal-stimulated gastrin (Table S1). Therefore, low basal gastrin and loss of meal-stimulated gastrin can be attributed specifically to the loss of CaR expression and not to reduced ambient Ca2+ concentration or another unknown effect of PTH gene deletion.
G-Cell CaRs Are the Predominant Chemosensors Mediating Gastrin Secretion.
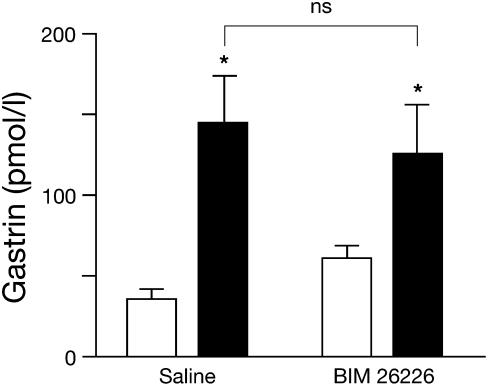
Although G-cell CaR expression and CaR-dependent response to extracellular Ca2+ (8, 16) suggest a direct chemosensory role, they do not preclude a role for neuronal CaR indirectly regulating the G cell. In fact, the rat gastric submucosal and myenteric neurons express CaR (9). GRP neuropetide-secreting intramural neurons project to the antral mucosa and stimulate gastrin secretion through G-cell GRPR-1 [bombesin subtype 1 receptor (BB1R)] receptors (45, 46). Pharmacologic studies using the BB1R subtype selective antagonist, [Leu13-ψ(CH2NH)−Leu14] bombesin, indicate that gastric GRP neurons in rat antral mucosal segments and isolated whole rat stomach (37, 47) mediate peptone-stimulated gastrin release. However, recent human studies with the potent BB1R selective antagonist, BIM 26226, indicate that GRP acts only at pharmacologic doses to regulate gastrin and has no physiologic affect during a meal (48). Although our data (Fig. S3) and data of others support a pharmacologic role for GRP stimulation of BB1R on antral G cells, the physiologic role of GRP neurons in peptone-stimulated gastrin secretion is still in question. However, i.v. infusion of BIM 26226 ([D-F5 Phe6, D-Ala11] bombesin (6–13) OMe) just before peptone gavage did not inhibit gastrin secretion (Fig. 4). This suggests that gastrin secretion is not physiologically regulated by CaR-expressing GRP neurons and is consistent with the findings in humans (47).
Fig. 4.
Inhibition of GRP has no significant effect on peptone-stimulated gastrin secretion. WT (C57BL/6) mice were fasted overnight, i.v. administered with either BIM 26226 or saline control, and immediately gavaged with peptone. Plasma gastrin was measured by RIA just before (open bars) and 30 min after (closed bars) peptone gavage. *P < 0.05, pre- vs. postgavage (ns, peptone plus saline vs. peptone plus BIM 26226; n = 6 and 10 mice for saline and BIM 26226, respectively). ns, nonsignificant.
Deletion of CaR in the Stomach Does Not Alter Acid Secretory Capacity.
To assess functional changes in the oxyntic mucosa in the absence of CaR, acid secretion was measured. In the basal fasting state, there was a marked (75%) reduction in acid secretion (Fig. S4A) and elevation in gastric pH (Fig. S4B) only in CaR-null mice. These results are consistent with earlier results in which only CaR-null mice exhibited lower basal gastrin secretion (Fig. 2). Although there was a trend to lower acid secretion in CaR-null mice, response to either gastrin or histamine was not significantly affected (Fig. S4C). These results suggest that low basal gastrin in CaR-null mice is sufficient to maintain adequate growth and development of the oxyntic mucosa.
CaR Is Necessary to Maintain Normal G-Cell Number and Gastrin Content.
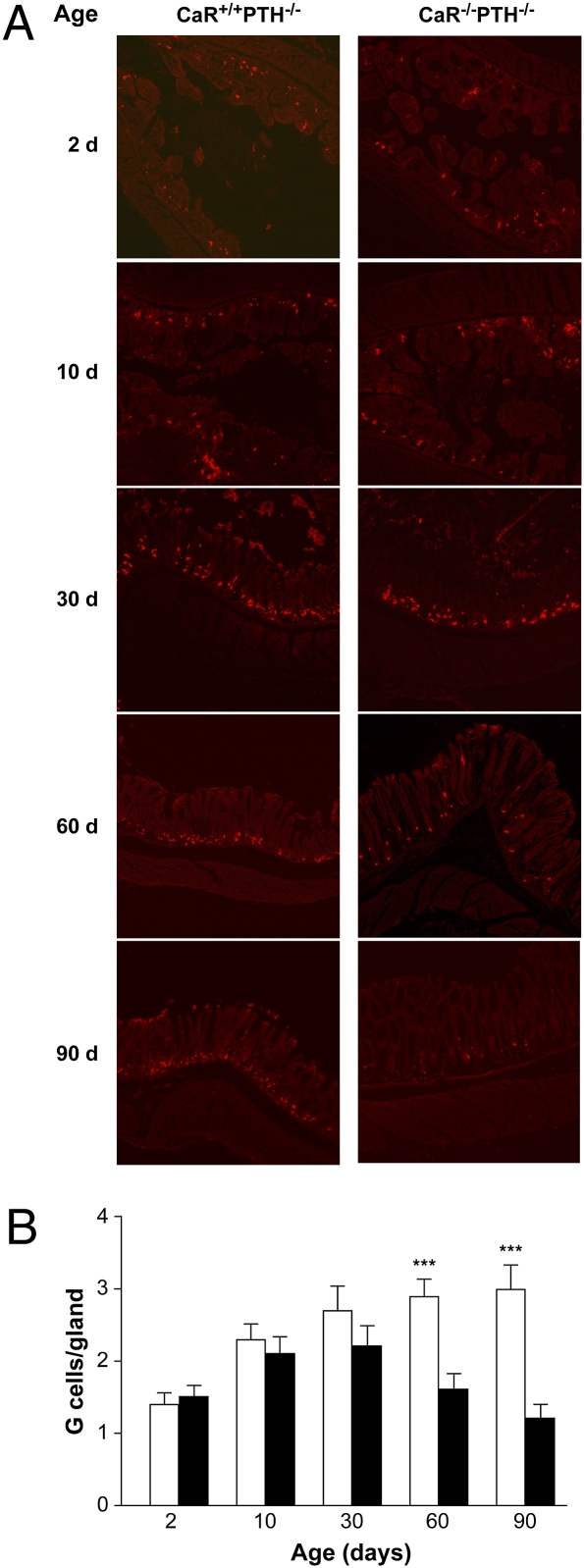
Although the G cell expresses CaR (Fig. 1) and the gastrin response to Ca2+, peptone, Phe, and pH requires CaR (Fig. 2), these data do not explain the low basal plasma gastrin in CaR-null mice (Fig. 2). To address this issue, H&E and gastrin immunofluorescence staining of the gastric antrum were performed. CaR-null mice antra were morphologically indistinguishable from the WT and heterozygous littermates. However, the number of G cells was reduced from an average of 3 cells per antral gland in WT, CaR WT, and heterozygous mice to 1 cell per gland in CaR-null mice (Fig. 5 A and B). This reduction in G-cell number was supported by a similar reduction in total gastric gastrin content (Fig. 5C). Therefore, the observed reduction in G-cell number is the most likely explanation for the reduced basal gastrin secretion. To determine whether the decrease in G-cell number in 60-d-old mice was a result of reduced development vs. progressive age-related loss, antral sections were immunostained for gastrin at postnatal days 2, 10, 30, 60, and 90. After 30 d of age, there was a progressive loss in G-cell number in CaR-null mice and no noticeable loss in CaR WT mice (Fig. 6). With G-cell number being normal up to 30 d of age, CaR-mediated differentiation is unlikely to account for progressive G-cell loss. However, there are ample data supporting a CaR-mediated proliferative effect in transfected HEK-293 cells (49), ovarian epithelial cells, and prostate and breast cancer cell lines, which is ERK1/2-mediated through a PLC/PKC-dependent pathway (50, 51) that may also play a role in the maintenance of G-cell number.
Fig. 5.
CaR-null (CaR−/−PTH−/−) mice have markedly reduced antral G cells and stomach gastrin content at age 60 d. (A) Immunostaining for gastrin in representative sections of antrum. (B) Average number of immunoreactive G cells per gland. Data are the mean number of G cells per gland ± SEM. ***P < 0.001 vs. CaR−/−PTH−/− (n = 50 glands/genotype). (C) Whole-stomach gastrin content. Data are the mean for the extraction of gastrin from the whole stomach ± SEM. ***P < 0.001 vs. CaR−/−PTH−/− (n = 5 stomachs/genotype).
Fig. 6.
CaR-null mice progressively lose gastrin-producing G cells between 30 and 90 d of age. (A) At the indicated age, gastric antral cryosections were prepared for gastrin immunohistochemistry for CaR-null (CaR−/−PTH−/−) and WT (CaR+/+PTH−/−) littermates. Representative images are presented from among multiple sections obtained from at least three mice for each genotype. (B) Average number of immunoreactive G cells per gland. Data are the mean number of G cells per gland ± SEM. ***P < 0.001, WT (CaR+/+PTH−/−, open bars) vs. knock out (CaR−/−PTH−/−, closed bars; n = 10 glands/age group).
The insensitivity of G-cell response to luminal nutrients and pH in the absence of CaR expression indicates that CaR plays a dominant role in the regulation of the gastric phase of acid secretion and therefore, warrants consideration along with accepted G-cell receptors for regulatory neurocrine and paracrine factors such as GRP and somatostatin. These findings and the dependence of G-cell number on CaR functional expression suggest that the calcilytics and calcimimetics acting on CaR should be evaluated for effects on gastric acid secretion.
Materials and Methods
Origin, Breeding, and Genotyping of Genetically Engineered Mice.
CaR and PTH double null mice.
As previously described (52), CaR-null mice die shortly after birth because of hyperparathyroidism, necessitating the use of CaR-null mice with compensatory deletion of the PTH gene (44). CaR−/−PTH−/− mice were derived as previously described (53); 8- to 10-wk-old male mice were used unless specifically stated otherwise.
Sweet and amino acid taste receptor null mice.
T1R2/3 null mice (a gift from Nick Ryba, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD) and (WT) littermate controls were obtained from double heterozygous matings (35).
In Vivo Experiments.
All studies were performed under an approved animal protocol under the supervision of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) animal care and use committee (ACUC) in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Gastrin Secretion Studies.
All secretion studies were performed in 8- to 10-wk-old littermates.
Luminal Studies.
Gastrin response to calcium, protein, phenylalanine, and pH.
Overnight fasted mice were gavaged with a bolus (1.5% of body weight) of 100 mM Ca2+ gluconate (Sigma-Aldrich), 8% peptone (Becton Dinkinson Co.), 100 mM l-phenylalanine (Sigma-Aldrich), or 150 mM (pH 7.0) Hepes buffer (Mediatech). Retro-orbital sinus blood was sampled before and 20–30 min after gavage at the previously determined peak gastrin response for each agent.
Gastrin response to the specific CaR agonist, cinacalcet.
CaR+/+PTH−/− mice were gavaged with either the CaR-specific agonist cinacalcet (HPLC purified from pharmaceutical tablets by C.J.T.; 100 mg/kg dissolved in 20% 2-hydroxypropyl-β-cyclodextrin as vehicle, 1.5% body weight) or with vehicle alone.
Affect of a high Ca2+ diet.
Mice were fed either a normal rodent chow and water diet or a high-Ca2+ diet for 1 mo. After mice were fasted overnight and fed with rat chow ad libitum, blood was sampled from the retro-orbital sinus before and 30 min after feeding for determination of total plasma Ca2+ and gastrin.
Parenteral Studies.
Affect of the CaR-specific antagonist, NPS 2143, on peptone-stimulated gastrin.
Overnight fasted mice were i.v. injected with either the CaR-specific antagonist NPS2143 (synthesized by J-J.K. and C.J.T.) (1 mg/kg dissolved in vehicle, 20% 2-hydroxypropyl-β-cyclodextrin; Sigma-Aldrich) as a 100-μL bolus or vehicle into the tail vein. Thirty minutes postinjection, mice were gavaged with a bolus (1.5% body weight) of 8% peptone.
Affect of the BB1R-specific antagonist, BIM 26226, on peptone-stimulated gastrin.
WT C57BL/6 mice were injected with either the BB1R-specific antagonist, BIM 26226 (500 μg/kg), dissolved in 100 μL saline or saline alone into the tail vein. Thirty minutes postinjection, mice were gavaged with a 1.5% body weight bolus of 8% peptone.
Total Plasma Calcium Levels.
Total plasma Ca2+ was determined using a SYNCHRON LX20 in ad libitum-fed mice on the specified diet.
Determination of Gastric Tissue and Plasma Gastrin.
The stomachs from overnight-fasted and anesthetized mice were collected, and whole-stomach gastrin was extracted as previously described (54). The plasma (20 μL) and stomach-extracted gastrins were measured by radioimmunoassay (Euro-Diagnostic AB).
Immunocytochemistry.
SI Materials and Methods has information on immunocytochemistry.
Statistical Analysis.
Paired Student t test was used to evaluate the differences between baseline and stimulated values. One- and two-way ANOVA was applied to analyze the differences between genotypes in response to single and multiple conditions, respectively. Statistical analysis was performed using GraphPad Prism version 3.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health and by Program Project Grant P01-0707056 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009078107/-/DCSupplemental.
References
- 1.Walsh JH, Grossman MI. Gastrin (first of two parts) N Engl J Med. 1975;292:1324–1334. doi: 10.1056/NEJM197506192922505. [DOI] [PubMed] [Google Scholar]
- 2.Taylor IL, Byrne WJ, Christie DL, Ament ME, Walsh JH. Effect of individual l-amino acids on gastric acid secretion and serum gastrin and pancreatic polypeptide release in humans. Gastroenterology. 1982;83:273–278. [PubMed] [Google Scholar]
- 3.McArthur KE, Isenberg JI, Hogan DL, Dreier SJ. Intravenous infusion of L-isomers of phenylalanine and tryptophan stimulate gastric acid secretion at physiologic plasma concentrations in normal subjects and after parietal cell vagotomy. J Clin Invest. 1983;71:1254–1262. doi: 10.1172/JCI110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Levant JA, Walsh JH, Isenberg JI. Stimulation of gastric secretion and gastrin release by single oral doses of calcium carbonate in man. N Engl J Med. 1973;289:555–558. doi: 10.1056/NEJM197309132891104. [DOI] [PubMed] [Google Scholar]
- 6.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 7.Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing receptors: Implications for GI physiology. Am J Physiol Gastrointest Liver Physiol. 2006;291:G753–G761. doi: 10.1152/ajpgi.00189.2006. [DOI] [PubMed] [Google Scholar]
- 8.Buchan AM, Squires PE, Ring M, Meloche RM. Mechanism of action of the calcium-sensing receptor in human antral gastrin cells. Gastroenterology. 2001;120:1128–1139. doi: 10.1053/gast.2001.23246. [DOI] [PubMed] [Google Scholar]
- 9.Cheng I, et al. Expression of an extracellular calcium-sensing receptor in rat stomach. Gastroenterology. 1999;116:118–126. doi: 10.1016/s0016-5085(99)70235-0. [DOI] [PubMed] [Google Scholar]
- 10.Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu Rev Physiol. 2009;71:205–217. doi: 10.1146/annurev.physiol.010908.163128. [DOI] [PubMed] [Google Scholar]
- 11.Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci USA. 2000;97:4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem. 2004;279:37241–37249. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- 13.Quinn SJ, et al. Sodium and ionic strength sensing by the calcium receptor. J Biol Chem. 1998;273:19579–19586. doi: 10.1074/jbc.273.31.19579. [DOI] [PubMed] [Google Scholar]
- 14.Conigrave AD, et al. L-amino acids regulate parathyroid hormone secretion. J Biol Chem. 2004;279:38151–38159. doi: 10.1074/jbc.M406373200. [DOI] [PubMed] [Google Scholar]
- 15.Goebel SU, et al. Expression of the calcium-sensing receptor in gastrinomas. J Clin Endocrinol Metab. 2000;85:4131–4137. doi: 10.1210/jcem.85.11.6963. [DOI] [PubMed] [Google Scholar]
- 16.Ray JM, Squires PE, Curtis SB, Meloche MR, Buchan AM. Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J Clin Invest. 1997;99:2328–2333. doi: 10.1172/JCI119413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dufner MM, et al. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1084–G1090. doi: 10.1152/ajpgi.00571.2004. [DOI] [PubMed] [Google Scholar]
- 18.Geibel JP, et al. The stomach divalent ion-sensing receptor scar is a modulator of gastric acid secretion. J Biol Chem. 2001;276:39549–39552. doi: 10.1074/jbc.M107315200. [DOI] [PubMed] [Google Scholar]
- 19.Jain RN, Samuelson LC. Differentiation of the gastric mucosa. II. Role of gastrin in gastric epithelial cell proliferation and maturation. Am J Physiol Gastrointest Liver Physiol. 2006;291:G762–G765. doi: 10.1152/ajpgi.00172.2006. [DOI] [PubMed] [Google Scholar]
- 20.Behar J, Hitchings M, Smyth RD. Calcium stimulation of gastrin and gastric acid secretion: Effect of small doses of calcium carbonate. Gut. 1977;18:442–448. doi: 10.1136/gut.18.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bevilacqua M, et al. Dissimilar PTH, gastrin, and calcitonin responses to oral calcium and peptones in hypocalciuric hypercalcemia, primary hyperparathyroidism, and normal subjects: A useful tool for differential diagnosis. J Bone Miner Res. 2006;21:406–412. doi: 10.1359/JBMR.051210. [DOI] [PubMed] [Google Scholar]
- 22.Schinke T, et al. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat Med. 2009;15:674–681. doi: 10.1038/nm.1963. [DOI] [PubMed] [Google Scholar]
- 23.Frauli M, Neuville P, Vol C, Pin JP, Prézeau L. Among the twenty classical L-amino acids, only glutamate directly activates metabotropic glutamate receptors. Neuropharmacology. 2006;50:245–253. doi: 10.1016/j.neuropharm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Pin JP, et al. Activation mechanism of the heterodimeric GABA(B) receptor. Biochem Pharmacol. 2004;68:1565–1572. doi: 10.1016/j.bcp.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 25.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 26.Pi M, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mun HC, et al. The Venus Fly Trap domain of the extracellular Ca2+ -sensing receptor is required for L-amino acid sensing. J Biol Chem. 2004;279:51739–51744. doi: 10.1074/jbc.M406164/200. [DOI] [PubMed] [Google Scholar]
- 28.Hira T, Nakajima S, Eto Y, Hara H. Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells. FEBS J. 2008;275:4620–4626. doi: 10.1111/j.1742-4658.2008.06604.x. [DOI] [PubMed] [Google Scholar]
- 29.Rasschaert J, Malaisse WJ. The G-protein-coupled, extracellular Ca(2+)-sensing receptor: Expression in pancreatic islet B-cells and possible role in the regulation of insulin release. Mol Genet Metab. 1999;68:328–331. doi: 10.1006/mgme.1999.2928. [DOI] [PubMed] [Google Scholar]
- 30.Kidd M, Hauso O, Drozdov I, Gustafsson BI, Modlin IM. Delineation of the chemomechanosensory regulation of gastrin secretion using pure rodent G cells. Gastroenterology. 2009;137:231–241. doi: 10.1053/j.gastro.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Schubert ML, Edwards NF, Makhlouf GM. Regulation of gastric somatostatin secretion in the mouse by luminal acidity: A local feedback mechanism. Gastroenterology. 1988;94:317–322. doi: 10.1016/0016-5085(88)90418-0. [DOI] [PubMed] [Google Scholar]
- 32.Piqueras L, Martínez V. Role of somatostatin receptors on gastric acid secretion in wild-type and somatostatin receptor type 2 knockout mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:510–520. doi: 10.1007/s00210-004-0992-8. [DOI] [PubMed] [Google Scholar]
- 33.Doroszewicz J, Waldegger P, Jeck N, Seyberth H, Waldegger S. pH dependence of extracellular calcium sensing receptor activity determined by a novel technique. Kidney Int. 2005;67:187–192. doi: 10.1111/j.1523-1755.2005.00069.x. [DOI] [PubMed] [Google Scholar]
- 34.Stim JA, Bernardo AA, Arruda JA. The role of parathyroid hormone and vitamin D in acid excretion and extrarenal buffer mobilization. Miner Electrolyte Metab. 1994;20:60–71. [PubMed] [Google Scholar]
- 35.Zhao GQ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 36.Berthoud HR. Morphological analysis of vagal input to gastrin releasing peptide and vasoactive intestinal peptide containing neurons in the rat glandular stomach. J Comp Neurol. 1996;370:61–70. doi: 10.1002/(SICI)1096-9861(19960617)370:1<61::AID-CNE6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 37.Schubert ML, Coy DH, Makhlouf GM. Peptone stimulates gastrin secretion from the stomach by activating bombesin/GRP and cholinergic neurons. Am J Physiol. 1992;262:G685–G689. doi: 10.1152/ajpgi.1992.262.4.G685. [DOI] [PubMed] [Google Scholar]
- 38.Conigrave AD, Mun HC, Brennan SC. Physiological significance of L-amino acid sensing by extracellular Ca(2+)-sensing receptors. Biochem Soc Trans. 2007;35:1195–1198. doi: 10.1042/BST0351195. [DOI] [PubMed] [Google Scholar]
- 39.Nemeth EF. Misconceptions about calcimimetics. Ann N Y Acad Sci. 2006;1068:471–476. doi: 10.1196/annals.1346.044. [DOI] [PubMed] [Google Scholar]
- 40.Nagano N, Nemeth EF. Functional proteins involved in regulation of intracellular Ca(2+) for drug development: The extracellular calcium receptor and an innovative medical approach to control secondary hyperparathyroidism by calcimimetics. J Pharmacol Sci. 2005;97:355–360. doi: 10.1254/jphs.fmj04007x6. [DOI] [PubMed] [Google Scholar]
- 41.Nemeth EF, et al. Calcilytic compounds: Potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther. 2001;299:323–331. [PubMed] [Google Scholar]
- 42.Ceglia L, Harris SS, Rasmussen HM, Dawson-Hughes B. Activation of the calcium sensing receptor stimulates gastrin and gastric acid secretion in healthy participants. Osteoporos Int. 2009;20:71–78. doi: 10.1007/s00198-008-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block GA, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 44.Kos CH, et al. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest. 2003;111:1021–1028. doi: 10.1172/JCI17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dockray GJ, Vaillant C, Walsh JH. The neuronal origin of bombesin-like immunoreactivity in the rat gastrointestinal tract. Neuroscience. 1979;4:1561–1568. doi: 10.1016/0306-4522(79)90019-8. [DOI] [PubMed] [Google Scholar]
- 46.DuVal JW, et al. Stimulation of gastrin and somatostatin secretion from the isolated rat stomach by bombesin. Am J Physiol. 1981;241:G242–G247. doi: 10.1152/ajpgi.1981.241.3.G242. [DOI] [PubMed] [Google Scholar]
- 47.Matsuno M, Matsui T, Iwasaki A, Arakawa Y. Role of acetylcholine and gastrin-releasing peptide (GRP) in gastrin secretion. J Gastroenterol. 1997;32:579–586. doi: 10.1007/BF02934105. [DOI] [PubMed] [Google Scholar]
- 48.Hildebrand P, et al. Regulation of gastric function by endogenous gastrin releasing peptide in humans: Studies with a specific gastrin releasing peptide receptor antagonist. Gut. 2001;49:23–28. doi: 10.1136/gut.49.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rey O, Young SH, Yuan JZ, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J Biol Chem. 2005;280:22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- 50.El Hiani Y, et al. Extracellular signal-regulated kinases 1 and 2 and TRPC1 channels are required for calcium-sensing receptor-stimulated MCF-7 breast cancer cell proliferation. Cell Physiol Biochem. 2009;23:335–346. doi: 10.1159/000218179. [DOI] [PubMed] [Google Scholar]
- 51.Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr Rev. 2009;30:178–195. doi: 10.1210/er.2008-0041. [DOI] [PubMed] [Google Scholar]
- 52.Ho C, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 53.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest. 2002;109:1173–1182. doi: 10.1172/JCI14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friis-Hansen L, Rehfeld JF. Ileal expression of gastrin and cholecystokinin. In search of a related hormone. FEBS Lett. 1994;343:115–119. doi: 10.1016/0014-5793(94)80301-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.