Abstract
Autotransporters are bacterial virulence factors consisting of an N-terminal “passenger domain” that is secreted in a C- to-N-terminal direction and a C-terminal “β domain” that resides in the outer membrane (OM). Although passenger domain secretion does not appear to use ATP, the energy source for this reaction is unknown. Here, we show that efficient secretion of the passenger domain of the Escherichia coli O157:H7 autotransporter EspP requires the stable folding of a C-terminal ≈17-kDa passenger domain segment. We found that mutations that perturb the folding of this segment do not affect its translocation across the OM but impair the secretion of the remainder of the passenger domain. Interestingly, an examination of kinetic folding mutants strongly suggested that the ≈17-kDa segment folds in the extracellular space. By mutagenizing the ≈17-kDa segment, we also fortuitously isolated a unique translocation intermediate. Analysis of this intermediate suggests that a heterooligomer that facilitates the membrane integration of OM proteins (the Bam complex) also promotes the surface exposure of the ≈17-kDa segment. Our results provide direct evidence that protein folding can drive translocation and help to clarify the mechanism of autotransporter secretion.
Keywords: autotransporters, Bam complex, outer membrane, protein folding, protein translocation
Autotransporters are a large superfamily of virulence factors produced by Gram-negative bacteria that consist of two domains, an N-terminal passenger domain that frequently exceeds 100 kDa and a C-terminal ≈30-kDa β domain (reviewed in ref. 1). The passenger domain is secreted into the extracellular space, where it mediates the virulence function of the protein, and is often released from the cell surface by a subsequent proteolytic cleavage. Structural and bioinformatic studies strongly suggest that almost all passenger domains form an elongated β helix (2–5). The β domain forms a 12-stranded β barrel that is localized to the outer membrane (OM) (6–8). The pore of the β barrel is traversed by an α-helical segment that typically protrudes into the extracellular space and connects the β domain to the passenger domain. In some cases, however, the α-helical segment is cleaved in an intrabarrel reaction that releases the passenger domain and leaves only a small α-helical fragment inside the β barrel (7, 9). The α-helical segment appears to be incorporated into the pore of the β barrel (which presumably acquires considerable tertiary structure in the periplasm) before its integration into the OM (10).
Recent work has shown that passenger domains are secreted in a C-to-N-terminal direction (11, 12), but the mechanism of secretion is unclear. Based on the observation that the deletion of the β domain abolishes secretion, it was originally proposed that the passenger domain is secreted through a channel formed by the covalently linked β domain (13). In this model, the C terminus of the passenger domain first inserts into the β domain pore as a hairpin, and N-terminal segments progressively slide past a static strand. Because the β domain pore is only ≈10 Å in diameter (6–8), the hairpin would most likely be in a fully extended conformation until translocation is complete, at which point the C terminus of the passenger domain would form an α helix. More recent data, however, have challenged the self-transport or “autotransporter” hypothesis. Most notably, folded polypeptides that cannot fit into the β barrel pore have been shown to be efficiently secreted via the autotransporter pathway (14, 15). Furthermore, a stalled passenger domain translocation intermediate can be cross-linked to BamA, a subunit of a protein complex (Bam complex) that facilitates the integration of β barrel proteins into the OM (12, 16). These observations have led to the proposal that the secretion of the passenger domain and membrane integration of the β domain are facilitated by the Bam complex in a concerted reaction (6, 12, 14). In this model, the β domain is required for secretion because it targets the passenger domain to the Bam complex.
The source of energy used for passenger domain secretion is also unknown. Although the periplasm is devoid of ATP, it is conceivable that passenger domain translocation is driven by an unidentified inner membrane (IM) protein that utilizes ATP hydrolysis or the IM membrane potential. Such a protein might act directly on the passenger domain or interact with periplasmic or OM factors. As an alternative, the folding of the passenger domain in the extracellular space might promote translocation, possibly by acting as a Brownian ratchet (17). Consistent with this hypothesis, it has been shown that two different purified passenger domains fold slowly in vitro and contain a protease-resistant ≈20- to 25-kDa C-terminal segment whose sequence is conserved (5, 18, 19). The data raise the possibility that a C-terminal stable core might nucleate vectorial folding of the β helix. The exact function of this C-terminal region, however, is unclear. Although in one case the introduction of specific mutations into this region appears to inhibit passenger domain secretion (20), in several other cases deletion of this segment perturbs passenger domain folding and stability but does not clearly affect secretion (19, 21, 22). Furthermore, recent studies suggest that the β helix folds in a concerted process involving the whole protein rather than a stepwise process that requires the formation of a stable C-terminal core (23).
In this study, we reexamined the role of the C-terminal segment of the passenger domain in secretion by using the Escherichia coli O157:H7 autotransporter EspP as a model protein. The EspP passenger domain is released from the cell surface by an intrabarrel cleavage after the completion of translocation (14, 24). By analyzing mutants of EspP we obtained evidence that a ≈17-kDa C-terminal fragment is initially exposed on the cell surface and that the stable folding of this segment in the extracellular space is the rate-limiting step in the translocation of the rest of the passenger domain. The results demonstrate a clear coupling of protein folding and secretion and strongly suggest that at least part of the passenger domain is secreted as an unfolded polypeptide. During the course of our experiments we isolated an early translocation intermediate in which only the ≈17-kDa C-terminal fragment was exposed. Examination of this intermediate strongly suggested that the initial phase of translocation is facilitated by the Bam complex.
Results
The Folding of a C-Terminal Stable Core Drives Secretion of the EspP Passenger Domain.
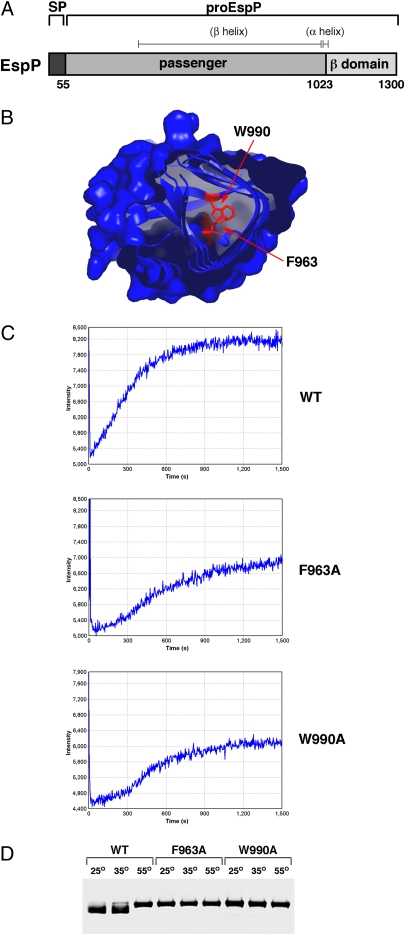
It has been shown that the mutation of large hydrophobic residues near the C terminus of the EspP passenger domain severely reduces the steady-state level of secreted protein in the culture medium (20). Based on the crystal structure of the passenger domain of Hbp (2), a closely related autotransporter, we surmised that these mutations might perturb the folding of a protease-resistant C-terminal fragment by destabilizing the hydrophobic core of the β helix. To test this idea, we first purified a fragment of the wild-type EspP passenger domain that corresponds to the β helix (residues 315–1023; Fig. 1A and ref. 25) and derivatives containing a mutation (F963A or W990A) that we predicted would disrupt the packing of the hydrophobic core (Fig. 1B). The purified mutant polypeptides appeared to be properly folded (Fig. S1). We next denatured the β-helical fragments and examined their refolding by Trp fluorescence. Consistent with our hypothesis, both mutant β helices refolded more slowly and less efficiently than the wild-type β helix (Fig. 1C). Because exclusively β-stranded proteins are often particularly resistant to SDS denaturation (26), we also compared the sensitivity of the wild-type and mutant β helices to this detergent. We found that although the wild-type β helix maintained a compact structure that migrated relatively rapidly on SDS/PAGE when it was heated at 35 °C, the mutant β helices were completely denatured at 25 °C (Fig. 1D). Taken together, the results indicate that residues F963 and W990 play a key role in the folding of at least the C terminus of the EspP passenger domain.
Fig. 1.
The mutation of C-terminal hydrophobic residues perturbs the folding of the EspP passenger domain. (A) Illustration of EspP showing the signal peptide (SP) (residues 1–55), the passenger domain (residues 56–1023), and the β domain (residues 1024–1300). ProEspP contains covalently linked passenger and β domains. The location of predicted structural elements is also shown. (B) Crystal structure of the C terminus of Hbp (2). The conserved residues that correspond to F963 and W990 in EspP are shown. (C) Refolding of the β helix of EspP, EspP(F963A), and EspP(W990A) was examined by monitoring Trp fluorescence after dilution of the polypeptides out of 4 M Gdn-HCl. Fluorescence intensity is in arbitrary units. (D) The β-helical domain of EspP, EspP(F963A), and EspP(W990A) was heated at the indicated temperature in sample buffer containing 0.1% SDS and resolved by SDS/PAGE.
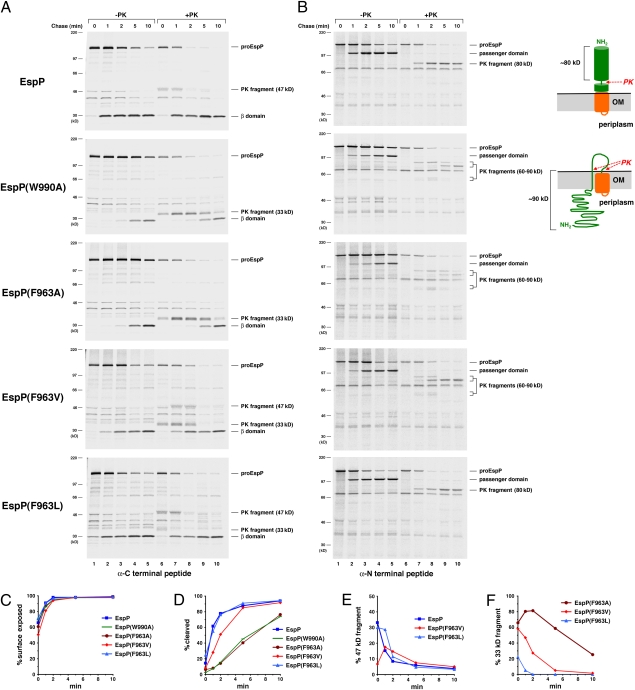
We next examined the effect of the F963A and W990A mutations on EspP passenger domain secretion. AD202 transformed with a plasmid encoding either wild-type or mutant EspP under the control of the trc promoter were grown in minimal medium. After espP expression was induced by the addition of isopropylthiogalactoside (IPTG), cells were subjected to pulse-chase labeling. Half of the cells were then treated with proteinase K (PK), and the other half were untreated. Finally, samples were subjected to immunoprecipitation with antisera directed against N-terminal and C-terminal EspP peptides and resolved by SDS/PAGE. We assessed the exposure of the passenger domain on the cell surface by quantitating the fraction of proEspP (the precleavage form of the protein that contains covalently linked passenger and β domains) that was sensitive to PK digestion. To assess the cleavage of the protein into discrete passenger and β domain fragments, we quantitated the amount of free β domain that was immunoprecipitated with the C-terminal antiserum. As previously observed, the wild-type passenger domain was exposed rapidly on the cell surface (Fig. 2A Top and C). Cleavage lagged behind cell surface exposure and appeared to occur only after translocation was complete (Fig. 2 A and B Top and D). Interestingly, a ≈47-kDa C-terminal fragment containing the β domain plus a ≈17-kDa C-terminal passenger domain fragment that presumably corresponds to the stable core of other autotransporters was observed at early time points (Fig. 2A Top, lanes 6 and 7, and E). This fragment was not detected at late time points as a consequence of passenger domain cleavage. As reported (12), an ≈80-kDa PK-resistant N-terminal fragment accumulated at essentially the same rate as the cleaved passenger domain and could also be generated from soluble passenger domain (Fig. 2B Top and Fig. S2). This fragment likely resulted from the folding of the N terminus of the passenger domain around the time of cleavage.
Fig. 2.
Point mutations that impair the folding of the EspP stable core delay passenger domain secretion. AD202 transformed with pRLS5 (Ptrc-espP) or a plasmid encoding the indicated EspP mutant were subjected to pulse–chase labeling after the addition IPTG. Half of the cells were treated with PK, and EspP-containing polypeptides were immunoprecipitated using C-terminal (A) and N-terminal (B) anti-EspP antisera. The samples in B were resolved on 4–12% NuPage/MOPS gels (Invitrogen). The percent of the passenger domain that was surface exposed or cleaved from proEspP in A is plotted in C and D, and the percent of each protein that was converted to a ≈47- or ≈33-kDa C-terminal fragment by PK treatment is plotted in E and F.
Although the F963A and W990A mutations did not affect the initial exposure of the passenger domain on the cell surface, they had a striking effect on EspP biogenesis and the pattern of fragments produced by PK digestion (Fig. 2A, gels 2 and 3 and C). Both mutations markedly delayed passenger domain cleavage (Fig. 2D). Furthermore, PK treatment generated a ≈33-kDa C-terminal fragment instead of a ≈47-kDa fragment that likewise declined in parallel with passenger domain cleavage (Fig. 2F). This fragment contained only the ≈30 residues at the extreme C terminus of the passenger domain that presumably reside inside the β domain as an α helix. The presence of the ≈33-kDa fragment implies that point mutations perturb the folding of the C-terminal stable core. PK treatment also produced unique ≈90- and ≈60-kDa N-terminal fragments that disappeared at late time points (Fig. 2B, gels 2 and 3). The finding that these fragments could not be generated from soluble passenger domain and could be degraded when the OM was permeabilized (Figs. S2 and S3) indicated that they were derived from proEspP and that they correspond to large segments of the passenger domain that were transiently trapped inside the periplasm. An ≈80-kDa fragment that could also be generated from soluble passenger domain was observed only at late time points (Fig. 2B, gels 2 and 3, lanes 9 and 10 and Fig. S2). These results indicate that by perturbing the folding of the C-terminal stable core, the point mutations delay the secretion of the entire polypeptide N-terminal to it and thereby delay other steps of EspP biogenesis (e.g., passenger domain cleavage) that require completion of translocation.
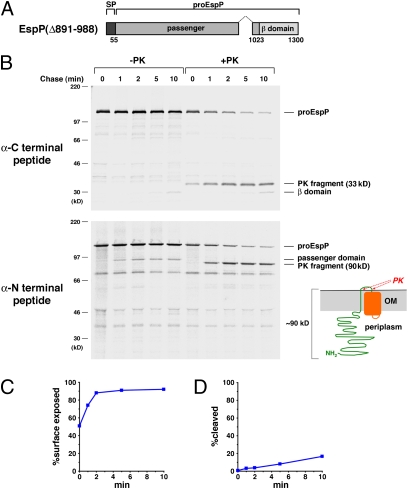
We next deleted ≈100 residues from the C terminus of the EspP passenger domain to examine the effect of a more drastic mutation on protein biogenesis. The EspP(Δ891–988) mutation (Fig. 3A) only slightly impaired the exposure of the C terminus of the passenger domain on the cell surface (Fig. 3 B and C). In contrast, the mutation almost completely abolished passenger domain cleavage (Fig. 3 B and D). PK treatment generated ≈33-kDa C-terminal and ≈90-kDa N-terminal fragments that persisted throughout the time course (Fig. 3B). The ≈90-kDa fragment was degraded when the OM was permeabilized (Fig. S3) and therefore corresponds to a large segment of proEspP that was trapped in the periplasm. The results imply that the EspP(Δ891–988) mutation exerts a stronger effect on EspP biogenesis than the F963A and W990A mutations by nearly completely blocking secretion of the bulk of the passenger domain. Curiously, the point mutations and the deletion both caused the retention of a ≈90-kDa N-terminal fragment inside the cell. Because the passenger domain of EspP(Δ891–988) is smaller, this observation implies that the deletion reduced the size of the C-terminal polypeptide that was exposed on the cell surface and suggests that the C terminus of the passenger domain has unique properties that facilitate its transport across the OM.
Fig. 3.
A large C-terminal deletion traps the majority of the EspP passenger domain in the periplasm. (A) Illustration of EspP Δ891–988. (B) AD202 transformed with pJH104 (Ptrc-espPΔ891–988) were treated as described in Fig. 2, and EspP-containing polypeptides were immunoprecipitated using C- and N-terminal anti-EspP antisera. The samples in B Lower were resolved on a 4–12% NuPage/MOPS gel. The percent of the passenger domain that was surface exposed or cleaved from proEspP in B is shown in C and D.
Taken together, the results strongly suggest that the translocation of the EspP passenger domain involves at least two steps. In the first step, a C-terminal ≈17-kDa fragment is rapidly exposed on the cell surface in a reaction that is independent of protein folding. Subsequently, translocation of the rest of the passenger domain is coupled to folding of the C-terminal fragment. Defects in the completion of translocation correlate with the severity of C-terminal mutations. Consistent with the notion that mutations that impair the folding of the stable core only affect the translocation of distal polypeptide segments, we found that the introduction of the F963A and W990A mutations into EspPΔ1, a truncated protein containing only the last 116 residues of the passenger domain, had no effect on either translocation or cleavage (Fig. S4). We also found that further deletion of the passenger domain impeded signal peptide processing but not passenger domain secretion (Fig. S5).
Folding of the EspP C-Terminal Stable Core in the Extracellular Space.
Although the above results demonstrated that the folding of a ≈17-kDa C-terminal fragment facilitates the secretion of the majority of the passenger domain, they did not indicate whether the folding of the stable core occurs before or after its exposure on the cell surface. To distinguish between these possibilities, we sought mutations that would simply slow the folding of the stable core. We conjectured that the introduction of hydrophobic residues at position 963 that are smaller than Phe but larger than Ala might slow the formation of a hydrophobic core and produce the desired kinetic effect. Consistent with our hypothesis, we found that the F963V mutation significantly delayed the appearance of the ≈47-kDa C-terminal fragment that was observed when wild-type EspP was digested with PK and that a milder F963L mutation produced a similar although much subtler effect (Fig. 2A, gels 4 and 5 and E). Presumably because the delay in folding slowed the completion of passenger domain translocation, the F963V mutation reduced the rate of passenger domain cleavage (Fig. 2D). Interestingly, PK treatment of the F963V mutant also generated a ≈33-kDa C-terminal and a ≈90-kDa N-terminal fragment, but these fragments disappeared more rapidly than the corresponding fragments generated by PK treatment of the F963A mutant (Fig. 2 A and B, gel 4 and F). Although PK treatment of the F963L mutant likewise generated a ≈33-kDa fragment, this fragment was observed only at the first time point (Fig. 2A, gel 5, lane 6). The early appearance of the ≈33-kDa fragment of both the F963V and F963L mutants followed by its disappearance and the gradual appearance of the ≈47-kDa fragment was highly reproducible (Fig. S6). This temporal pattern implies that the C terminus of the mutants is initially exposed on the cell surface as an unfolded polypeptide and subsequently folds in the extracellular space.
The EspP Passenger Domain Interacts with BamA Upon exposure of Its C Terminus.
By transiently stalling translocation after exposure of a C-terminal ≈17-kDa passenger domain fragment, the EspP(F963A) mutation creates a translocation intermediate that can be used to examine early steps in secretion. We wanted to use this intermediate to determine whether the translocation of the C terminus of the passenger domain might be facilitated by an unlinked factor. The autotransporter hypothesis predicts that when translocation stalls, a polypeptide segment that is in an extended conformation (≈3.5 Å per residue) traverses the β barrel. Given that the distance from the passenger domain cleavage site inside the β barrel to the top of the extracellular loops is ≈40 Å (7), PK digestion would be expected to protect only ≈12 residues of the passenger domain if its extreme C terminus is fully extended. PK treatment of EspP(F963A) during the stalling of translocation, however, produced a ≈33-kDa fragment that appears to be ≈30 residues larger than the β domain. After complete translocation of the passenger domain, PK digestion of a truncated version of EspP that cannot undergo passenger domain cleavage (EspP*Δ1) also produces a ≈33-kDa fragment (refs. 14 and 24 and Fig. S3). The size of this fragment agrees well with the prediction that a ≈30-residue segment of the passenger domain resides inside the EspP*Δ1 β barrel as an α helix (≈1.5 Å per residue) following translocation. The similarity of the EspP(F963A) and EspP*Δ1 PK products suggests that the β barrel is traversed by an α-helical polypeptide at an early stage of translocation.
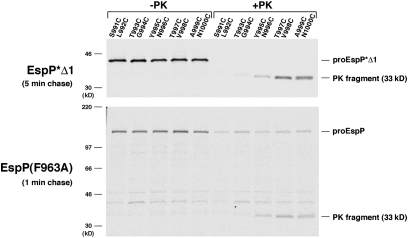
To compare the conformation of the polypeptide that resides inside the EspP β barrel before and after the completion of translocation, we mutated pairs of residues near the predicted PK cleavage site in EspP*Δ1 (which lacks cysteines) to cysteine. Cells that produced the mutants were pulse-labeled with [35S]cysteine and subjected to a 5-min chase to ensure that passenger domain translocation was complete. Half of the cells were treated with PK, and immunoprecipitations were performed with the C-terminal anti-EspP antiserum. Although radiolabeled residues C991–C994 were completely digested by PK and some of the C995 and C996 was also digested, residues C997–C1000 were completely protected (Fig. 4, Top). Based on these results, we estimate that the last ≈27 residues of the passenger domain (residues 997–1023) reside inside the β barrel after translocation. When the same set of mutations was introduced into EspP(F963A) (which contains two cysteines) and cells were subjected to a 1-min chase to trap the passenger domain in a partially translocated state, cysteines at the same positions were protected from digestion (Fig. 4, Bottom). This finding shows that the conformation of the peptide embedded inside the EspP β barrel is established before the completion of translocation. Experiments in which the helix-promoting alanines in the segment 999ANKEATRNAAA1009 of EspP(F963A) were mutated to helix-breaking glycines also yielded data that were consistent with the notion that the peptide is in an α-helical conformation when translocation stalls (Fig. S7). Although the results strongly suggest that the EspP β barrel contains a static α-helical peptide during the entire translocation reaction, they do not exclude the possibility that the β barrel is in an expanded conformation in which it can also accommodate an extended hairpin.
Fig. 4.
An α-helical segment traverses the EspP β barrel before the completion of passenger domain translocation. AD202 transformed with a plasmid encoding the indicated derivative of EspP*Δ1 or EspP(F963A) were pulse-labeled with [35S]cysteine after the addition IPTG and subjected to a 1- or 5-min chase. Half of the cells were treated with PK, and EspP-containing polypeptides were immunoprecipitated using the C-terminal anti-EspP antisera.
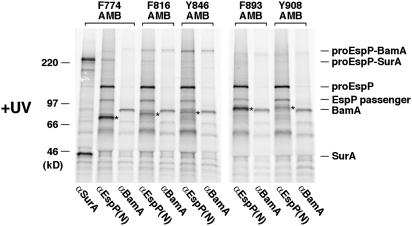
We next used a site-specific in vivo photocrosslinking method to identify factors that might promote the first stage of passenger domain translocation. This technique employs the incorporation of the photoactivatable amino acid analog p-benzoyl-l-phenylalanine (Bpa) at amber codons and was used to show that the EspP passenger domain interacts with BamA when translocation stalls at residue ≈590 (12). Because the F963A mutation stalls translocation at residue ≈860 (≈160 residues from the passenger domain C terminus), we introduced an amber codon into EspP or EspP(F963A) at one of five positions between residues 774 and 908. AD202 were transformed with plasmids that encode an amber mutant and the amber suppression system (pDULEBpa). Two equal samples were removed from radiolabeled cultures after a 1-min chase and one sample was exposed to UV light. EspP-containing polypeptides were then immunoprecipitated using the N-terminal antiserum. Interestingly, UV-irradiation of the EspP(F963A) derivatives contained an amber codon immediately N-terminal to the site of stalling (residues 816 and 846) but none of the wild-type derivatives yielded a high molecular weight adduct that could be immunoprecipitated with an anti-BamA antiserum (Fig. 5 and Fig. S8). This adduct could also be immunoprecipitated with the C-terminal anti-EspP antiserum and therefore contained proEspP (Fig. S9). In contrast, no significant cross-linking was observed to residues that were exposed on the cell surface (residues 893 and 908). Consistent with previous results (12), a periplasmically disposed residue that was far from the site of stalling (residue 774) was cross-linked to the chaperone SurA. These results show that EspP(F963A) interacts with BamA when translocation stalls early and suggest that the Bam complex promotes exposure of the C-terminal ≈17-kDa fragment.
Fig. 5.
Cross-linking of an early EspP secretion intermediate to BamA. AD202 were transformed with pDULEBpa and a derivative of pRI22 [Plac-espP] harboring the F963A mutation and an amber codon at the indicated position. Cells were pulse-labeled and subjected to a 1-min chase after the addition of IPTG. Half of each sample was UV-irradiated, and equal portions were used for immunoprecipitations with the N-terminal anti-EspP antiserum or anti-BamA or anti-SurA antisera. Truncated forms of EspP that resulted from translation termination at the amber codon are denoted (*).
Discussion
In this study, we used mutants of EspP to obtain evidence that the secretion of an autotransporter passenger domain occurs in at least two distinct stages. In the first stage, a C-terminal segment is translocated across the OM. The folding of this fragment then facilitates the secretion of the remainder of the polypeptide. We found that the EspP F963A and W990A mutations impaired the refolding of the purified β helix and prevented the folding of the ≈17-kDa C-terminal stable core in vivo. Although these mutations had no effect on the cell surface exposure of the C terminus or the secretion of a truncated passenger domain, they delayed the secretion of the N-terminal ≈90-kDa of the full-length passenger domain. Furthermore, we found that the deletion of a large portion of the ≈17-kDa fragment did not significantly impair the initiation of translocation (and therefore did not cause a global folding defect), but blocked the second stage of translocation. The results show that the severity of C-terminal mutations correlates with defects in the completion of the translocation reaction. The results also suggest that although the C terminus of passenger domains containing point mutations like F963A does not fold stably, it adopts a conformation (perhaps transiently) that is compatible with the secretion and folding of the N terminus. Based on the analysis of two mutations (F963V and F963L) that slowed the folding of the ≈17-kDa fragment (but produced no other discernable effect), it is very likely that the C terminus of the native passenger domain traverses the OM in an unfolded conformation and then folds in the extracellular space.
Although protein translocation reactions generally require ATP hydrolysis or a proton motive force, our results show that the transport of a polypeptide across a membrane can be promoted by protein folding and does not necessarily require the input of external energy. Presumably, the C terminus of the passenger domain acts as a Brownian ratchet that moves passively across the OM but then becomes trapped in the extracellular space as a consequence of folding. Although chaperones may keep the C terminus of the passenger domain in an unfolded conformation in the periplasm, our results suggest that the presence of large hydrophobic residues maximizes the rate of folding in the extracellular space. Consistent with this notion, large hydrophobic residues are found at positions that are equivalent to F963 and W990 in a wide range of autotransporters (19).
Based on our examination of the C terminus of the EspP passenger domain, it is tempting to speculate that the translocation of the entire passenger domain proceeds through a vectorial diffusion and folding process nucleated by the formation of a C-terminal core. Indeed, the repetitive β-helical architecture of passenger domains may have evolved to promote the sequential movement of a polypeptide in an environment devoid of ATP. In support of this hypothesis, the introduction of a linker at residue 586 of EspP was shown to transiently stall translocation near the site of the insertion (12). This observation suggests that folding drives the secretion of segments far from the C terminus. The folding of the C-terminal stable core, however, may not be absolutely essential for passenger domain secretion. We found that even a large C-terminal deletion did not completely abolish secretion. We also found that point mutations such as F963A lead to the transient generation of an N-terminal ≈60-kDa PK fragment. This fragment may result from a scenario in which >160 residues initially diffuse across the OM. Subsequently, N-terminal segments of the exposed fragment fold and bypass the function of the C-terminal stable core. Indeed, the relatively efficient secretion of passenger domains of proteins like SSP and BrkA whose C terminus has been deleted (19, 22) may be due to an unusual ability of N-terminal segments to fold and replace the C-terminal core. Furthermore, the efficient secretion of polypeptides that fold in the periplasm via the autotransporter pathway (14) raises the possibility that the folding of the passenger domain in the extracellular space is not the only force that drives translocation.
Because the F963A mutation created an intermediate that was transiently stalled at an early stage of translocation, we were able to obtain insights into the secretion mechanism. Our results strongly suggest that residues ≈997–1023 are embedded in the β barrel in a static α-helical conformation both during and after translocation. The data are consistent with previous results that indicated that the same segment is incorporated into the EspP β barrel as an α helix in the periplasm (10). Thus, although the finding that the EspP(Δ891–988) mutation reduced the number of exposed residues suggests that the C terminus of the passenger domain has distinct properties, this segment does not appear to be required to form a hairpin that initiates translocation. Instead, this segment may simply diffuse more readily across the OM than adjacent segments. The observation that the EspP passenger domain was cross-linked to BamA after exposure of the C-terminal ≈17-kDa suggests that the Bam complex facilitates the initial step of translocation. Indeed, it is conceivable that the Bam complex fortuitously drags the C terminus of the passenger domain across the OM in the process of integrating the β domain into the lipid bilayer, perhaps in the same way that it localizes the loops of β barrel proteins in the extracellular space. In any case, although the role of the Bam complex in passenger domain secretion is not yet clear, our results and previous results showing that EspP could be cross-linked to BamA when translocation was stalled at a relatively late stage suggest that BamA interacts continuously with the passenger domain during translocation.
Materials and Methods
Bacterial Strains, Antibiotics, and Antisera.
E. coli strain AD202 (MC4100 ompT::kan) was used in all experiments. Ampicillin (100 μg/mL) and tetracycline (5 μg/mL) were added as needed. Polyclonal antisera generated against EspP N- and C-terminal peptides and BamA have been described (12, 27), and anti-SurA was provided by Rajeev Misra (Arizona State University, Tempe, AZ).
Pulse–Chase Labeling and Photocrosslinking.
Unless otherwise noted, cells were grown at 37 °C in M9 containing 0.2% glycerol and all of the l-amino acids except methionine and cysteine (40 μg/mL). Overnight cultures were washed and diluted into fresh M9 at OD550 = 0.02. When the cultures reached OD550 = 0.2, EspP synthesis was induced by the addition of 10 μM IPTG. After 30 min, pulse–chase labeling with Tran35S-label (MP Biomedicals) and PK digestion was performed as described (12). In experiments involving cysteine labeling, cells were grown in M9 containing 0.2% glycerol and all of the l-amino acids except cysteine and were radiolabeled with [35S]cysteine (MP Biomedicals). In photocrosslinking experiments, 1 mM Bpa was added along with 200 μM IPTG, and cells were UV irradiated after a 1-min chase as described (12). In all experiments, proteins were collected by TCA precipitation, and immunoprecipitations were conducted as described (12). Except as noted, proteins were resolved by SDS/PAGE on 8–16% minigels (Invitrogen). The results of immunoprecipitations with the C-terminal anti-EspP antiserum were used for quantitative analysis. Cell surface exposure was defined as 1-[proEspP (+PK)/proEspP (−PK) + β domain (−PK)] and passenger domain cleavage was defined as [β domain (−PK)/β domain (−PK) + proEspP (−PK)]. In all calculations, the signal from each band was normalized to account for differences in the number of radioactive amino acids.
Supplementary Material
Acknowledgments
We thank Travis Barnard for helping to construct Fig. 1 and Susan Buchanan for providing valuable comments on the manuscript. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009491107/-/DCSupplemental.
References
- 1.Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala'Aldeen D. Type V protein secretion pathway: The autotransporter story. Microbiol Mol Biol Rev. 2004;68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otto BR, et al. Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J Biol Chem. 2005;280:17339–17345. doi: 10.1074/jbc.M412885200. [DOI] [PubMed] [Google Scholar]
- 3.Emsley P, Charles IG, Fairweather NF, Isaacs NW. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature. 1996;381:90–92. doi: 10.1038/381090a0. [DOI] [PubMed] [Google Scholar]
- 4.Gangwer KA, et al. Crystal structure of the Helicobacter pylori vacuolating toxin p55 domain. Proc Natl Acad Sci USA. 2007;104:16293–16298. doi: 10.1073/pnas.0707447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Junker M, et al. Pertactin β helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc Natl Acad Sci USA. 2006;103:4918–4923. doi: 10.1073/pnas.0507923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oomen CJ, et al. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 2004;23:1257–1266. doi: 10.1038/sj.emboj.7600148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnard TJ, Dautin N, Lukacik P, Bernstein HD, Buchanan SK. Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat Struct Mol Biol. 2007;14:1214–1220. doi: 10.1038/nsmb1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Berg B. Crystal structure of a full-length autotransporter. J Mol Biol. 2010;396:627–633. doi: 10.1016/j.jmb.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 9.Tajima N, Kawai F, Park SY, Tame JR. A novel intein-like autoproteolytic mechanism in autotransporter proteins. J Mol Biol. July 6, 2010 doi: 10.1016/j.jmb.2010.06.068. 10.1016/j/jmb/2010.06.068. [DOI] [PubMed] [Google Scholar]
- 10.Ieva R, Skillman KM, Bernstein HD. Incorporation of a polypeptide segment into the β domain pore during the assembly of a bacterial autotransporter. Mol Microbiol. 2008;67:188–201. doi: 10.1111/j.1365-2958.2007.06048.x. [DOI] [PubMed] [Google Scholar]
- 11.Junker M, Besingi RN, Clark PL. Vectorial transport and folding of an autotransporter virulence protein during outer membrane secretion. Mol Microbiol. 2009;71:1323–1332. doi: 10.1111/j.1365-2958.2009.06607.x. [DOI] [PubMed] [Google Scholar]
- 12.Ieva R, Bernstein HD. Interaction of an autotransporter passenger domain with BamA during its translocation across the bacterial outer membrane. Proc Natl Acad Sci USA. 2009;106:19120–19125. doi: 10.1073/pnas.0907912106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pohlner J, Halter R, Beyreuther K, Meyer TF. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature. 1987;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 14.Skillman KM, Barnard TJ, Peterson JH, Ghirlando R, Bernstein HD. Efficient secretion of a folded protein domain by a monomeric bacterial autotransporter. Mol Microbiol. 2005;58:945–958. doi: 10.1111/j.1365-2958.2005.04885.x. [DOI] [PubMed] [Google Scholar]
- 15.Jong WS, et al. Limited tolerance towards folded elements during secretion of the autotransporter Hbp. Mol Microbiol. 2007;63:1524–1536. doi: 10.1111/j.1365-2958.2007.05605.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu T, et al. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Klauser T, Pohlner J, Meyer TF. Selective extracellular release of cholera toxin B subunit by Escherichia coli: Dissection of Neisseria Igaβ-mediated outer membrane transport. EMBO J. 1992;11:2327–2335. doi: 10.1002/j.1460-2075.1992.tb05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renn JP, Clark PL. A conserved stable core structure in the passenger domain β helix of autotransporter virulence proteins. Biopolymers. 2008;89:420–427. doi: 10.1002/bip.20924. [DOI] [PubMed] [Google Scholar]
- 19.Oliver DC, Huang G, Nodel E, Pleasance S, Fernandez RC. A conserved region within the Bordetella pertussis autotransporter BrkA is necessary for folding of its passenger domain. Mol Microbiol. 2003;47:1367–1383. doi: 10.1046/j.1365-2958.2003.03377.x. [DOI] [PubMed] [Google Scholar]
- 20.Velarde JJ, Nataro JP. Hydrophobic residues of the autotransporter EspP linker domain are important for outer membrane translocation of its passenger. J Biol Chem. 2004;279:31495–31504. doi: 10.1074/jbc.M404424200. [DOI] [PubMed] [Google Scholar]
- 21.Klauser T, Krämer J, Otzelberger K, Pohlner J, Meyer TF. Characterization of the Neisseria Iga β-core. The essential unit for outer membrane targeting and extracellular protein secretion. J Mol Biol. 1993;234:579–593. doi: 10.1006/jmbi.1993.1613. [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi Y, Nishiyama M, Horinouchi S, Beppu T. Involvement of the COOH-terminal pro-sequence of Serratia marcescens serine protease in the folding of the mature enzyme. J Biol Chem. 1994;269:32800–32806. [PubMed] [Google Scholar]
- 23.Junker M, Clark PL. Slow formation of aggregation-resistant β-sheet folding intermediates. Proteins. 2010;78:812–824. doi: 10.1002/prot.22609. [DOI] [PubMed] [Google Scholar]
- 24.Dautin N, Barnard TJ, Anderson DE, Bernstein HD. Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J. 2007;26:1942–1952. doi: 10.1038/sj.emboj.7601638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brockmeyer J, Spelten S, Kuczius T, Bielaszewska M, Karch H. Structure and function relationship of the autotransport and proteolytic activity of EspP from Shiga toxin-producing Escherichia coli. PLoS ONE. 2009;4:e6100. doi: 10.1371/journal.pone.0006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen MM, Andersen KK, Westh P, Otzen DE. Unfolding of β-sheet proteins in SDS. Biophys J. 2007;92:3674–3685. doi: 10.1529/biophysj.106.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabady RL, Peterson JH, Skillman KM, Bernstein HD. An unusual signal peptide facilitates late steps in the biogenesis of a bacterial autotransporter. Proc Natl Acad Sci USA. 2005;102:221–226. doi: 10.1073/pnas.0406055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.