Abstract
The development of transgenic technologies in monkeys is important for creating valuable animal models of human physiology so that the etiology of diseases can be studied and potential therapies for their amelioration may be developed. However, the efficiency of producing transgenic primate animals is presently very low, and there are few reports of success. We have developed an improved methodology for the production of transgenic rhesus monkeys, making use of a simian immunodeficiency virus (SIV)-based vector that encodes EGFP and a protocol for infection of early-cleavage–stage embryos. We show that infection does not alter embryo development. Moreover, the timing of infection, either before or during embryonic genome activation, has no observable effect on the level and stability of transgene expression. Of 70 embryos injected with concentrated virus at the one- to two-cell stage or the four- to eight-cell stage and showing fluorescence, 30 were transferred to surrogate mothers. One transgenic fetus was obtained from a fraternal triple pregnancy. Four infant monkeys were produced from four singleton pregnancies, of which two expressed EGFP throughout the whole body. These results demonstrate the usefulness of SIV-based lentiviral vectors for the generation of transgenic monkeys and improve the efficiency of transgenic technology in nonhuman primates.
Keywords: lentiviral vector, transgenesis
Because of their genetic and physiological similarities to humans, nonhuman primates provide powerful experimental models to study human development and diseases. Monkeys are particularly appropriate for the study of cognitive functions and disorders as well as complex behavior (1–3). However, many diseases afflicting humans do not occur naturally in monkeys; therefore transgenic animals are needed. Specific genes could be examined in transgenic monkeys to ascertain their possible roles in causing diseases. In this way, the etiology of diseases could be studied, and potential therapies might be developed. To date, there have been few reported successes in producing transgenic monkeys (4–6). The low efficiency of transgenesis in monkeys at present hinders its application to clinically relevant disease studies. More work on transgenesis in monkeys would be useful to increase its efficiency and establish new models for human diseases, such as a recently developed transgenic rhesus monkey model for Huntington's disease that expresses exon I of the mutant Huntington (htt) gene (4).
A major obstacle to generating transgenic nonhuman primates is the low efficiency of assisted reproductive technologies (ARTs) in producing oocytes and embryos suitable for genetic engineering, embryonic stem cell derivation, and cloning. The first birth of a rhesus macaque after in vitro fertilization (IVF) was reported in 1984 (7), but the development of ARTs in nonhuman primates has been slow compared with its human counterpart. The efficacy of IVF methods is critically dependent on the protocols used for ovarian stimulation, in vitro embryo culture, embryo transfer, and uterine–embryo synchrony. During the past decade, we and others have developed and improved these protocols in the rhesus monkey (8–18). Recently, IVF and intracytoplasmic sperm injection also were achieved in the marmoset (19) and in the cynomolgus monkey (20).
In this article, we describe the generation of transgenic rhesus monkeys using a simian immunodeficiency virus (SIV)-based vector to infect early-cleavage–stage embryos. We produced two living infant monkeys that stably express EGFP.
Results
Embryo Development After Infection with a Lentiviral Vector in Vitro.
A high-titer [8 × 107 infectious particles/mL] SIV-based vector was used for gene transfer. The vector carried the sequence encoding EGFP, driven by a CMV-enhanced chicken β-actin (CAG) promoter. Eighty-one in vitro-fertilized embryos were infected at the one- or two-cell stage or at the four- to eight-cell stage by injecting 50–100 pL of virus suspension (four to eight viral particles) into the perivitelline space. After 3–5 d in culture, 70 embryos (86%) displayed fluorescence, indicating a high infection rate. To monitor the development potential of the injected embryos, embryos at the one- or two-cell stage from each of three monkeys were pooled and randomly allocated to the group injected at the one- or two-cell stage, the group injected at the four- to eight-cell stage, or the control group (Table 1). There were no significant differences in developmental rates in the two experimental groups and the control group (Table 1).
Table 1.
In vitro development of rhesus monkey embryo after injection of lentiviral vector
| Development stage (%) |
|||||||
| Group | Embryos used/monkeys* | One-cell stage | Two-cell stage | Eight-cell stage | 16-Cell stage | Morula | Blastocyst |
| Four to eight cells | 12/3 | 12 | 10 (83.3) | 8 (66.7) | 6 (50.0) | 6 (50.0) | 6 (50.0) |
| One or two cells | 19/3 | 19 | 17 (89.5) | 16 (84.2) | 14 (73.7) | 11 (57.9) | 10 (52.6) |
| Control | 15/3 | 15 | 13 (86.7) | 10(66.7) | 10 (66.7) | 9 (60.0) | 9 (60.0) |
*The one-cell–stage embryos from three monkeys were collected and pooled. They were randomly assigned to each of three groups: one- or two-cell stage with lentiviral infection, four- to eight-cell stage with lentiviral infection, or one- or two-cell stage control group (noninjected IVF embryos). There were no significant differences in developmental rates in either experimental group or in the control group (P > 0.05).
Production of Transgenic Rhesus Fetuses and Newborns.
Eight EGFP-positive embryos derived from embryos injected at the one- or two-cell stage and 22 EGFP-positive embryos derived from embryos injected at the four- to eight- cell stage were implanted into eight surrogate mothers (Table 2). One triple pregnancy resulted from the transfer of the eight embryos infected at the one- or two-cell stage. Four pregnancies resulted from the transfer of 22 blastocysts infected at the four- to eight-cell stage. Both pregnancy and embryo development rates are consistent with those observed with normal IVF embryos, indicating that the rate of successful pregnancies is not altered by lentivirus infection.
Table 2.
Pregnancy rate, live births, and transgenic outcomes
| Stage | No. of embryos | No. of surrogate mothers | No. of pregnancies | No. of pregnancies/ surrogate mothers | Multiple pregnancy | Live birth | Spontaneous miscarriage | Transgenic pregnancy | Transgenesis/ pregnancies |
| Four to eight cells | 22 | 6 | 4 | 67% | 0 | 4 | 0 | 2 | 50% |
| One or two cells | 8 | 2 | 1 | 50% | 1 (triple) | 0 | 1 | 1 | 33% |
| Control | 20 | 5 | 3 | 60% | 0 | 2 | 1 | — |
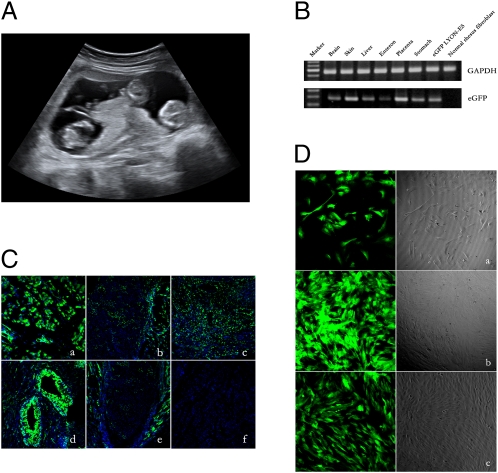
A fraternal triple pregnancy (Fig. 1A) resulting from embryos infected at the one- or two-cell stage miscarried at 66 d of gestation. The miscarriage probably was caused by the triple pregnancy, which rarely happens in the rhesus monkey. Examination of aborted fetuses under UV light revealed that one fetus was positive for GFP expression, and the other two were negative. Transgene integration in the positive fetus was found in all tissues examined, including placenta, brain, liver, skin, and stomach (Fig. 1B). Transgene expression was detected in the different tissues, both in sections of organs (Fig. 1C) and in cultured cells derived from those organs (Fig. 1D). Thus, tissues originating from all three germ layers expressed the transgene in the developing fetus.
Fig. 1.
Analysis of the triple pregnancy that miscarried at 66 d of gestation. (A) Ultrasound image showing the triple pregnancy at 50 d of gestation. (B) PCR analysis of genomic DNA showing the presence of the EGFP transgene in six different organs of the EGFP-positive fetus. (C) Confocal microscope analysis of GFP expression (green) on frozen sections of brain (a), liver (b), muscle (c), bone (d), and gristle (e) prepared from the GFP-positive fetus. The frozen section of bladder (f) from a normal monkey was performed as control. Nuclei are labeled with Hoechst (blue). (Scale bar: 50 μM.) (D) Cultured cells prepared from brain (a), muscle (b), and liver (c) of the GFP transgenic fetus, showing GFP expression.
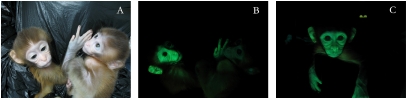
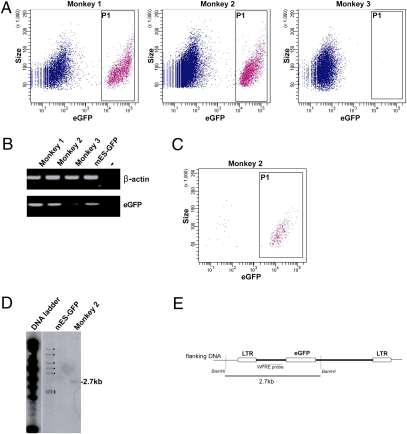
Four pregnancies resulting from embryos infected at the four- to eight-cell stage were concluded naturally, and two offspring exhibited whole-body expression of the EGFP reporter (Fig. 2). To characterize transgene expression further in these two transgenic animals, fibroblasts were prepared from skin biopsies at 16 wk of age. Fibroblasts analyzed by flow cytometry showed that 28% and 17% of the population exhibited EGPF expression in transgenic monkeys 1 and 2, respectively (Fig. 3A). These results indicate that not all blastomeres of the four- to eight-cell–stage embryos were infected by the lentivirus and that both transgenic monkeys are chimeric for EGFP expression. A PCR analysis of genomic DNA confirmed the presence of integrated proviral DNA in the fibroblasts of both transgenic newborns (Fig. 3B). To determine the number of integrated proviral copies, the EGFP-expressing fibroblasts of transgenic monkey 2 were sorted by flow cytometry (Fig. 3C), amplified in culture, and the genomic DNA subsequently was analyzed by Southern blot (Fig. 3D). Because proviral DNA has only a BamHI site, BamHI digestion cuts the proviral DNA into two halves, the size of which is determined by the position of the next BamHI site in the flanking genomic sequences. The woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) probe revealed a single 2.7-kb BamHI fragment, thus revealing a single proviral integration site in the genome of transgenic monkey 2.
Fig. 2.
(A) Transmission light image of two transgenic-positive monkeys. (B) Fluorescent image showing GFP expression in the monkey on the left in A. (C) Fluorescent image of another transgenic-positive animal.
Fig. 3.
Analysis of the two transgenic monkeys (monkey 1 and monkey 2) and control monkey (monkey 3) after birth. (A) Flow cytometry analysis of EGFP expression in fibroblasts. (B) PCR analysis of genomic DNA from the three newborns. (C) Flow cytometry analysis of EGFP expression in fibroblasts from transgenic monkey 2 after cell sorting. (D) Southern blot analysis of proviral DNA integration (→) in EGFP-positive cells after cell sorting. Control DNA is from mouse ES cells infected with the GAE-CAG-EGFP/WPRE lentiviral vector and showing seven independent integration sites (marked with dots). (E) Schematic representation of the integrated proviral DNA showing the position of the WPRE probe to identify a single 2.7-kb BamHI fragment encompassing the DNA junction between proviral DNA and the host genome. LTR, Long Terminal Repeat.
The four animals underwent a physical checkup every 4 mo. No difference in body weight was observed between transgenic and nontransgenic animals during their first 20 mo of life (Table 3).
Table 3.
Summary of transgenic and monkeys
| Body weight (kg) |
||||||
| Animal | Date of birth | Sex | Expression of GFP | 4 mo | 10 mo | 20 mo |
| Transgenic monkey | ||||||
| 1 | 2008–07-15 | Male | + | 1.24 | 2.11 | 3.00 |
| 2 | 2008–07-08 | Female | + | 0.92 | 1.64 | 2.87 |
| 3 | 2008–06-26 | Female | − | 1.12 | 1.74 | 2.80 |
| 4 | 2008–07-16 | Male | − | 1.17 | 1.94 | 3.05 |
| Normal monkey | ||||||
| 1 | 2008–07-20 | Female | − | 1.06 | 1.65 | 2.90 |
| 2 | 2008–07-20 | Male | − | 1.21 | 1.91 | 2.95 |
Discussion
In this report, we describe efficient production of transgenic rhesus monkeys by infection of early-cleavage–stage embryos with an SIV-based lentiviral vector. Over the past few years, we have successfully developed ARTs in the rhesus monkey, including controlled follicular stimulation (14, 15, 17), oocyte in vitro maturation, and culture of preimplantation embryos (9, 12, 13, 18), and, more recently, embryo transfer to surrogate mothers (this report). We achieved a pregnancy rate of 62% (five of eight), and 27% of the transferred embryos (4 of 15) developed into healthy newborns. Fifteen embryos were transferred into five pregnant monkeys, who gave birth to four healthy offspring. These rates are significantly higher than those published by most laboratories so far (7, 21, 22).
We used an SIV-based lentiviral vector to transfer the EGFP gene into the developing preimplantation embryo. We made use of GAE-CAG-EGFP/WPRE, a highly crippled SIVmac251-based lentiviral vector (23) expressing EGFP under the control of the CAG promoter (24). The use of CAG promoter ensures both high and stable expression of the transgene in all tissue types, including nondividing cells (24). SIVmac251- and HIV-derived lentiviral vectors are equally efficient at transfecting primate cells and expressing a transgene (25). Most importantly, we observed that treated and control embryos developed to the blastocyst stage with similar efficiency, indicating that neither the injection procedure nor the lentiviral vector itself induce significant harm to the embryos.
In the rhesus monkey, embryonic genome activation takes place at the six- to eight-cell stage (8, 26). We thus infected preimplantation embryos at the one- or two-cell stage and at the four- to eight-cell stage to compare the rates of gene transfer and of development to the blastocyst stage when proviral integration occurs before or during embryonic genome activation. No difference could be observed between the two experimental groups, suggesting that embryonic genome activity has no observable effect on the capacity of blastomeres to be infected and to express the transgene. Previously, metaphase II oocytes were used for infection with retroviral and lentiviral vectors (4, 27). The unfertilized oocyte was targeted because the disassembly of the nuclear membrane at metaphase was believed to facilitate integration of retroviral vectors. By contrast, lentiviral vectors do not require disruption of the nuclear membrane during early-cleavage–stage development. In addition, the early-cleavage–stage embryo is more resistant than the oocyte to the microinjection procedure.
Injecting the lentiviral vector into four- to eight-cell–stage embryos led to chimeric rather than fully transgenic newborns, indicating that only one or a few blastomeres were infected after virus injection into the perivitelline space. Southern blot analysis of genomic DNA from transgenic monkey 2 revealed only one integration site, indicating that only one blastomere had been infected after virus injection into the perivitelline space. Injection of virus into the unfertilized oocyte is more likely to produce fully transgenic monkey newborns, although chimerism also could be observed in this situation because lentiviral vectors have the capacity to remain nonintegrated for one or several cell divisions before integration into the genome. This issue was not addressed in the reports using this strategy (4, 27).
It is surprising that not every miscarried fetus and newborn offspring was transgenic, because only blastocysts showing clearly visible EGFP-positive cells were transferred to surrogate mothers. It is conceivable that the cells deriving from the infected blastomere underwent negative selection during inner-cell-mass and epiblast development. Whether this selection particularly targets and eliminates infected cells unfit for future development or reveals a more general mechanism of stem cell pool reduction around the time of implantation remains to be determined.
In conclusion, we have developed an efficient strategy for producing transgenic monkeys that will be instrumental both in developmental studies and in the generation of monkey models of human monogenic diseases. The efficiency of embryo infection with lentiviral vectors needs to be improved further to increase both the number of transgenic animals and the rate of chimerism in the newborns.
Materials and Methods
Collection of Rhesus Monkey Oocytes and in Vitro Fertilization.
All animal procedures were approved in advance by the Institutional Animal Care and Use Committee of Kunming Primate Research Center and Kunming Institute of Zoology, Chinese Academy of Sciences. Cycling females (6 to 12 y old) were subjected to follicular stimulation using twice-daily intramuscular injections of 18 IU of recombinant human FSH (rhFSH) (Gonal FTM Laboratories) for 8 d; then 1,000 IU of human chorionic gonadotropin (hCG) (Lizhu Groups) were injected on day 9 as described by Yang et al. (17). Cumulus–oocyte complexes were collected from animals by laparoscopic follicular aspiration 30–34 h following hCG administration. Follicular contents were placed in Hepes-buffered Tyrode's albumin lactate pyruvate (TALP) medium (28) containing 0.3% BSA at 37 °C. Oocytes were stripped of cumulus cells by pipetting after brief exposure (<1 min) to hyaluronidase (0.5 mg/mL) in TALP-Hepes to allow visual classification of nuclear maturity as prophase I (PI; intact germinal vesicle), metaphase I (MI; no germinal vesicle, no polar body), metaphase II (MII; first polar body present), and atretic (presence of fragmentation or vacuoles in ooplasm). Immature oocytes in either MI or PI stages were cultured in a 50-μL drop of CMRL-1066 medium (Invitrogen) containing 10% FBS, 10 IU/mL porcine FSH, and 10 IU/mL ovine luteinizing hormone at 37 °C in humidified air (5% CO2) for up to 24 h. Oocytes that were mature (MII) at collection were placed in chemically defined, protein-free hamster embryo culture medium-10 (HECM-10) (29) at 37 °C in 5% CO2 until inseminated (36 h) with capacitated, hyperactivated spermatozoa diluted to a final concentration of 2 × 105 /mL in 50-μL drops of TALP for fertilization. After coincubation of oocytes and spermatozoa for 12–16 h, oocytes were examined for the presence of two pronuclei and two polar bodies as evidence of fertilization. Fertilized ova were washed to remove spermatozoa and then cultured in HECM-10 (3) containing 10% FCS (HyClone Laboratories Inc.) to allow embryo development. Culture medium was replaced every other day.
Selection of Surrogate Mothers and Embryo Transfer.
Embryos at the 16-cell to blastocyst stage were selected for embryo transfer. Surrogate females exhibiting normal menstrual cycles were identified based on their steroid hormone profiles and observation of menses. Embryo transfer into the oviduct was conducted by the laparoscopic approach. In brief, monkeys were anesthetized with ketamine (10–12 mg/kg). After sterile skin preparation and draping, the abdomen was insufflated with CO2 at 15 mm Hg pressure, and the endoscope was inserted through the corresponding trocar cannula via a small supraumbilical incision. Typically, three or four embryos were transferred unilaterally into each female using a polythene catheter connected to a 1-mL syringe filled with TH3 medium. The catheter containing the embryos was inserted transabdominally by threading it through a 25-gauge hypodermic needle and was advanced through the fimbrium into the oviductal ampulla to a distance of about 3 cm, where the embryos were released. After transfer, the catheter was removed and carefully examined and rinsed to ensure that all embryos had been expelled. In the event of a retained embryo, a second transfer was attempted. To detect pregnancy, serum estradiol and progesterone concentrations were monitored, and a clinical pregnancy was confirmed by fetal cardiac activity detected by ultrasonography. The progression of pregnancies was monitored periodically by ultrasonography.
Virus Production and Gene Delivery in Rhesus Monkey Preimplantation Embryos.
The method for producing SIV-based viruses in 293FT (American Tissue Culture Collection) cells is fully described elsewhere (25). Briefly, 2.5 × 106 293FT cells were seeded in a dish 10 cm in diameter 24 h before transfection. Cells were transfected by the calcium phosphate method with a mixture of DNAs containing 6.5 μg pPax2 plasmid encoding the vesicular stomatitis virus glycoprotein (VSV-G) envelope, 3.5 μg pMD2G plasmid encoding the gag, pol, tat, and rev proteins, and 10 μg GAE-CAG-EGFP/WPRE lentiviral vector (24). The following day, cells were fed again with fresh medium and further cultured for 24–30 h. The supernatant then was clarified by centrifugation (1,000 × g, 15 min), passed through a cellulose acetate filter (pore size, 0.8 μm), and concentrated by ultracentrifugation (25,000 RPM, 2 h) on a 20% (wt/vol) sucrose gradient. The viral pellet was resuspended in PBS, frozen, and titrated by infection of 293T cells followed by counting of EGFP-positive cells. Early-cleavage–stage (one- or two- and four- to eight-cell) embryos were selected for perivitelline space injection. A lentiviral suspension was loaded into the injection needle by micropipette before injection into the perivitelline space. After virus injection, the embryos were cultured in HECM-10 until the blastocyst stage, before embryo transfer into surrogate mothers.
PCR and Southern Blot Analysis of Proviral DNA.
To detect the EGFP gene, GFP-F forward primer (5′-GACGTAAACGGCCACAAGT T-3′) and GFP-R reverse primer (5′-GGTCTTGTAGTTGCCGTCGT-3′) were used to yield a 264-bp product after amplification of genomic DNA from monkey tissues. Genomic DNA from different tissues was subjected to PCR for 30 cycles at 94 °C for 5 min, 94 °C for 45 s, 55 °C for 45 s, 72 °C for 45 s, and then 72 °C for 5 min. For Southern blot detection, 10 μg of BamHI-digested genomic DNA was separated on a 0.8% agarose gel, transferred to a nylon membrane (Hybond-N+; Amersham), and hybridized with a 32P-labeled probe (Ready-to-Go Labeling Kit; Amersham) encompassing the WPRE region of the GAE-CAG-eGFP/WPRE vector.
Monitoring of GFP Expression in Newborns Monkeys, Immunohistochemistry, and Flow Cytometry.
Live newborns were placed under an epifluorescent light (475 nm), and images were captured using a digital camera equipped with a 520-nm wavelength emission filter. Tissues of transgenic monkeys were fixed in 4% paraformaldehyde (PFA) overnight at 4 °C and then cryoprotected in 30% sucrose before sectioning at 5 μm. EGFP immunostaining was performed as described (24). Briefly, the sections were postfixed in 4% PFA for 5 min and then permeabilized in 0.5% Triton X-100 for 10 min. Nonspecific binding was blocked with 5% sheep serum for 1 h before incubation with Alexa Fluor 488-conjugated anti-GFP antibody (Invitrogen) at 4 °C overnight. Then sections were incubated with Hoechst 33342 for 30 min. For flow cytometry analysis, single-cell suspensions were resuspended in PBS, and the percentage of GFP-positive cells was counted using a FACS-CantoII (Becton Dickinson). Data acquisition was performed with Diva software (Becton Dickinson).
Statistical Analysis.
Results are expressed as mean ± SD.
The rates of embryo development were transformed by arcsine of square root. The development potential of experimental groups and controls was compared by a paired-sample t test. Statistical analysis was done using SPSS 11.5 software (SPSS Inc.).
Acknowledgments
We thank Dr. Barry Bavister for his comments on the revision of the manuscript. W.J. was supported by Grants KSCX1-05-02 and KSCX2-YW-R-47from the Chinese Academy of Sciences; 2006CB701505 and 2007CB947701 from the Major State Basic Development Program; 2006AA02A116 and 2006AA02A101 from the National High Technology Research and Development Program; 2007BAI33B00 from the National Key Technology R&D Program; 30701059 and 30700425 from the China National Science Foundation; 2006PT08-2 from the R&D Infrastructure and Facility Development Program of Yunnan Province; 2007GH from the Social Science and Technology Development Program of Yunnan Province; and 2009ZX09501-028 from the National Science and Technology Major Project. K.Z. is supported by the Chinese National 985 Project to Sichuan University, by the US National Eye Institute/National Institutes of Health, by a Veterans Administration Merit Award, Research to Prevent Blindness, and by a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Varki A. A chimpanzee genome project is a biomedical imperative. Genome Res. 2000;10:1065–1070. doi: 10.1101/gr.10.8.1065. [DOI] [PubMed] [Google Scholar]
- 2.Vaitukaitis JL. Animal models of human disease for the 21st century. Lab Anim Sci. 1998;48:562–564. [PubMed] [Google Scholar]
- 3.McKiernan SH, Bavister BD. Culture of one-cell hamster embryos with water soluble vitamins: Pantothenate stimulates blastocyst production. Hum Reprod. 2000;15:157–164. doi: 10.1093/humrep/15.1.157. [DOI] [PubMed] [Google Scholar]
- 4.Yang SH, et al. Towards a transgenic model of Huntington's disease in a non-human primate. Nature. 2008;453:921–924. doi: 10.1038/nature06975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan AW. Transgenic nonhuman primates for neurodegenerative diseases. Reprod Biol Endocrinol. 2004;2:39. doi: 10.1186/1477-7827-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki E, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- 7.Bavister BD, Boatman DE, Collins K, Dierschke DJ, Eisele SG. Birth of rhesus monkey infant after in vitro fertilization and nonsurgical embryo transfer. Proc Natl Acad Sci USA. 1984;81:2218–2222. doi: 10.1073/pnas.81.7.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niu Y, et al. Impairments in embryonic genome activation in rhesus monkey somatic cell nuclear transfer embryos. Cloning Stem Cells. 2008;10:25–36. doi: 10.1089/clo.2007.0040. [DOI] [PubMed] [Google Scholar]
- 9.Zheng P, Bavister BD, Ji WZ. Amino acid requirements for maturation of rhesus monkey (Macacca mulatta) oocytes in culture. Reproduction. 2002;124:515–522. doi: 10.1530/rep.0.1240515. [DOI] [PubMed] [Google Scholar]
- 10.Zheng P, et al. Effect of age and breeding season on the developmental capacity of oocytes from unstimulated and follicle-stimulating hormone-stimulated rhesus monkeys. Biol Reprod. 2001;64:1417–1421. doi: 10.1095/biolreprod64.5.1417. [DOI] [PubMed] [Google Scholar]
- 11.Zheng P, Bavister BD, Ji W. Energy substrate requirement for in vitro maturation of oocytes from unstimulated adult rhesus monkeys. Mol Reprod Dev. 2001;58:348–355. doi: 10.1002/1098-2795(200103)58:3<348::AID-MRD14>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Zheng P, Wang H, Bavister BD, Ji W. Maturation of rhesus monkey oocytes in chemically defined culture media and their functional assessment by IVF and embryo development. Hum Reprod. 2001;16:300–305. doi: 10.1093/humrep/16.2.300. [DOI] [PubMed] [Google Scholar]
- 13.Zheng P, et al. 17Beta-estradiol and progesterone improve in-vitro cytoplasmic maturation of oocytes from unstimulated prepubertal and adult rhesus monkeys. Hum Reprod. 2003;18:2137–2144. doi: 10.1093/humrep/deg410. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, et al. Effects of rhFSH regimen and time interval on ovarian responses to repeated stimulation cycles in rhesus monkeys during a physiologic breeding season. Theriogenology. 2008;70:108–114. doi: 10.1016/j.theriogenology.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Yang S, et al. Ovarian response to gonadotropin stimulation in juvenile rhesus monkeys. Theriogenology. 2009;72:243–250. doi: 10.1016/j.theriogenology.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Yang S, He X, Hildebrandt TB, Zhou Q, Ji W. Superovulatory response to a low dose single-daily treatment of rhFSH dissolved in polyvinylpyrrolidone in rhesus monkeys. Am J Primatol. 2007;69:1278–1284. doi: 10.1002/ajp.20433. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, et al. Effects of rhFSH dose on ovarian follicular response, oocyte recovery and embryo development in rhesus monkeys. Theriogenology. 2007;67:1194–1201. doi: 10.1016/j.theriogenology.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Si W, et al. Cryopreservation of rhesus macaque (Macaca mulatta) spermatozoa and their functional assessment by in vitro fertilization. Cryobiology. 2000;41:232–240. doi: 10.1006/cryo.2000.2283. [DOI] [PubMed] [Google Scholar]
- 19.Gilchrist RB, Nayudu PL, Hodges JK. Maturation, fertilization, and development of marmoset monkey oocytes in vitro. Biol Reprod. 1997;56:238–246. doi: 10.1095/biolreprod56.1.238. [DOI] [PubMed] [Google Scholar]
- 20.Sun Q, et al. Efficient reproduction of cynomolgus monkey using pronuclear embryo transfer technique. Proc Natl Acad Sci USA. 2008;105:12956–12960. doi: 10.1073/pnas.0805639105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bavister BD. ARTs in action in nonhuman primates: Symposium summary—advances and remaining issues. Reprod Biol Endocrinol. 2004;2:43. doi: 10.1186/1477-7827-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf DP, et al. Use of assisted reproductive technologies in the propagation of rhesus macaque offspring. Biol Reprod. 2004;71:486–493. doi: 10.1095/biolreprod.103.025932. [DOI] [PubMed] [Google Scholar]
- 23.Mangeot PE, et al. Development of minimal lentivirus vectors derived from simian immunodeficiency virus (SIVmac251) and their use for gene transfer into human dendritic cells. J Virol. 2000;74:8307–8315. doi: 10.1128/jvi.74.18.8307-8315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wianny F, et al. Derivation and cloning of a novel rhesus embryonic stem cell line stably expressing tau-green fluorescent protein. Stem Cells. 2008;26:1444–1453. doi: 10.1634/stemcells.2007-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nègre D, et al. Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Ther. 2000;7:1613–1623. doi: 10.1038/sj.gt.3301292. [DOI] [PubMed] [Google Scholar]
- 26.Schramm RD, Bavister BD. Onset of nucleolar and extranucleolar transcription and expression of fibrillarin in macaque embryos developing in vitro. Biol Reprod. 1999;60:721–728. doi: 10.1095/biolreprod60.3.721. [DOI] [PubMed] [Google Scholar]
- 27.Chan AW, Chong KY, Martinovich C, Simerly C, Schatten G. Transgenic monkeys produced by retroviral gene transfer into mature oocytes. Science. 2001;291:309–312. doi: 10.1126/science.291.5502.309. [DOI] [PubMed] [Google Scholar]
- 28.Mitalipov SM, Yeoman RR, Nusser KD, Wolf DP. Rhesus monkey embryos produced by nuclear transfer from embryonic blastomeres or somatic cells. Biol Reprod. 2002;66:1367–1373. doi: 10.1095/biolreprod66.5.1367. [DOI] [PubMed] [Google Scholar]
- 29.McKiernan SH, Bavister BD. Culture of one-cell hamster embryos with water soluble vitamins: Pantothenate stimulates blastocyst production. Hum Reprod. 2000;15:157–164. doi: 10.1093/humrep/15.1.157. [DOI] [PubMed] [Google Scholar]