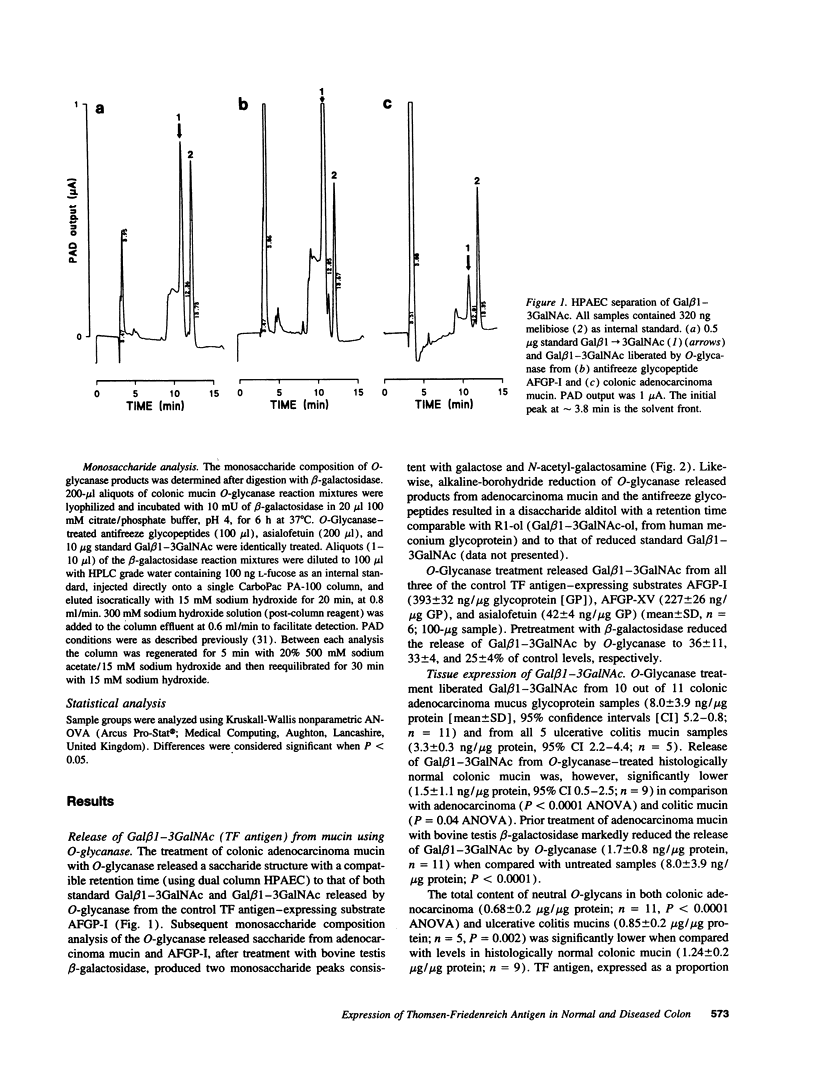
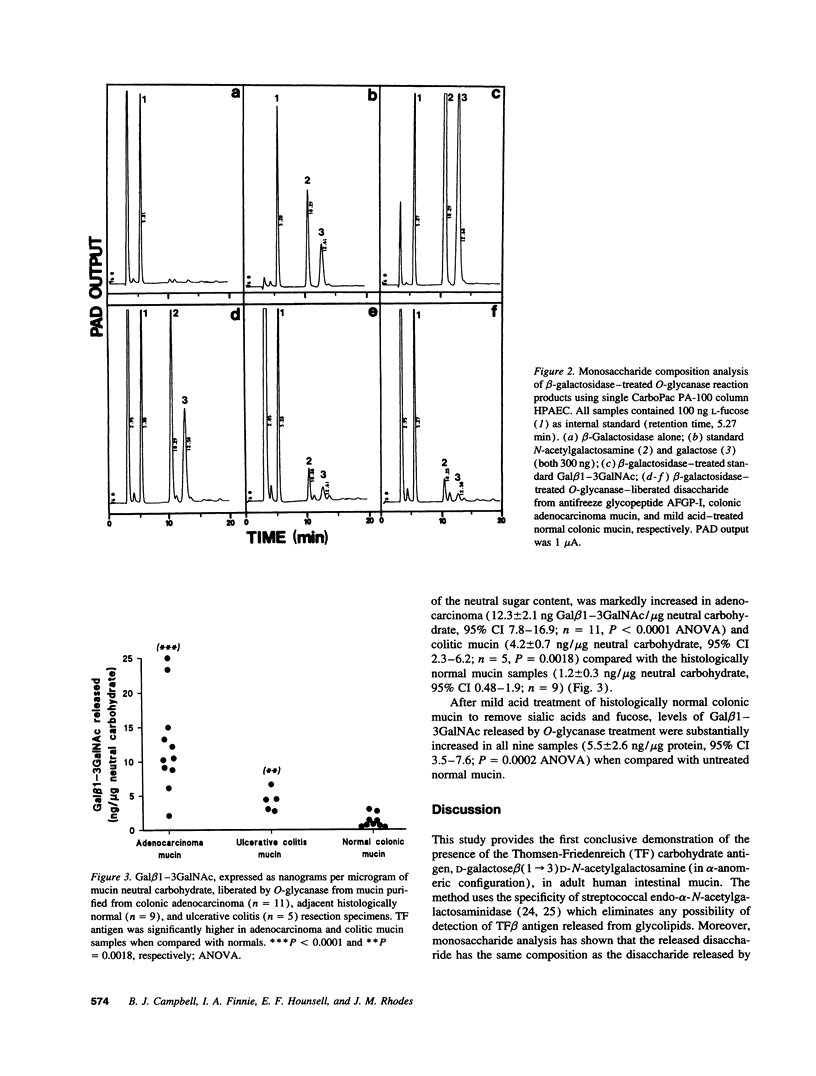
Abstract
Increased binding of the lectin peanut agglutinin is a common feature in epithelial malignancy and hyperplasia. This may have considerable functional importance in the intestine by allowing interaction between the epithelium and mitogenic lectins of dietary or microbial origin. Peanut agglutinin binds the disaccharide Thomsen-Friedenreich (TF, T or core 1) blood group antigen, Gal beta (1-3) GalNAc alpha-, but is not totally specific for this site. Consequently, there has been controversy about the presence of this structure in colon cancer; studies with anti-TF monoclonal antibodies have failed to detect it. We have examined the presence of TF antigen in colonic mucus glycoprotein (mucin) using endo-alpha-N-acetylgalactosaminidase (O-Glycanase), which specifically catalyzes the hydrolysis of TF antigen from glycoconjugates. Samples of adenocarcinoma, inflammatory bowel disease (ulcerative colitis), and normal mucin were treated with O-glycanase, the liberated disaccharide was separated from the glycoprotein and analyzed using dual CarboPac PA-100 column high performance anion-exchange chromatography coupled with pulsed amperometric detection. O-Glycanase treatment released increased amounts of TF antigen from both colonic adenocarcinoma (8.0 +/- 3.9 ng/micrograms protein, n = 11; P < 0.0001 ANOVA) and ulcerative colitis mucin (3.3 +/- 0.3 ng/micrograms protein, n = 5; P = 0.04) compared with mucin samples from histologically normal mucosa distant from carcinoma (1.5 +/- 1.1 ng/micrograms protein, n = 9). However, after mild acid treatment to remove sialic acids and fucose, releasable TF antigen was increased in all nine of these histologically normal mucin samples (5.5 +/- 2.6 ng/micrograms protein, P < 0.0002). We conclude that TF antigen is an oncofetal antigen which is expressed in colon cancer, but is concealed by further glycosylation (sialylation and/or fucosylation) in the normal colonic mucosa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland C. R., Deshmukh G. D. The carbohydrate composition of mucin in colonic cancer. Gastroenterology. 1990 May;98(5 Pt 1):1170–1177. doi: 10.1016/0016-5085(90)90330-4. [DOI] [PubMed] [Google Scholar]
- Boland C. R., Lance P., Levin B., Riddell R. H., Kim Y. S. Abnormal goblet cell glycoconjugates in rectal biopsies associated with an increased risk of neoplasia in patients with ulcerative colitis: early results of a prospective study. Gut. 1984 Dec;25(12):1364–1371. doi: 10.1136/gut.25.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland C. R. Lectin histochemistry in colorectal polyps. Prog Clin Biol Res. 1988;279:277–287. [PubMed] [Google Scholar]
- Boland C. R., Montgomery C. K., Kim Y. S. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2051–2055. doi: 10.1073/pnas.79.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B. J., Davies M. J., Rhodes J. M., Hounsell E. F. Separation of neutral oligosaccharide alditols from human meconium using high-pH anion-exchange chromatography. J Chromatogr. 1993 Dec 22;622(2):137–146. doi: 10.1016/0378-4347(93)80259-7. [DOI] [PubMed] [Google Scholar]
- Chatterjee B. P., Ahmed H., Uhlenbruck G., Janssen E., Kolar C., Seiler F. R. Jackfruit (Artocarpus integrifolia) and the Agaricus mushroom lectin fit also to the so-called peanut receptor. Behring Inst Mitt. 1985 Dec;(78):148–158. [PubMed] [Google Scholar]
- Ching C. K., Rhodes J. M. Purification and characterization of a peanut-agglutinin-binding pancreatic-cancer-related serum mucus glycoprotein. Int J Cancer. 1990 Jun 15;45(6):1022–1027. doi: 10.1002/ijc.2910450607. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Fraser G., Read A. E. Study of the carbohydrate content of mucus glycoproteins from normal and diseased colons. Clin Sci (Lond) 1981 Aug;61(2):229–234. doi: 10.1042/cs0610229. [DOI] [PubMed] [Google Scholar]
- Cooper H. S. Peanut lectin-binding sites in large bowel carcinoma. Lab Invest. 1982 Oct;47(4):383–390. [PubMed] [Google Scholar]
- Cooper H. S., Reuter V. E. Peanut lectin-binding sites in polyps of the colon and rectum. Adenomas, hyperplastic polyps, and adenomas with in situ carcinoma. Lab Invest. 1983 Dec;49(6):655–661. [PubMed] [Google Scholar]
- Delannoy P., Pelczar H., Vandamme V., Verbert A. Sialyltransferase activity in FR3T3 cells transformed with ras oncogene: decreased CMP-Neu5Ac:Gal beta 1-3GalNAc alpha-2,3-sialyltransferase. Glycoconj J. 1993 Feb;10(1):91–98. doi: 10.1007/BF00731192. [DOI] [PubMed] [Google Scholar]
- Endo Y., Kobata A. Partial purification and characterization of an endo-alpha-N-acetylgalactosaminidase from the culture of medium of Diplococcus pneumoniae. J Biochem. 1976 Jul;80(1):1–8. doi: 10.1093/oxfordjournals.jbchem.a131240. [DOI] [PubMed] [Google Scholar]
- Hoskins L. C., Boulding E. T. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981 Jan;67(1):163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsell E. F., Feizi T. Gastrointestinal mucins. Structures and antigenicities of their carbohydrate chains in health and disease. Med Biol. 1982 Oct;60(5):227–236. [PubMed] [Google Scholar]
- Hounsell E. F., Lawson A. M., Feeney J., Gooi H. C., Pickering N. J., Stoll M. S., Lui S. C., Feizi T. Structural analysis of the O-glycosidically linked core-region oligosaccharides of human meconium glycoproteins which express oncofoetal antigens. Eur J Biochem. 1985 Apr 15;148(2):367–377. doi: 10.1111/j.1432-1033.1985.tb08848.x. [DOI] [PubMed] [Google Scholar]
- Itzkowitz S. H., Yuan M., Montgomery C. K., Kjeldsen T., Takahashi H. K., Bigbee W. L., Kim Y. S. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989 Jan 1;49(1):197–204. [PubMed] [Google Scholar]
- Jacobs L. R., Huber P. W. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985 Jan;75(1):112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. J., Chan A., Roe R., Warren B. F., Dell A., Morris H. R., Bartolo D. C., Durdey P., Corfield A. P. Two different glycosyltransferase defects that result in GalNAc alpha-O-peptide (Tn) expression. Glycobiology. 1994 Jun;4(3):267–279. doi: 10.1093/glycob/4.3.267. [DOI] [PubMed] [Google Scholar]
- Lance P., Lev R. Colonic oligosaccharide structures deduced from lectin-binding studies before and after desialylation. Hum Pathol. 1991 Apr;22(4):307–312. doi: 10.1016/0046-8177(91)90077-3. [DOI] [PubMed] [Google Scholar]
- Longenecker B. M., Willans D. J., MacLean G. D., Selvaraj S., Suresh M. R., Noujaim A. A. Monoclonal antibodies and synthetic tumor-associated glycoconjugates in the study of the expression of Thomsen-Friedenreich-like and Tn-like antigens on human cancers. J Natl Cancer Inst. 1987 Mar;78(3):489–496. [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Okada Y., Sotozono M., Sakai N., Yonei T., Nakanishi S., Tsuji T. Fucosylated Thomsen-Friedenreich antigen in alpha-anomeric configuration in human gastric surface epithelia: an allogeneic carbohydrate antigen possibly controlled by the Se gene. J Histochem Cytochem. 1994 Mar;42(3):371–376. doi: 10.1177/42.3.8308254. [DOI] [PubMed] [Google Scholar]
- Orntoft T. F., Harving N., Langkilde N. C. O-linked mucin-type glycoproteins in normal and malignant colon mucosa: lack of T-antigen expression and accumulation of Tn and sialosyl-Tn antigens in carcinomas. Int J Cancer. 1990 Apr 15;45(4):666–672. doi: 10.1002/ijc.2910450416. [DOI] [PubMed] [Google Scholar]
- Parker N., Finnie I. A., Raouf A. H., Ryder S. D., Campbell B. J., Tsai H. H., Iddon D., Milton J. D., Rhodes J. M. High performance gel filtration using monodisperse highly cross-linked agarose as a one-step system for mucin purification. Biomed Chromatogr. 1993 Mar-Apr;7(2):68–74. doi: 10.1002/bmc.1130070204. [DOI] [PubMed] [Google Scholar]
- Picard J. K., Feizi T. Peanut lectin and anti-Ii antibodies reveal structural differences among human gastrointestinal glycoproteins. Mol Immunol. 1983 Nov;20(11):1215–1220. doi: 10.1016/0161-5890(83)90145-1. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A. Emergence of antigenic glycoprotein structures in ulcerative colitis detected through monoclonal antibodies. Gastroenterology. 1988 Aug;95(2):371–378. doi: 10.1016/0016-5085(88)90493-3. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985 Jul 15;260(14):8262–8271. [PubMed] [Google Scholar]
- Pullan R. D., Thomas G. A., Rhodes M., Newcombe R. G., Williams G. T., Allen A., Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994 Mar;35(3):353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M., Black R. R., Savage A. Altered lectin binding by colonic epithelial glycoconjugates in ulcerative colitis and Crohn's disease. Dig Dis Sci. 1988 Nov;33(11):1359–1363. doi: 10.1007/BF01536988. [DOI] [PubMed] [Google Scholar]
- Rhodes J. M., Black R. R., Savage A. Glycoprotein abnormalities in colonic carcinomata, adenomata, and hyperplastic polyps shown by lectin peroxidase histochemistry. J Clin Pathol. 1986 Dec;39(12):1331–1334. doi: 10.1136/jcp.39.12.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M. Colonic mucus and mucosal glycoproteins: the key to colitis and cancer? Gut. 1989 Dec;30(12):1660–1666. doi: 10.1136/gut.30.12.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder S. D., Parker N., Ecclestone D., Haqqani M. T., Rhodes J. M. Peanut lectin stimulates proliferation in colonic explants from patients with inflammatory bowel disease and colon polyps. Gastroenterology. 1994 Jan;106(1):117–124. doi: 10.1016/s0016-5085(94)94775-9. [DOI] [PubMed] [Google Scholar]
- Ryder S. D., Smith J. A., Rhodes E. G., Parker N., Rhodes J. M. Proliferative responses of HT29 and Caco2 human colorectal cancer cells to a panel of lectins. Gastroenterology. 1994 Jan;106(1):85–93. doi: 10.1016/s0016-5085(94)94527-6. [DOI] [PubMed] [Google Scholar]
- Ryder S. D., Smith J. A., Rhodes J. M. Peanut lectin: a mitogen for normal human colonic epithelium and human HT29 colorectal cancer cells. J Natl Cancer Inst. 1992 Sep 16;84(18):1410–1416. doi: 10.1093/jnci/84.18.1410. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Lin Y., DeVries A. L. Structure of the carbohydrate of antifreeze glycoproteins from an antartic fish. FEBS Lett. 1975 Jun 15;54(2):135–138. doi: 10.1016/0014-5793(75)80060-3. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Springer G. F. T and Tn, general carcinoma autoantigens. Science. 1984 Jun 15;224(4654):1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]
- Umemoto J., Matta K. L., Barlow J. J., Bhavanandan V. P. Action of endo-alpha-N-acetyl-D-galactosaminidase on synthetic glycosides including chromogenic substrates. Anal Biochem. 1978 Nov;91(1):186–193. doi: 10.1016/0003-2697(78)90830-8. [DOI] [PubMed] [Google Scholar]
- Xu H., Sakamoto K., Shamsuddin A. M. Detection of the tumor marker D-galactose-beta-(1-->3)-N-acetyl-D-galactosamine in colonic cancer and precancer. Arch Pathol Lab Med. 1992 Nov;116(11):1234–1238. [PubMed] [Google Scholar]
- Yu L., Fernig D. G., Smith J. A., Milton J. D., Rhodes J. M. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993 Oct 1;53(19):4627–4632. [PubMed] [Google Scholar]
- Yuan M., Itzkowitz S. H., Boland C. R., Kim Y. D., Tomita J. T., Palekar A., Bennington J. L., Trump B. F., Kim Y. S. Comparison of T-antigen expression in normal, premalignant, and malignant human colonic tissue using lectin and antibody immunohistochemistry. Cancer Res. 1986 Sep;46(9):4841–4847. [PubMed] [Google Scholar]


