Abstract
Neuroblastomas are pediatric tumors which develop from sympathetic precursors and express neuronal proteins, such as neuropeptide Y (NPY). NPY is a sympathetic neurotransmitter acting via multiple receptors (Y1-Y5R). Both NPY and Y2Rs are commonly expressed in neuroblastoma cell lines and tissues. The peptide secreted from neuroblastomas stimulates tumor cell proliferation and angiogenesis. Since both processes are Y2R-mediated, the goal of this study was to assess Y2R as a potential therapeutic target for neuroblastoma. In vitro, Y2R antagonist (BIIE0246) prevented activation of p44/42 MAPK induced by endogenous NPY, which resulted in decreased proliferation and induction of Bim-mediated apoptosis. Similar growth-inhibitory effects were achieved with NPY siRNA and Y2R siRNA. In vivo, Y2R antagonist significantly inhibited growth of SK-N-BE(2) and SK-N-AS xenografts, which was associated with decreased activation of p44/42 MAPK, as well as reduced proliferation (Ki67) and increased apoptosis (TUNEL). The Y2R antagonist also exerted an anti-angiogenic effect. In vitro, it reduced the proliferation of endothelial cells induced by neuroblastoma-conditioned media. Consequently, the Y2R antagonist-treated xenografts had decreased vascularization and a high degree of focal fibrosis. In human neuroblastoma tissues, the expression of Y2R was observed in both tumor and endothelial cells, while NPY was predominantly expressed in neuroblastoma cells. In summary, Y2R is a promising new target for neuroblastoma therapy affecting both cancer cells and tumor vasculature.
Keywords: Neuropeptide Y, neuroblastoma, angiogenesis
Introduction
Neuroblastomas are pediatric tumors of neuroendocrine origin with very diverse phenotypes ranging from spontaneously regressing tumors in infants to very aggressive malignancies, which account for approximately 15% of all childhood cancer deaths (Maris, 2005; Maris et al., 2007; Park et al., 2008). These tumors develop from precursors of sympathetic neurons and express neuronal markers, such as the sympathetic neurotransmitter neuropeptide Y (NPY) and its receptors (Biedler et al., 1978; Kitlinska et al., 2005; O’Hare & Schwartz, 1989a; O’Hare & Schwartz, 1989b). NPY, acting through multiple G protein-coupled receptors (Y1–Y5), is a growth factor for a variety of cells, such as neuronal precursors, vascular smooth muscle and endothelial cells (Hansel et al., 2001; Movafagh et al., 2006; Pons et al., 2008; Pons et al., 2003; Zukowska-Grojec et al., 1998; Zukowska-Grojec et al., 1993). The peptide has also been shown to stimulate angiogenesis in a variety of physiological and pathological conditions (Ekstrand et al., 2003; Kitlinska et al., 2002; Koulu et al., 2004; Lee et al., 2003b; Yoon et al., 2002).
Due to their sympathetic origin, neuroblastomas release high levels of NPY, which often results in increased peptide concentrations in a patient’s plasma. These elevated plasma NPY levels are associated with poor clinical outcome of the disease in children over one year of age, with advanced stage neuroblastomas (Cohen et al., 1990; Dotsch et al., 1998; Kogner et al., 1994). These data correlate with our previous findings that exogenous NPY enhances neuroblastoma growth via two processes – a direct autocrine stimulation of tumor cell proliferation and a paracrine angiogenic effect (Kitlinska et al., 2005). Both processes are mediated mainly by Y2 receptors (Y2Rs), with some contribution from Y5R. Since Y2R is the main angiogenic receptor in the NPY system (Ekstrand et al., 2003; Koulu et al., 2004; Lee et al., 2003a) and the most commonly expressed NPY receptor in neuroblastoma cells (Kitlinska et al., 2005; Korner et al., 2004), we focused on assessing Y2Rs as potential therapeutic targets. We sought to determine whether blocking actions of endogenous NPY by targeting Y2R will be sufficient to inhibit neuroblastoma growth. We show that Y2R blockage in neuroblastoma cells leads to decreased p44/42 MAPK activation, which results in a reduced proliferation rate, as well as increased Bim levels and enhanced apoptosis. Moreover, blocking the Y2R pathway impairs tumor vascularization, which, together with the direct effect on neuroblastoma cells, leads to significant inhibition of tumor growth in vivo.
Results
NPY is an autocrine growth factor for neuroblastomas
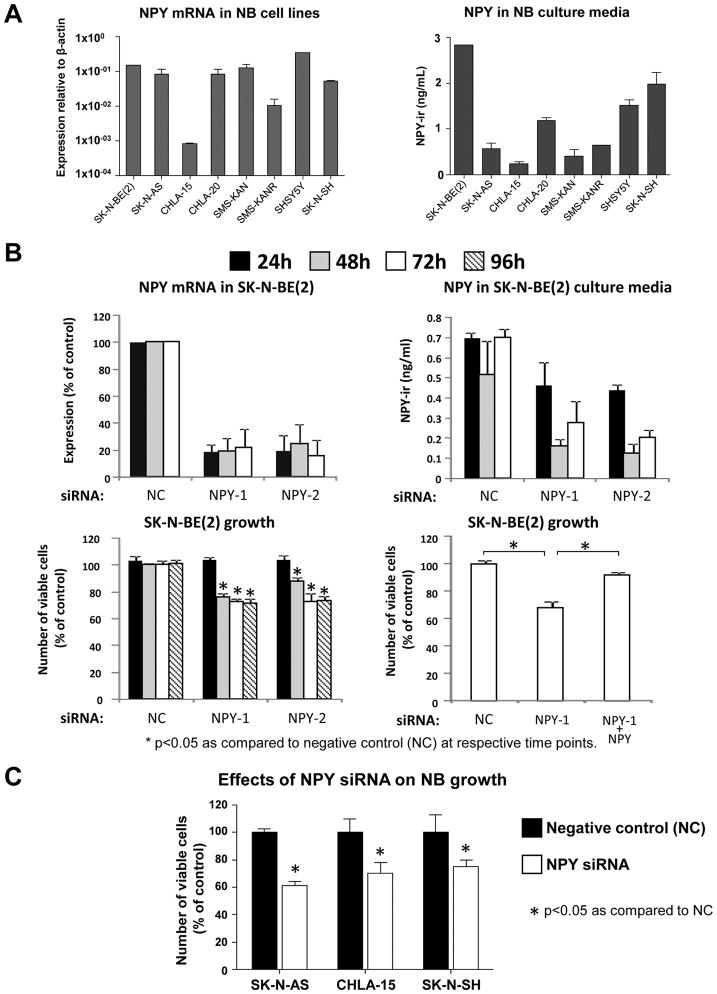
Our previous data indicated that exogenous NPY stimulates proliferation of SK-N-BE(2) neuroblastoma cells (Kitlinska et al., 2005). In the current study, high NPY mRNA levels were detected by real-time RT-PCR in eight neuroblastoma cell lines and release of the peptide was confirmed by ELISA on their corresponding culture media (Fig. 1A). To determine if blocking endogenous NPY will be sufficient to inhibit neuroblastoma growth, SK-N-BE(2) cells were transfected with two different NPY siRNAs. Both siRNAs decreased NPY mRNA levels by approximately 80%, as measured by real time RT-PCR, which resulted in approximately 70% decreased NPY concentration in the culture media (Fig. 1B). These decreases in NPY levels led to a reduction in SK-N-BE(2) cell growth measured by MTS assay (Fig. 1B). The growth inhibition caused by NPY siRNA was rescued by exogenous NPY (10−7M). In line with this, in three other neuroblastoma cell lines, NPY siRNA had growth inhibitory effects comparable to that observed in SK-N-BE(2) cells (Fig. 1C).
Fig. 1. Down-regulation of endogenous NPY reduces neuroblastoma growth in vitro.
A. Expression of NPY was detected by real-time RT-PCR in eight tested neuroblastoma cell lines and its release confirmed by ELISA.
B. In SK-N-BE(2) cells, NPY siRNA inhibited expression of the peptide (real-time RT-PCR) and its release into the cell culture media (ELISA), which resulted in a decreased number of viable cells (MTS assay). The growth-inhibitory effect of NPY siRNA was rescued by exogenous NPY (10−7M).
(NC – negative control siRNA; NPY-1 and NPY-2 – two different siRNAs for NPY)
C. In three selected cell lines, NPY siRNA exerted growth-inhibitory effects comparable to this observed in SK-N-BE(2) cells.
Blocking Y2R prevents actions of endogenous NPY and inhibits neuroblastoma growth in vitro
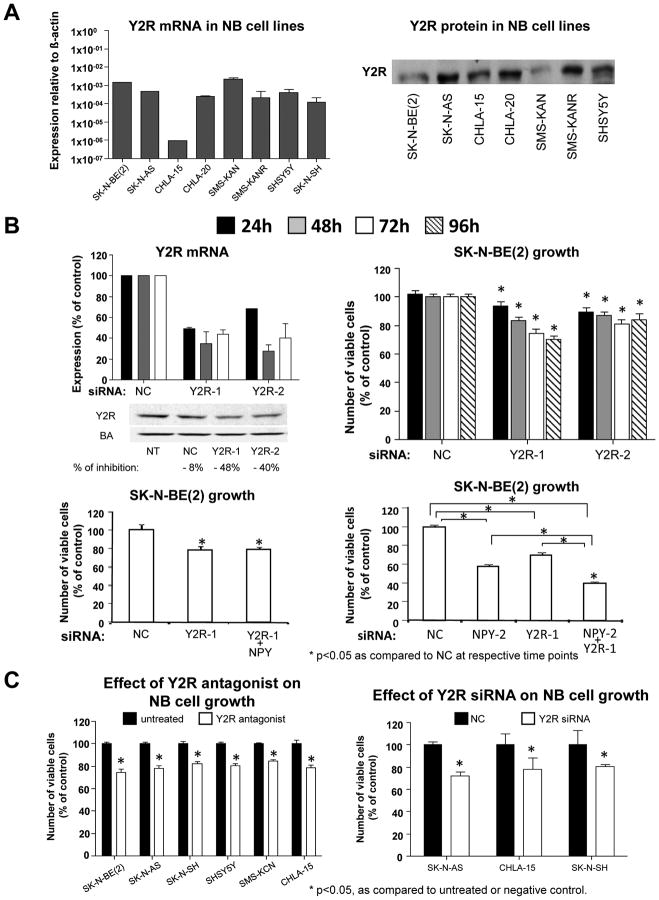
Like NPY, Y2Rs were commonly expressed in various neuroblastoma cells (Fig. 2A). To determine if a reduction in Y2R levels prevents the growth-promoting effect of endogenous NPY, SK-N-BE(2) cells were transfected with two different Y2R siRNAs. The Y2R knockdown (approximately 70% reduction in mRNA and 40–50% decrease in protein levels) resulted in statistically significant decreases in SK-N-BE(2) cell growth (Fig. 2B). In contrast to the effect with NPY siRNA, growth inhibition induced by Y2R siRNA was not rescued by exogenous NPY (Fig. 2B). To increase the efficiency of blocking the NPY system, the most effective NPY and Y2R siRNAs were co-transfected into SK-N-BE(2) cells. The resulting growth inhibition (approximately 60%) was significantly greater than that observed with single siRNAs (Fig. 2B), indicating that efficient blockage of the NPY system exerts a strong inhibitory effect on neuroblastoma cell growth.
Fig. 2. Blocking Y2Rs mimics the growth-inhibitory effect of NPY siRNA.
A. Y2Rs were detected by RT-PCR and Western blot in selected neuroblastoma cell lines.
B. In SK-N-BE(2) cells, Y2R siRNA decreased Y2R mRNA (real time RT-PCR) and protein (Western blot) levels and reduced neuroblastoma cell growth (MTS assay). The growth-inhibitory effect of Y2R siRNA, was not rescued by exogenous NPY (10−7M). Co-transfection with both NPY and Y2R siRNAs augmented the effects of the single siRNAs (MTS assay).
(NT – non-transfected; NC – negative control siRNA; Y2R-1 and Y2R-2 – two different siRNAs for Y2R)
C. In all selected cell lines, 48h treatment with Y2R antagonist (BIIE0246, 10−6M) or 96h transfection with Y2R siRNA decreased the number of viable cells (MTS assay).
The effect of Y2R blockage on neuroblastoma cell growth was also tested in six other cell lines. 48h treatment with Y2R antagonist at concentration 10−6M (dose based on previous studies (Kitlinska et al., 2005)) resulted in modest, but statistically significant growth-inhibitory effects in all tested cell lines (Fig. 2C). This was further confirmed by the growth–inhibitory effect of Y2R siRNA in three selected cell lines.
Y2R antagonist inhibits growth of neuroblastoma cells by decreasing endogenous proliferation and inducing apoptosis
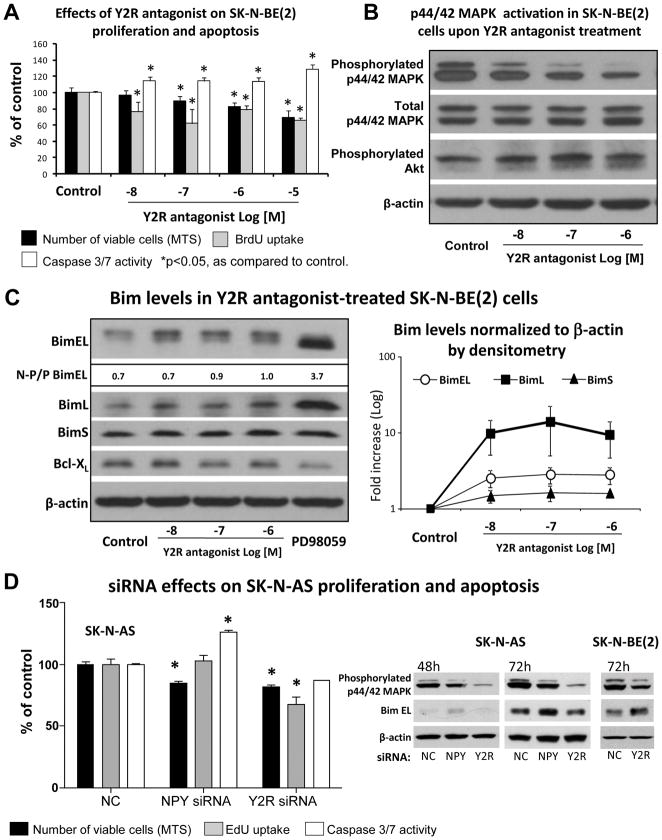
To determine a mechanism of the growth inhibitory effect resulting from Y2R blockade in neuroblastoma, SK-N-BE(2) cells were treated with Y2R antagonist (10−8 to 10−6M) and assayed for levels of proliferation and apoptosis. The treatment resulted in a dose-dependent inhibition of cell growth measured by MTS assay, which was associated with a decreased percent of BrdU positive cells (Fig. 3A). These data indicate that Y2R antagonist acts by inhibiting neuroblastoma cell proliferation induced by endogenous NPY. Simultaneously, however, an increase in apoptosis measured by caspase 3/7 activity was also observed (Fig. 3A). This mechanism was further confirmed in SK-N-AS cells treated with NPY and Y2R siRNAs. Interestingly, although both types of siRNA induced comparable growth inhibitory effects (MTS assay), increased apoptosis was the main mechanism of NPY siRNA actions, while decreased proliferation prevailed in the Y2R siRNA-transfected cells (Fig. 3D). However, the same Y2R siRNA caused a 28% increase in apoptosis in SK-N-BE(2) cells (data not shown).
Fig. 3. Y2R antagonist prevents p44/42 MAPK activation induced by endogenous NPY, which leads to decreased cell proliferation and induction of Bim-mediated apoptosis.
A. In SK-N-BE(2) cells Y2R antagonist at concentrations 10−8–10−5M decreased the number of viable cells (MTS assay) and proliferation rate (BrdU uptake), as well as increased apoptosis (caspase 3/7 activity). The measurements were performed 48, 12 and 24h after treatment, respectively.
B. In SK-N-BE(2) cells treated as above, 12h after treatment, Y2R antagonist dose dependently decreased levels of phosphorylated p44/42 MAPK (Western blot). No significant differences in phosphorylated Akt were observed.
C. 24h after treatment, the reduction in p44/42 MAPK was followed by an increase in protein levels of three isoforms of Bim (Western blot followed by densitometry). The level of BimEL phosphorylation decreased with treatment, as measured by the ratio of non-phosphorylated (lower band) to phosphorylated (upper band) form of Bim EL (N-P/P BimL). This effect was mimicked by MEK 1 inhibitor, PD98059, (5×10−5M). No changes in Bcl-XL were observed.
D. In SK-N-AS cells, transfection with NPY siRNA or Y2R siRNA decreased the number of viable cells (72h, MTS assay) and levels of active p44/42 MAPK (Western blot). In NPY siRNA-treated cells this was associated with increased caspase 3/7 activity and up-regulation of Bim (Western blot), while in Y2R siRNA-transfected cells, with decrease in proliferation (EdU uptake). The same Y2R siRNA up-regulated Bim in SK-N-BE(2) cells. NC – negative control siRNA.
The growth inhibitory effect of Y2R antagonist is mediated by a decrease in p44/42 MAPK activation and an up-regulation of Bim
Previously, we have shown that exogenous NPY stimulates neuroblastoma proliferation via activation of the p44/42 MAPK pathway and that this effect can be blocked by Y2R antagonist (Kitlinska et al., 2005). To determine whether disruption of endogenous NPY stimulation via Y2R blockage affects basal p44/42 MAPK activity, SK-N-BE(2) cells were treated with Y2R antagonist at concentrations ranging from 10−8 to 10−6M for 6, 12 and 24h. After 12h, a significant, dose-dependent decrease in phospho-p44/42 MAPK levels was observed (Fig. 3B), supporting the anti-proliferative actions of Y2R antagonist. In contrast, no significant changes in Akt activation were detected. 24h after treatment, the decrease in MAPK activation was followed by an increase in levels of Bim (Fig. 3C), a pro-apoptotic protein known to be regulated by p44/42 MAPK. This change was observed for all three known isoforms of Bim – BimEL, BimL and BimS. The Y2R antagonist-induced increase in Bim protein levels were mimicked by an inhibitor of p44/42 MAPK pathway, PD098059. Treatment with this inhibitor resulted in a change in BimEL gel migration, with only one detectable band corresponding to its non-phosphorylated form (Ley et al., 2003). Similarly, increased relative intensity of the lower, non-phosphorylated BimEL band was observed in Y2R antagonist-treated samples (Fig. 3C). p44/42 MAPK-mediated phosphorylation of Bad, which can also contribute to the anti-apoptotic effects of this MAPK, was not detected. Also, no difference in levels of Bcl-xl, a pro-survival protein implicated in regulation of neuronal cell death, was observed (Fig. 3C). These results were corroborated by a decrease in the endogenous levels of activated p44/42 MAPK observed in SK-N-AS cells transfected with NPY and Y2R siRNAs (Fig. 3D). Moreover, in NPY siRNA-treated SK-N-AS cells and Y2R siRNA-treated SK-N-BE(2) cells, a significant increase in apoptosis was associated with elevated levels of Bim (Fig. 3D).
Y2R antagonist inhibits growth of neuroblastoma xenografts
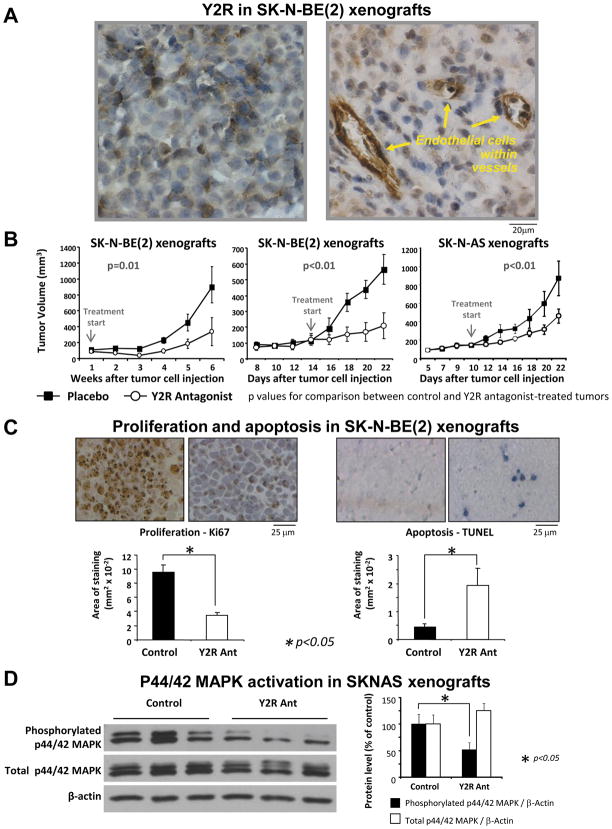
To validate our findings in vivo, Y2R antagonist was tested in a neuroblastoma xenograft model. SK-N-BE(2) cells were injected subcutaneously into nude mice and treated with daily local injections of Y2R antagonist (10−6M). In the first experiment, the treatment started 1 day after tumor cell inoculation and lasted for 6 weeks (Fig. 4B). At 33 days after initiation of treatment, the median of tumor growth was significantly higher for the control group (606.5 mm3) than for the Y2R antagonist group (53.2 mm3).
Fig. 4. Y2R antagonist inhibits growth of neuroblastoma xenografts in vivo.
A. In SK-N-BE(2) xenografts, positive immunostaining for Y2R was detected in tumor cells and endothelium within tumor vasculature.
B. SK-N-BE(2) or SK-N-AS cells were subcutaneously injected into nude mice (n = 8–10). Daily injections of Y2R antagonists (100μl of 10−6M in saline) were initiated either 1 day after tumor cell inoculation and continued for 6 weeks (left panel) or started 10–14 days after cell inoculation and continued until day 22 (middle and right panels). In all experiments, Y2R antagonist significantly inhibited xenograft growth.
C. In SK-N-BE(2) xenografts, the Y2R antagonist significantly decreased tumor cell proliferation and increased apoptosis, measured as an area of Ki67 or TUNEL staining.
D. In SK-N-AS tumors, Y2R antagonist decreased levels of phosphorylated p44/42 MAPK (Western blot, representative samples shown).
In the second experiment, the treatment started 14 days after tumor cell inoculation - when increases in tumor size were first observed. 9 days following initial treatment, the median tumor growth was 516.3 mm3 for the control group, and 44.2 mm3 for the Y2R antagonist group (Fig. 4B). A similar growth-inhibitory effect was observed in SK-N-AS xenografts treated in the same way (Fig. 4B).
Y2R antagonist decreases proliferation rate and increases apoptosis in neuroblastoma xenografts in vivo
To determine the mechanisms by which Y2R antagonist inhibits neuroblastoma growth in vivo, SK-N-BE(2) tumors were stained for markers of proliferation (Ki67) and apoptosis (TUNEL). The proliferation levels were significantly lower in Y2R antagonist-treated tumors, as compared to the controls (Fig. 4C). This effect was also associated with a significant increase in apoptosis levels (Fig. 4C). Consistent with these results and our findings in vitro, the levels of activated p44/42 MAPK in Y2R antagonist-treated tumors were significantly decreased, as compared to the controls (Fig. 4D). However, no evident differences in Bim levels were observed (data not shown).
Y2R antagonist reduces the angiogenic potential of neuroblastomas
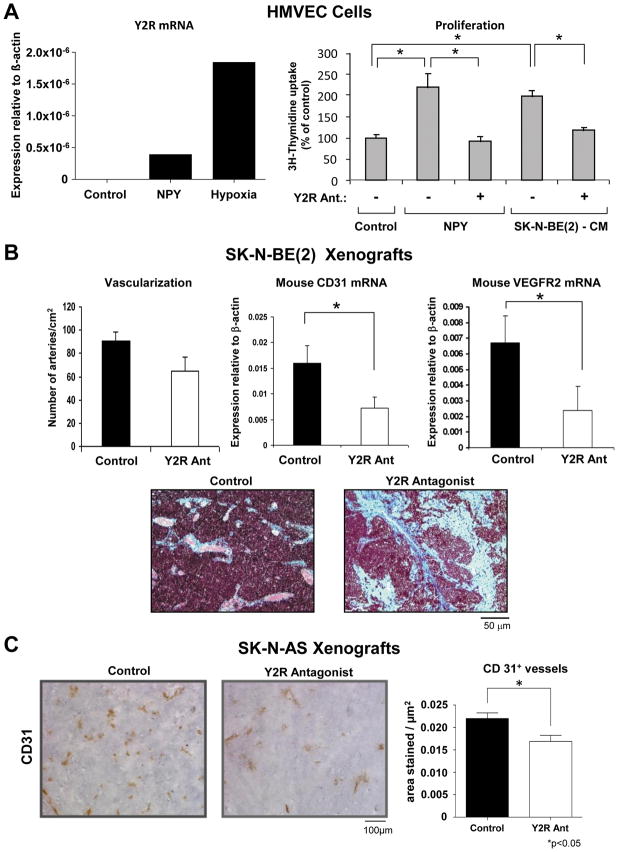
Our previous data indicated that NPY released from neuroblastomas promotes tumor vascularization (Kitlinska et al., 2005). To determine if this effect is mediated by Y2R, we assessed HMVEC expression levels for this receptor. Although not detectable under basal conditions, Y2R mRNA was induced by hypoxia (0.1% oxygen for 24h) and NPY (10−7M for 6h) treatment (Fig. 5A). Moreover, in HMVECs, Y2R antagonist (10−6M) completely blocked the proliferative effect of NPY (10−8M, optimal dose based on previous studies (Kitlinska et al., 2002)) and significantly reduced proliferation induced by SK-N-BE(2)-conditioned media (Fig. 5A). Thus, the mitogenic actions of neuroblastoma-derived NPY on endothelial cells are highly dependent on Y2R.
Fig. 5. Y2R antagonist exerts an anti-angiogenic effect.
A. In HMVECs, Y2R mRNA was induced by NPY and hypoxia (real time RT-PCR). Y2R antagonist (10−6M) blocked HMVEC proliferation (3H-thymidine uptake) induced by NPY (10−8M) and significantly reduced the mitogenic effect of SK-N-BE(2)-conditioned media.
B. In SK-N-BE(2) xenografts, the difference in the number of arteries per cm2 between Y2R antagonist and control groups did not reach statistical significance. However, expression of mouse endothelial markers, CD31 and VEGFR2 (real time RT-PCR), was significantly down-regulated in the treatment group, which was associated with marked focal fibrosis (Masson;s trichrome stain).
C. In Y2R antagonist-treated SK-N-AS xenografts, area of CD31-positive vessels was significantly decreased, as compared to the control.
In line with these results, in SK-N-BE(2) xenografts, Y2R was detected not only in tumor cells, but also in endothelium of tumor vasculature (Fig. 4A). Moreover, Y2R antagonist-treated SK-N-BE(2) xenografts appeared to have lower vascularization, as measured by the number of arteries per cm2, though differences did not reach statistical significance (Fig. 5B). However, there were statistically significant differences in mRNA levels of mouse endothelial markers – CD31 and VEGFR2, suggesting anti-angiogenic effects of Y2R antagonist (Fig. 5B). Moreover, the treated tumors exhibited high levels of focal fibrosis (Fig. 5B), which is considered a sign of tumor necrosis following ischemia and its subsequent regression. This anti-angiogenic effect of Y2R antagonist was further confirmed in SK-N-AS xenografts, where a significant decrease in the area of CD31-positive vessels was observed (Fig. 5C).
NPY and its Y2Rs are expressed in human neuroblastomas
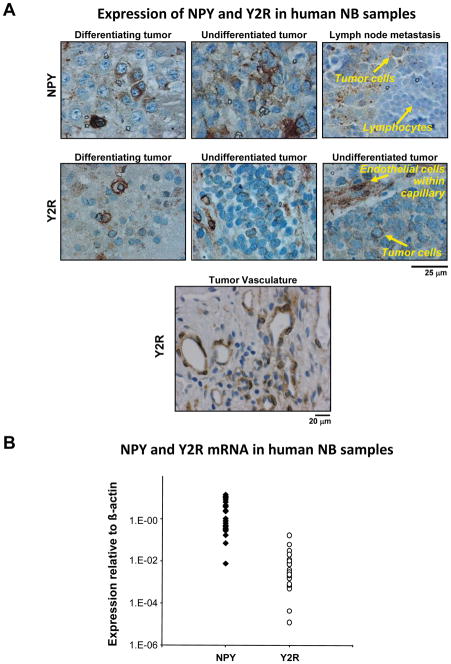
To validate our findings in experimental models, we tested the expression of NPY and Y2R in human neuroblastoma samples by immunohistochemistry and real time RT-PCR. Out of 20 tumor samples derived from neuroblastoma patients at different stages of the disease, 85% stained positively for NPY. The peptide was detected in primary tumors, both differentiating and undifferentiated, as well as in lymph node metastases (Fig. 6A). Positive immunostaining for Y2R was observed in 80% of neuroblastoma cases. As with NPY, Y2R staining was detected in both differentiating tumors and their undifferentiated, aggressive counterparts (Fig. 6A). Strong Y2R staining was also observed in endothelial cells within the tumor vasculature. Expression of NPY and Y2R in neuroblastoma tissues was also confirmed by real time RT-PCR. All tissues expressed variable, but very high levels of NPY and Y2R mRNA (Fig. 6B).
Fig. 6. NPY and Y2Rs are expressed in human neuroblastoma tissues.
A. Human neuroblastoma tissues were immunostained for NPY and its Y2Rs. NPY was detected in most cases of neuroblastoma with different degrees of differentiation, as well as in lymph node metastases. Y2Rs were present in both differentiating and undifferentiated tumors. The receptors were also detected in endothelial cells within the tumor vasculature.
B. Real time RT-PCR confirmed high expression of NPY and Y2Rs in all human neuroblastoma samples (n=20).
Discussion
High expression of NPY and its receptors in neuroblastomas has previously only been considered as a marker of neuronal differentiation (Magni et al., 2000; Sheriff et al., 1998) with little attention given to the role of NPY signaling in neuroblastoma biology. However, reports correlating elevated NPY plasma levels with poor clinical outcome of the disease (Cohen et al., 1990; Dotsch et al., 1998; Kogner et al., 1994), as well as emerging evidence on growth-promoting and angiogenic effects of the peptide (Hansel et al., 2001; Lee et al., 2003b; Movafagh et al., 2006; Zukowska-Grojec et al., 1998) suggest that NPY does play an integral role in the regulation of neuroblastoma growth. Our previous studies indicated that NPY stimulates growth of neuroblastomas by an autocrine mitogenic effect on tumor cells and by its angiogenic activity (Kitlinska et al., 2005). Since both processes are mediated mainly by Y2R, here we assessed this receptor as a potential therapeutic target for the treatment of aggressive neuroblastomas. Our goal was to determine the extent at which the endogenous NPY system is essential to the stimulation of neuroblastoma growth and, consequently, if blocking its effects by targeting Y2Rs will be sufficient to inhibit neuroblastoma growth in vivo.
Previously, we have found that exogenous NPY stimulates neuroblastoma cell proliferation via p44/42 MAPK activation (Kitlinska et al., 2005). In the current study, we demonstrated that blockade of the endogenous NPY/Y2R pathway results in reduced levels of active p44/42 MAPK and marked decreases in the proliferation rate of neuroblastoma cells, as well as induction of Bim-mediated apoptosis. Bim is a member of the Bcl-2 family known to stimulate apoptosis upon growth factor withdrawal (Lei & Davis, 2003; Putcha et al., 2003; Whitfield et al., 2001). p44/42 MAPK has been shown to phosphorylate BimEL, which promotes its degradation and prevents apoptosis (Ley et al., 2003). In neuroblastoma cells, p44/42 MAPK-driven regulation of Bim has been implicated in pro-survival activity of brain-derived neurotrophic factor (BDNF) (Li et al., 2007). Here, we have shown that in SK-N-BE(2) cells, a selective inhibitor of p44/42 MAPK activation, PD098059, not only significantly increases levels of all three forms of Bim, but also converts BimEL to its more stable, non-phosphorylated form. Consequently, in the same cells treated with Y2R antagonist or Y2R siRNA, decreases in p44/42 activation resulted in similar changes in Bim levels and phosphorylation.
Interestingly, the extent to which inhibition of proliferation and apoptosis contribute to the observed growth-inhibitory effects may vary. For example, in SK-N-AS cells, NPY siRNA upregulated Bim and induced apoptosis, while Y2R siRNA caused a decrease in cell proliferation. In Y2R antagonist-treated SK-N-BE(2) cells, both growth-inhibitory processes were observed. Thus, decrease in p44/42 MAPK activation is a direct consequence of blocking NPY/Y2R pathway, which in turn results in a decrease in proliferation and/or an increase in Bim-mediated apoptosis. The balance between these two processes may depend on the efficiency of inhibition, basal Y2R and NPY levels, and other factors.
In line with this, in neuroblastoma xenografts, treatment with Y2R antagonist resulted in marked reduction of phospho-p44/42 MAPK, but no detectable changes in Bim levels. This may result from the fact that despite a statistically significant increase in apoptosis with Y2R antagonist treatment, overall levels of cell death were relatively low, possibly below the detection level of the Western blot. Thus, even though apoptosis seems to contribute to the growth-inhibitory effect of Y2R antagonist in vivo, the marked reduction in cell proliferation appears to be the major consequence of decreased p44/42 MAPK activation and the main mechanism of this phenomenon.
Although the mechanisms of Y2R antagonist actions in vitro and in vivo seemed to be consistent, the magnitude of the effect was strikingly different. The modest effects of Y2R antagonist on neuroblastoma cells in culture translated into highly effective growth inhibition in vivo, suggesting that actions of endogenous NPY are enhanced by factors present in the tumor microenvironment. In addition, in vivo, the anti-proliferative effect of Y2R antagonist on neuroblastoma cells is further augmented by its anti-angiogenic actions. Our previous results indicated that exogenous NPY enhances vascularization of neuroblastoma xenografts (Kitlinska et al., 2005). In the current study, we showed that endothelial cells within neuroblastoma xenografts and human neuroblastoma vasculature are highly Y2R positive and that Y2R antagonist decreases basal angiogenesis in neuroblastoma xenografts.
Neuroblastomas are highly vascularized and angiogenesis-dependent tumors in which the degree of vascularization has been linked to adverse prognosis and a poor outcome of the disease (Meitar et al., 1996). Combinations of different anti-proliferative and anti-angiogenic agents have already been tested and proven effective in animal models of the disease and in clinical studies (Ribatti & Ponzoni, 2005; Rossler et al., 2008; Shusterman & Maris, 2005). Thus, the Y2R antagonist treatment, targeting both formation of blood vessels and cancer cell proliferation seems to be a promising approach, in line with other currently tested neuroblastoma therapies. Notably, NPY does not seem to be involved in developmental angiogenesis, since both Y2 and NPY knockout mice do not have any known organ defects. This is particularly important since neuroblastoma therapy is applied to children with active angiogenesis-dependent developmental processes.
As discussed above, treatment with Y2R antagonist, BIIE0246, significantly inhibited neuroblastoma xenograft growth. Notably, this antagonist is known to be extremely unstable, with an in vivo half-life of only 30 min (Malmstrom, 2001). As indicated by the enhanced growth–inhibitory effect with combined NPY and Y2R siRNAs, the efficiency of NPY pathway inhibition is an important factor determining the magnitude of the response. Thus, the success of Y2R-targeted treatment could be increased by developing new, more efficient and stable antagonists. Moreover, the role of other NPY receptors also expressed in some neuroblastoma cells (Kitlinska et al., 2005) and the effect of therapies targeting multiple NPY receptors remain to be investigated.
The clinical relevance of our experimental findings is supported by the expression of NPY and its Y2Rs in human neuroblastoma tissues shown here and previously reported by others (Korner et al., 2004). The fact that expression of both NPY and Y2R was detected in all tested neuroblastoma cell lines and in a high percent of neuroblastoma tissues proves their value as universal therapeutic targets. This is in contrast to some other molecules implicated in neuroblastoma, such as ALK. ALK is a recently discovered, very promising target in neuroblastoma therapy. However, the inhibitors of this molecule affect only a subset of tumors with ALK mutations (Chen et al., 2008; George et al., 2008; Mosse et al., 2008). For example, the growth of SK-N-AS cells, which was significantly inhibited by Y2R antagonist in vitro and in vivo, is not affected by ALK inhibitors (Mosse et al., 2008). Thus, due to the universal nature of the NPY growth-promoting effects, targeting its Y2R may be a supplementary treatment to those more selective therapies.
Taken together, we have shown for the first time that blocking Y2R is sufficient to inhibit neuroblastoma growth in vivo. The main mechanisms of this effect include inhibition of tumor cell proliferation with some contribution of apoptosis and impairment of angiogenesis. The efficiency of this treatment can be further enhanced by designing more potent Y2R antagonists. Therefore, NPY and its Y2R are promising new targets in neuroblastoma therapy.
Materials and methods
Materials
NPY was purchased from Bachem (San Carlos, CA), Y2R antagonist, BIIE0246, from Tocris (Ellisville, MO) and MEK 1 inhibitor, PD089059, from Sigma (St. Louis, MO).
Cell culture
Human neuroblastoma cells – SK-N-BE(2), SK-N-AS, SK-N-SH, SHSY5Y were obtained from ATCC (Manassas, VA) and SMS-KAN, SMS-KANR, CHLA-15 and CHLA-20 from Dr. Patrick Reynolds (Children’s Hospital of Los Angeles, CA). Human dermal microvascular endothelial cells (HMVEC) were purchased from Lonza (Basel, Switzerland). All cell lines were cultured according to the supplier’s recommendation. To create hypoxic conditions, HMVEC cells were cultured in hypoxic chamber containing 0.1% oxygen for 24h.
Cell viability and apoptosis assays
The cells cultured in 96 well plates were put into 1% FBS media and 24h later treated with the Y2R antagonist. After 24h, the caspase 3/7 activity was measured using Apo-ONE Homogenous Caspase 3/7 reagent (Promega, Madison, WI), while the number of viable cells was measured 48h after treatment using CellTiter 96®AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI).
Cell proliferation
SK-N-BE(2) cells cultured in 1% FBS for 24h were treated with Y2R antagonist at desired concentrations. 4h after treatment, 10μM BrdU was added to the media. 8h later, the cells were trypsinized and stained with anti-BrdU antibody using BrdU Flow Kit (BD Biosciences, San Diego, CA). Flow cytometric analysis was performed on FACSort (Becton Dickinson, Franklin Lakes, NJ) and data analyzed using CellQuest and ModFit LT softwares (Verity Software House Inc., Topsham, ME). The siRNA-treated cells were incubated for one hour with 5μM EdU, fixed and stained using Click-it EdU proliferation kit (Invitrogen, Carlsbad, CA). The percent of stained cells was evaluated by fluorescence microscopy.
Real time RT-PCR
RNA from culture cells was isolated using High Pure RNA Isolation Kit (Roche Applied Science, Indianapollis, IN) and from tissues using TRI reagent (Sigma, St. Louis, MO). cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) and amplified using ICycler iQ Detection System (Bio-Rad Laboratories, Hercules, CA). The PCR reactions were carried out using TaqMan Universal PCR Master Mix and pre-designed primers and fluorescein-labeled probes (Applied Biosystems, Foster City, CA). The results were calculated by the comparative CT method using β-actin as an endogenous reference gene.
siRNA transfection
NB cells were transfected with 30nM pre-designed siRNAs for NPY, Y2R or negative control (Applied Biosystems, Foster City, CA) using Trans IT-TKO reagent (Mirus, Madison, WI) and 24h later media was changed to fresh 10% or 1% FBS media. The efficiency of siRNA inhibition was tested by real time RT-PCR, Western blot (Y2R) or Neuropeptide Y Enzyme Immunoassay (Bachem, San Carlos, CA) and cell viability, apoptosis and proliferation were assessed as above.
Western blot
SK-N-BE(2) cells cultured in 1% or 5% FBS media for 24h were stimulated with Y2R antagonist (10−8M–10−6M) or MEK 1 inhibitor, PD089059 (5 × 10−5M) and harvested after 6, 12 and 24h. The siRNA transfected cells at the desired time points and tissue samples were lysed as previously described (Kitlinska et al., 2005). The proteins of interest were detected using the following antibodies: Phospho p44/42 MAPK E10 mouse monoclonal antibody, rabbit polyclonal antibodies against p44/42 MAPK, Bim, phospho-Bad (Ser155), phospho-Akt (Ser473), rabbit monoclonal antibody against Bcl-xl (Cell Signaling Technology, Inc., Beverly, MA), goat polyclonal anti-Npy2r antibody (Biosource, Camarillo, CA) and anti-β-actin mouse monoclonal antibody (Sigma, St. Louis, MO). Densitometry was performed using NIH Scion Image software (Scion Corp., Frederick, MD). Each immunoblot was repeated at least three times and representative results are shown in figures.
Nude mice xenograft model
Seven-ten week old nude mice (Taconic, Hudson, NY) were subcutaneously injected into their right flank with 5×106 of SK-N-BE(2) or SK-N-AS cells suspended in 0.1 ml of Matrigel (BD Biosciences, San Diego, CA). One day or ten-fourteen days after tumor cell inoculation, the daily treatment with Y2R antagonist administered as local injection (approximately 1 cm from the tumor) of 100μl of 10−6M solution in saline or saline alone was started. Tumor size was measured periodically and volume calculated by the formula: 0.44 × length × width2 (Wassberg, 1999). At the end of the experiment, mice were euthanized and tumors were harvested, measured and weighed. The animal protocolwas approved by the Georgetown University Animal Care and Use Committee.
Human neuroblastoma tissues
Samples of 20 human neuroblastoma tumors (matched formalin fixed tissues and RNA samples) from different stages of the disease were received from Children’s Oncology Group.
Immunohistochemistry and tumor vascularization
Immunostaining was performed using the following antibodies: rabbit polyclonal anti-NPY and anti-Y2R antibody provided by Astra Zeneca (Mölndal, Sweden) (Ekstrand et al., 2003; Jonsson-Rylander et al., 2003; Naveilhan et al., 1999; Uddman et al., 2002), Ki67 mouse monoclonal Ab (Oncogene Research Products, Boston, MA) and CD31 rat monoclonal Ab (BD Pharmingen, San Jose, CA). TUNEL was performed using In Situ Cell Detection Kit (Roche Diagnostic, Indianapolis, IN). The proliferation and apoptosis rates and tissue vascularization were calculated as an area of positive staining using NIH Scion Image software (Scion Corp., Frederick, MD) and NIH Image J. Vascularization of SK-N-BE(2) xenografts was measured as the number of arteries per cm2, based on H&E stained slides, using the Automated Cellular Imaging System II (Clarient, Inc, San Juan Capistrano, CA).
HMVEC proliferation
SK-N-BE(2) cells were grown in HMVEC culture media with 0.25% FBS for 24h. Then, the medium was collected and used to stimulate HMVEC cell proliferation at dilution 0.5x. HMVECs at passages 4 to 8 were plated onto 96-well plates, growth-arrested in serum-free media for 24h, and then treated for 24h with NPY (10−8M) or SK-N-BE(2)-conditioned media, with or without Y2R antagonist (10−6 M) in media supplemented with 0.25% FBS. The proliferation was measured using [3H] thymidine, as previously described (Kitlinska et al., 2005).
Statistical analysis
Statistical analysis was performed using SigmaStat® software. One-way repeated measure ANOVA with post-hoc t-test (P<0.05) using Dunnett’s method was used for data analysis. Data is presented as mean ± standard errors. The comparison of tumor growth in the in vivo experiments on SK-N-BE(2) cells was performed using Wilcoxon rank sum test to compare the differences of increases in tumor volumes from baseline. For the analysis of the SK-N-AS xenografts, the log-transformed tumor volumes were compared using linear mixed models to account for the repeated measures of each animal.
Acknowledgments
This work was supported by NIH grant 1R01CA123211-01 and funding from Children’s Cancer Foundation (Baltimore, MD) to Joanna Kitlinska The authors would like to thank John Styliaris and David Hur for help in performing experiments. We also thank the Flow Cytometry/Cell Sorting Shared Resources of Lombardi Comprehensive Cancer Center for technical assistance and Children’s Oncology Group for providing human samples.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. [PubMed] [Google Scholar]
- Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, Nakagawara A, Hayashi Y, Mano H, Ogawa S. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- Cohen PS, Cooper MJ, Helman LJ, Thiele CJ, Seeger RC, Israel MA. Neuropeptide Y expression in the developing adrenal gland and in childhood neuroblastoma tumors. Cancer Res. 1990;50:6055–6061. [PubMed] [Google Scholar]
- Dotsch J, Christiansen H, Hanze J, Lampert F, Rascher W. Plasma neuropeptide Y of children with neuroblastoma in relation to stage, age and prognosis, and tissue neuropeptide Y. Regul Pept. 1998;75–76:185–190. doi: 10.1016/s0167-0115(98)00067-6. [DOI] [PubMed] [Google Scholar]
- Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, Jonsson-Rylander AC, Hassani H, Hallberg B, Nordlander M, Cao Y. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003;100:6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George RE, Sanda T, Hanna M, Frohling S, Luther W, 2nd, Zhang J, Ahn Y, Zhou W, London WB, McGrady P, Xue L, Zozulya S, Gregor VE, Webb TR, Gray NS, Gilliland DG, Diller L, Greulich H, Morris SW, Meyerson M, Look AT. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–944. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- Jonsson-Rylander AC, Nordlander M, Svindland A, Ilebekk A. Distribution of neuropeptide Y Y1 and Y2 receptors in the postmortem human heart. Peptides. 2003;24:255–262. doi: 10.1016/s0196-9781(03)00041-x. [DOI] [PubMed] [Google Scholar]
- Kitlinska J, Abe K, Kuo L, Pons J, Yu M, Li L, Tilan J, Everhart L, Lee EW, Zukowska Z, Toretsky JA. Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Res. 2005;65:1719–1728. doi: 10.1158/0008-5472.CAN-04-2192. [DOI] [PubMed] [Google Scholar]
- Kitlinska J, Lee EW, Movafagh S, Pons J, Zukowska Z. Neuropeptide Y-induced angiogenesis in aging. Peptides. 2002;23:71–77. doi: 10.1016/s0196-9781(01)00581-2. [DOI] [PubMed] [Google Scholar]
- Kogner P, Bjork O, Theodorsson E. Plasma neuropeptide Y in healthy children: influence of age, anaesthesia and the establishment of an age-adjusted reference interval. Acta Paediatr. 1994;83:423–427. doi: 10.1111/j.1651-2227.1994.tb18134.x. [DOI] [PubMed] [Google Scholar]
- Korner M, Waser B, Reubi JC. High expression of neuropeptide y receptors in tumors of the human adrenal gland and extra-adrenal paraganglia. Clin Cancer Res. 2004;10:8426–8433. doi: 10.1158/1078-0432.CCR-04-0821. [DOI] [PubMed] [Google Scholar]
- Koulu M, Movafagh S, Tuohimaa J, Jaakkola U, Kallio J, Pesonen U, Geng Y, Karvonen MK, Vainio-Jylha E, Pollonen M, Kaipio-Salmi K, Seppala H, Lee EW, Higgins RD, Zukowska Z. Neuropeptide Y and Y2-receptor are involved in development of diabetic retinopathy and retinal neovascularization. Ann Med. 2004;36:232–240. doi: 10.1080/07853890410031236. [DOI] [PubMed] [Google Scholar]
- Lee EW, Grant DS, Movafagh S, Zukowska Z. Impaired angiogenesis in neuropeptide Y (NPY)-Y2 receptor knockout mice. Peptides. 2003a;24:99–106. doi: 10.1016/s0196-9781(02)00281-4. [DOI] [PubMed] [Google Scholar]
- Lee EW, Michalkiewicz M, Kitlinska J, Kalezic I, Switalska H, Yoo P, Sangkharat A, Ji H, Li L, Michalkiewicz T, Ljubisavljevic M, Johansson H, Grant DS, Zukowska Z. Neuropeptide Y induces ischemic angiogenesis and restores function of ischemic skeletal muscles. J Clin Invest. 2003b;111:1853–1862. doi: 10.1172/JCI16929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang J, Liu Z, Woo CW, Thiele CJ. Downregulation of Bim by brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from paclitaxel but not etoposide or cisplatin-induced cell death. Cell Death Differ. 2007;14:318–326. doi: 10.1038/sj.cdd.4401983. [DOI] [PubMed] [Google Scholar]
- Magni P, Beretta E, Scaccianoce E, Motta M. Retinoic acid negatively regulates neuropeptide Y expression in human neuroblastoma cells. Neuropharmacology. 2000;39:1628–1636. doi: 10.1016/s0028-3908(99)00231-2. [DOI] [PubMed] [Google Scholar]
- Malmstrom RE. Vascular pharmacology of BIIE0246, the first selective non-peptide neuropeptide Y Y(2) receptor antagonist, in vivo. Br J Pharmacol. 2001;133:1073–1080. doi: 10.1038/sj.bjp.0704171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005;17:7–13. doi: 10.1097/01.mop.0000150631.60571.89. [DOI] [PubMed] [Google Scholar]
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- Meitar D, Crawford SE, Rademaker AW, Cohn SL. Tumor angiogenesis correlates with metastatic disease, N-myc amplification, and poor outcome in human neuroblastoma. J Clin Oncol. 1996;14:405–414. doi: 10.1200/JCO.1996.14.2.405. [DOI] [PubMed] [Google Scholar]
- Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou C, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, Maris JM. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movafagh S, Hobson JP, Spiegel S, Kleinman HK, Zukowska Z. Neuropeptide Y induces migration, proliferation, and tube formation of endothelial cells bimodally via Y1, Y2, and Y5 receptors. Faseb J. 2006;20:1924–1926. doi: 10.1096/fj.05-4770fje. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P, Ernfors P. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med. 1999;5:1188–1193. doi: 10.1038/13514. [DOI] [PubMed] [Google Scholar]
- O’Hare MM, Schwartz TW. Expression and precursor processing of neuropeptide Y in human and murine neuroblastoma and pheochromocytoma cell lines. Cancer Res. 1989a;49:7015–7019. [PubMed] [Google Scholar]
- O’Hare MM, Schwartz TW. Expression and precursor processing of neuropeptide Y in human pheochromocytoma and neuroblastoma tumors. Cancer Res. 1989b;49:7010–7014. [PubMed] [Google Scholar]
- Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin North Am. 2008;55:97–120. x. doi: 10.1016/j.pcl.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Pons J, Kitlinska J, Jacques D, Perreault C, Nader M, Everhart L, Zhang Y, Zukowska Z. Interactions of multiple signaling pathways in neuropeptide Y-mediated bimodal vascular smooth muscle cell growth. Can J Physiol Pharmacol. 2008;86:438–448. doi: 10.1139/y08-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons J, Kitlinska J, Ji H, Lee EW, Zukowska Z. Mitogenic actions of neuropeptide Y in vascular smooth muscle cells: synergetic interactions with the beta-adrenergic system. Can J Physiol Pharmacol. 2003;81:177–185. doi: 10.1139/y02-166. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson EM., Jr JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Ponzoni M. Antiangiogenic strategies in neuroblastoma. Cancer Treat Rev. 2005;31:27–34. doi: 10.1016/j.ctrv.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Rossler J, Taylor M, Geoerger B, Farace F, Lagodny J, Peschka-Suss R, Niemeyer CM, Vassal G. Angiogenesis as a target in neuroblastoma. Eur J Cancer. 2008;44:1645–1656. doi: 10.1016/j.ejca.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Sheriff S, Dayal R, Kasckow J, Regmi A, Chance W, Fischer J, Balasubramaniam A. NPY upregulates genes containing cyclic AMP response element in human neuroblastoma cell lines bearing Y1 and Y2 receptors: involvement of CREB. Regul Pept. 1998;75–76:309–318. doi: 10.1016/s0167-0115(98)00083-4. [DOI] [PubMed] [Google Scholar]
- Shusterman S, Maris JM. Prospects for therapeutic inhibition of neuroblastoma angiogenesis. Cancer Lett. 2005;228:171–179. doi: 10.1016/j.canlet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Uddman R, Moller S, Nilsson T, Nystrom S, Ekstrand J, Edvinsson L. Neuropeptide Y Y1 and neuropeptide Y Y2 receptors in human cardiovascular tissues. Peptides. 2002;23:927–934. doi: 10.1016/s0196-9781(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Wassberg E. Angiostatic treatment of neuroblastoma. Ups J Med Sci. 1999;104:1–24. doi: 10.3109/03009739909178953. [DOI] [PubMed] [Google Scholar]
- Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- Yoon HZ, Yan Y, Geng Y, Higgins RD. Neuropeptide Y expression in a mouse model of oxygen-induced retinopathy. Clin Experiment Ophthalmol. 2002;30:424–429. doi: 10.1046/j.1442-9071.2002.00573.x. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, Rone J, Movafagh S, Ji H, Yeh Y, Chen WT, Kleinman HK, Grouzmann E, Grant DS. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–195. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]
- Zukowska-Grojec Z, Pruszczyk P, Colton C, Yao J, Shen GH, Myers AK, Wahlestedt C. Mitogenic effect of neuropeptide Y in rat vascular smooth muscle cells. Peptides. 1993;14:263–268. doi: 10.1016/0196-9781(93)90040-n. [DOI] [PubMed] [Google Scholar]