Abstract
The metabolic syndrome is conceptualized as a clustering of risk factors, including insulin resistance, dyslipidemia, central adiposity and elevated blood pressure (BP), that increase risk for cardiovascular disease and type 2 diabetes. Recent evidence suggests that markers of systemic inflammation may be included in the definition of the syndrome and play some role in its pathogenesis. In this study, we use a statistical modeling technique, confirmatory factor analyses, to evaluate relationships of systemic inflammation, as measured by plasma concentrations of C-reactive protein and interleukin-6, with the component factors of the metabolic syndrome (insulin resistance, dyslipidemia, central adiposity, and elevated BP) and to examine whether inflammation is a potential common pathway linking established components to the full syndrome. Subjects were 645 community volunteers aged 30–54 years (48% male; 82% European American; 18% African American). Consistent with existing literature, structural equation modeling adjusting for age, sex, and race confirmed a higher-order common factor underlying the covariation of insulin resistance, dyslipidemia, adiposity, and BP. Inflammation was positively associated with this common factor, accounting for 54% of its variance and partially mediating statistical aggregation of the component factors comprising the metabolic syndrome. These results were particularly strong for adiposity, raising the possibility that inflammatory processes stimulated by intraabdominal adipose tissue contribute to the development of the metabolic syndrome. The inclusion of inflammatory markers in the clinical definition of metabolic syndrome seems warranted and may improve prognostic assessment of risk of type 2 diabetes and cardiovascular disease.
Key Terms: metabolic syndrome, inflammation, interleukin-6, C-reactive protein
Introduction
The metabolic syndrome has been conceptualized as a clustering of metabolic risk factors, including insulin resistance, dyslipidemia, central adiposity, and elevated blood pressure (BP) that increase risk for cardiovascular disease (CVD) and type 2 diabetes (1, 2). These risk factors covary in epidemiological investigations (3) and, when combined, predict incident disease, disease course, and mortality, with the aggregate syndrome accounting for cardiovascular risk beyond that associated with the component risk factors (4).
The clinical definition of metabolic syndrome has undergone several iterations (1, 5–7). The current guidelines promulgated by the American Heart Association and the National Heart, Lung, and Blood Institute (5) largely overlap with those recommended by the International Diabetes Federation (1) and require evidence of three of the following five criteria: elevated fasting glucose, elevated BP, large waist circumference, elevated triglycerides and reduced high-density lipoprotein (HDL) cholesterol. Recently, it has also been proposed that markers of systemic inflammation be included in the definition of the syndrome (8, 9). In this regard, elevated peripheral levels of proinflammatory mediators, such as C-reactive protein (CRP) and interleukin (IL)-6, correlate with individual components of the metabolic syndrome and confer cardiovascular and metabolic risk beyond that associated with the clinically defined syndrome (9–12). Furthermore, mounting evidence suggests that inflammation plays a causal role in the development of both obesity and insulin resistance (13, 14) and may provide a common link between established components of the syndrome (15).
The epidemiologic covariation of components of the metabolic syndrome implies that one or another primary etiologic process exists. To date, there has been much speculation but no consensus regarding an underlying process that gives rise to the clinical syndrome. Structural equation modeling (SEM) and a derivative methodology, confirmatory factor analysis (CFA), can test whether a single factor contributes to associations between multiple variables and whether additional variables correlate with this underlying factor. Findings from recent studies employing these approaches provide consistent evidence that four subfactors, insulin resistance, adiposity, dyslipidemia, and elevated BP, load on a single latent factor that is consistent with currently accepted definitions of the metabolic syndrome (16–18). To date, no studies have used these techniques to examine the possibility that inflammation is related to the structure of the common latent factor.
Accordingly, the purpose of the current study was to use SEM to evaluate relationships between low grade systemic inflammation, as measured by CRP and IL-6, and the metabolic syndrome factor and to examine whether inflammation is a potential common pathway linking established metabolic components to the full syndrome. In light of existing literature, the common factor was defined as a single factor unifying four subfactors: insulin resistance, dyslipidemia, adiposity, and elevated BP. Our hypothesis was that inflammation would be positively associated with the common factor and would at least partially account for relationships between insulin resistance, dyslipidemia, adiposity and elevated BP.
Methods
Participants
Data for the present study were derived from the University of Pittsburgh Adult Health and Behavior project, a registry of behavioral and biological measurements on non-Hispanic Caucasian and African American individuals (30–54 years old) recruited via mass-mail solicitation from communities of southwestern Pennsylvania, USA (principally Allegheny County). Exclusion criterion for entry into the parent study included a reported history of atherosclerotic cardiovascular disease, chronic kidney or liver disease, cancer treatment in the preceding year, neurological disorders, or psychotic illness. Other exclusions included pregnancy and the use of insulin, nitrates, glucocorticoid, antiarrhythmic, psychotropic, or prescription weight-loss medications. Informed consent was obtained in accordance with approved protocol guidelines of the University of Pittsburgh Institutional Review Board.
Additional exclusion critera for the current analyses included use of antihypertensives, oral hypoglycemics, cholesterol-lowering medications, immunosuppressants, cold medications, or anti-histamines. Of the 1007 members of the parent project who met the above criteria, IL-6 and CRP measurements were available on 723 individuals. Of these, 73 individuals with IL-6 levels greater than 10pg/ml or CRP levels greater than 10 mg/L, suggesting the presence of acute illness (e.g., colds), and 5 individuals who were missing components of the metabolic syndrome were excluded, resulting in a final sample of 645 individuals.
Metabolic and inflammatory assessments
Participants were asked to fast overnight for 8 hours and avoid exercise for 12 hours and alcohol for 24 hours before coming into the laboratory in the morning to have blood drawn. At this visit, a nurse completed a medical history and medication use interview, obtained measurements of height and weight for the determination of BMI (kg/m2), took two manual BP measurements after the subject had been seated for 20 minutes, obtained a measurement of waist circumference, and drew a 40 cc blood sample. Serum glucose, HDL cholesterol, and triglyceride concentrations were measured by the Heinz Nutrition Laboratory, School of Public Health, University of Pittsburgh, which has met criteria for the Centers for Disease Control and Prevention – National Heart, Lung, and Blood Institute Standardization Program since 1982. Insulin concentration was measured in duplicate with a radioimmunoassay (Code-a-count; Diagnostic Products, Inc, Los Angeles, CA).
For the assessment of inflammatory markers, blood was collected in citrated tubes, with harvested plasma frozen at −80°C until analysis in batches. IL-6 levels were determined using a high sensitivity quantitative sandwich enzyme immunoassay kit (R & D Systems) according to manufacturer’s directions. The assay standard range is from 0.156 to 10 pg/mL. IL-6 levels were extrapolated from a standard curve with linear regression from a log-linear curve. All samples were run in duplicate and the average coefficient of variation (CV) between samples was 5%. Reciprocal transformation was applied to normalize raw score distributions of the IL-6 values. For ease of interpretation, the signs of correlations involving reciprocally transformed measurements of IL-6 were reversed.
CRP was measured at the University of Vermont’s Laboratory of Clinical Biochemistry Research with the BNII nephelometer from Dade Behring utilizing a particle enhanced immunonephelometric assay. In this procedure, polystyrene particles are coated with monoclonal antibodies to CRP, which, in the presence of antigen agglutinate cause an increase in the intensity of scattered light. The increase in scattered light is proportional to the amount of CRP in the sample. The assay range is 0.175–1100 mg/L. Intra-assay CVs range from 2.3–4.4% and inter-assay CVs range from 2.1–5.7%. Final CRP values were log normal (base e) transformed to better approximate a normal distribution.
Data analysis
Structural Equation Modeling was conducted based on Bentler and Weeks’ model (19) using the EQS program (20). Tests of significance were set at .01 (two-tailed). The ratio of cases to variables was over 60:1, and the ratio of cases to parameters was over 20:1. Both were sufficient for conducting SEM. A chi-square test was used to evaluate the congruency between the hypothesized model and empirical data. In addition, 3 other model fit indices were used: comparative fit index (CFI; .95 or above indicative of good fit), average absolute standardized residuals ( .05 or less indicative of good fit), and root mean square error of approximation (RMSEA; .05 or less indicative of good fit)(20).
We used SEM to examine the association of inflammation with the metabolic syndrome, with age, sex and race statistically adjusted in each analysis. The first step was a confirmatory factor analysis based on our prior model (16,17) to confirm the factor analytic structure of the metabolic syndrome in this sample (metabolic syndrome CFA model). Specifically, fasting insulin and glucose, BMI, waist circumference, fasting HDL and triglycerides, SBP and DBP, were arranged to load on four component factors, “insulin resistance”, “adiposity”, “dyslipidemia”, and “elevated BP”. The model included a higher (second-) order latent factor, hypothesized to underlie common variability among the four component factors consistent with the conceptualization of the metabolic syndrome.
For the next model, we created an inflammation factor, defined by IL-6 and CRP, and examined the strength of association between this factor and the second-order factor reflecting the metabolic syndrome (association model). We also examined whether inflammation accounted for some of the correlation between individual components of the metabolic syndrome (partial mediation model). Here, we allowed the insulin resistance, adiposity, dyslipidemia and BP subfactors to load on the higher-order factor and, independently, to be correlated with the inflammation factor. We then examined whether the components of the metabolic syndrome continued to load on the common factor once their association with inflammation was taken into account.
Finally, we examined whether the inflammation factor alone could account for the covariation among insulin resistance, adiposity, dyslipidemia and BP by fitting a model in which these factors were correlated with the inflammation factor but not permitted to load on an underlying second-order factor (full mediation model). A significant decrement in model fit in this nested model relative to the partial mediation model above would indicate that the partial mediation model is a better fit to the data than the full mediation model.
Results
Sample characteristics
Demographic characteristics of the sample and descriptive statistics of the metabolic and inflammatory variables are displayed in Table 1. Table 2 displays Pearson correlations between each pair of variables included in the analyses. As expected, high correlations were observed between clusters of factors proposed to load on the metabolic syndrome and between metabolic factors and inflammatory markers (all p’s < .01).
Table 1.
Descriptive statistics of participants’ demographic and biomedical characteristics
| Mean or % | SD | |
|---|---|---|
| Age | 44.65 | 6.55 |
| Sex (male/female) | 48% / 52% | |
| Race (European-Americans/African Americans) | 82% / 18% | |
| Education (years) | 15.60 | 2.74 |
| Current smokers | 15.8% | |
| Insulin (uU/ml) | 12.79 | 6.53 |
| Glucose (mg/dl) | 95.81 | 16.68 |
| Body mass index (kg/m2) | 27.16 | 5.38 |
| Waist circumference (inches) | 35.85 | 5.87 |
| High density lipoprotein cholesterol (mg/dl) | 54.05 | 14.19 |
| Triglycerides (mg/dl) | 120.55 | 86.93 |
| Systolic blood pressure (mmHg) | 116.35 | 13.27 |
| Diastolic blood pressure (mmHg) | 78.50 | 9.43 |
| Interleukin-6 (pg/ml) | 1.79 | 1.68 |
| C-reactive protein (mg/L) | 1.65 | 1.79 |
Table 2.
Intercorrelations of components of the metabolic syndrome and markers of inflammation
| SBP | DBP | Insulin | Glucose | BMI | Waist | HDL | TRI | IL-6 | |
|---|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure (SBP) | |||||||||
| Diastolic blood pressure (DBP) | .77** | ||||||||
| Insulin # | .28** | .33** | |||||||
| Glucose # | .16** | .17** | .28** | ||||||
| Body mass index (BMI) | .36** | .36** | .51** | .21** | |||||
| Waist | .40** | .45** | .54** | .32** | .85** | ||||
| High density lipoprotein cholesterol |
−.18** | −.20** | −.35** | −.21** | −.34** | −.46** | |||
| Triglycerides (TRI) # | .31** | .30** | .39** | .31** | .28** | .41** | −.46** | ||
| Interleukin-6 (IL-6) ## | .19** | .21** | .17** | .15** | .29** | .31** | −.20** | .12* | |
| C-reactive protein (CRP) # | .19** | .17** | .34** | .13* | .48** | .41** | −.18** | .21** | .33*** |
p < .01,
p < .001
Transformed with a natural log function,
Reciprocally transformed with the sign of the correlation reversed for ease of interpretation
Confirmatory factor analysis of metabolic syndrome
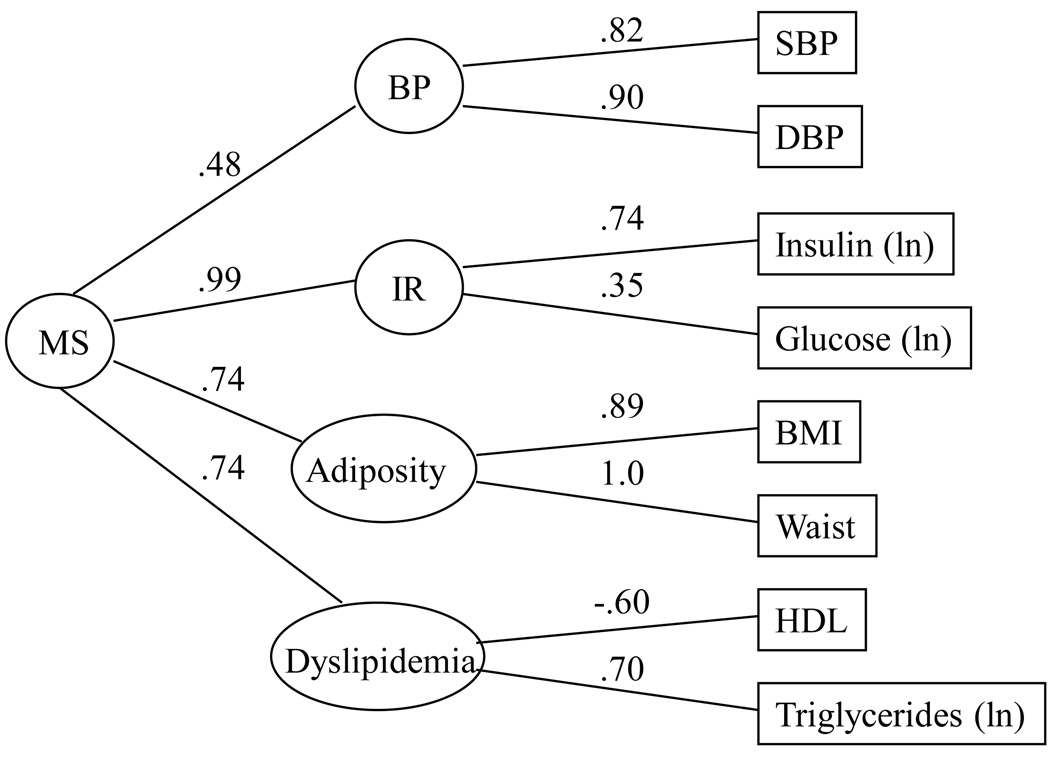
In the metabolic syndrome CFA model, we replicated in this sample our prior confirmatory factor analytic model of the metabolic syndrome (16, 17) (Figure 1). Overall, the model provided a good fit to the data. Although the statistically significant chi-square test (χ2 = 57.51, df = 12, N = 645, p < 0.01) indicated some difference between the estimated and observed variance-covariance matrices; this was likely due to the large sample size. The CFI of the model was .98, with the average absolute standardized residual = 0.02 and RMSEA = 0.08. These findings suggest that the factor structure proposed fit the data reasonably well. Consistent with previous work, the measured variables tended to load strongly on their respective factors, with the potential exception of the loading of glucose on the insulin resistance factor. Each of the subfactors loaded significantly on the second-order factor. As seen in prior studies, the least strongly loading factor was elevated BP (16). Overall the results indicate that the pattern of covariation among measured components of the metabolic syndrome is consistent with a model that includes four subfactors, reflecting “insulin resistance”, “dyslipidemia”, “adiposity” and “elevated BP”, which, in turn, load on a single, common, second-order factor, which we hypothesize to reflect the metabolic syndrome.
Figure 1.
Confirmatory factor analysis model: Factor structure of the metabolic syndrome with age, sex and race covaried. C2 = 57.51, df = 12, N = 645; CFI = .98, average absolute standardized residuals = .02, RMSEA = .08.
Association of inflammation with the metabolic syndrome
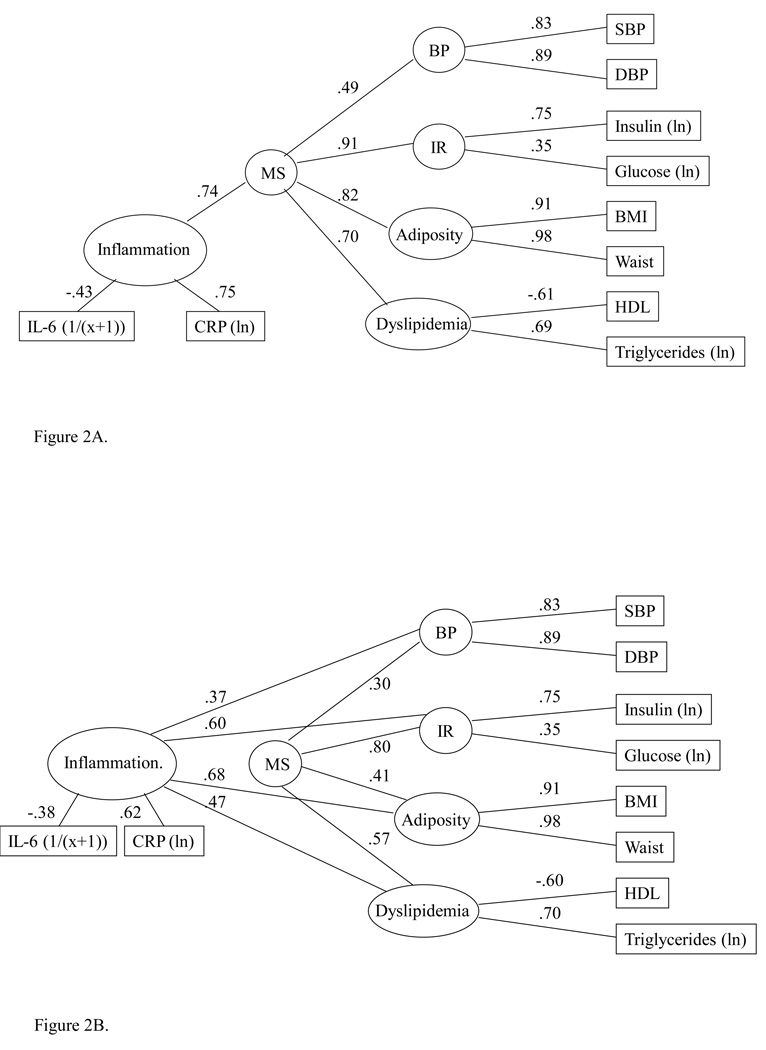
In the next model, we examined the association between an inflammation factor and the common, second-order factor (Figure 2A). Again, the model fit indices suggest that the factor structure proposed fit the data reasonably well, with CFI, average absolute standardized residual and RMSEA within the acceptable range. IL-6 and CRP showed a moderate to strong association with the inflammation factor. The path coefficient for the association between inflammation and the common factor was substantial, suggesting that the inflammation factor accounted for over half of the variance (r2 = (0.74)2, or 0.54) in the common, second-order factor underlying components of the metabolic syndrome. Secondary analyses showed that the association of the inflammatory factor with the common factor was similar in men and women (data not shown).
Figure 2.
A. Association model: Assocation between circulating inflammatory markers and metabolic syndrome with age, sex and race covaried. C2 = 97.44, df = 25, N = 645; CFI = .97, average absolute standardized residuals = .03, RMSEA = .07. B. Partial mediation model: Circulating inflammatory markers and metabolic syndrome with age, sex and race covaried. C2 = 79.93, df = 22, N = 645; CFI = .98, average absolute standardized residuals = .02, RMSEA = .06. From previous model: DC2 = 17.51, df = 3, p = .0006.
Mediation models
Given the strong association between the inflammation factor and the common factor, we next sought to determine whether inflammation could account for some portion of the association between components of the metabolic syndrome. The partial mediation model (Figure 2B), in which the subfactors of the metabolic syndrome were permitted to both correlate with the inflammation factor and to load on the common factor, provided good fit to the data. Within the model, the inflammation factor was associated significantly with each of the component factors of the metabolic syndrome (path co-efficients 0.37 – 0.68, r2s = 0.14 – 0.46), with the strongest association for the adiposity factor. Each of the component factors also continued to load significantly on the common, second-order factor, although the strength of these associations was now generally diminished. Relative to the metabolic syndrome CFA model (Figure 1), the most substantial change in loading on the common factor was for the adiposity factor, which dropped from a factor loading of 0.74 to 0.41. For BP, insulin resistance and dyslipidemia, the factor loadings dropped from 0.48 to 0.30, 0.99 to 0.80 and 0.74 to 0.57, respectively. These results suggest that inflammation does account for some of the association between insulin resistance, dyslipidemia, adiposity and elevated BP, suggesting that inflammation may be one factor accounting for correlation among metabolic syndrome components.
Finally, we examined a full mediation model, in which the inflammation factor accounted for all covariation among components of the metabolic syndrome. Relative to the partial mediation model, this nested model caused a significant decrement in model fit (Δχ2 (5) = 37.21, p < 0.0001), indicating that the full mediation model provides a less adequate fit to the data than the partial mediation model. This confirms that, although the inflammation factor does appear to contribute to associations between components of the metabolic syndrome, it does not solely account for these relationships.
Discussion
A consistent body of evidence shows (i) that the metabolic syndrome is a clinical phenomenon, (ii) that its component risk factors of insulin resistance, adiposity, dyslipidemia, and elevated BP aggregate within populations, and (iii) that it confers cardiovascular risk beyond that associated with these component risk factors (4). In regard to the clustering of traditional metabolic risk factors, our findings lend support to the established hierarchical four-factor structure of the metabolic syndrome (16–18). Consistent with this literature, we found a higher-order common factor underlying the covariation of insulin resistance, adiposity, dyslipidemia, and BP, with strong associations between the common factor and insulin resistance, adiposity, and dyslipidemia, and a more moderate contribution of BP. This model supports the currently accepted clinical definitions of the metabolic syndrome (1, 5–7) and provides further support for common processes that unite these core risk factors.
Both the epidemiologic aggregation of risk factors and the findings of structural equation modeling suggest the existence of an underlying, common etiologic process. Identification of this unidentified factor would both improve our understanding of the pathophysiology of the metabolic syndrome and also likely improve our ability to predict disease (9). One candidate factor is low grade systemic inflammation, itself a known participant in the development of obesity, insulin resistance and atherosclerosis. The current findings show that two markers of systemic inflammation, IL-6 and CRP, load on a single factor that is associated significantly with the factor analytically derived index of metabolic syndrome, accounting for 54% of its variance among 645 relatively-healthy, mid-life men and women. Indeed, the association of the common factor with inflammation was stronger than its associations with lipid levels or BP, which accounted for 49% and 24% of the variance, respectively. These findings are consistent with prior studies that show an association between inflammatory markers and both individual components and clinical definitions of the metabolic syndrome (9, 21–23). Taken together, these findings raise the possibility that systemic inflammation is a shared etiologic process that underlies the co-expression of insulin resistance, central adiposity, elevated BP and dyslipidemia. Additionally, the relationships between the metabolic syndrome and increased vulnerability to CVD and type 2 diabetes could be mediated, in part, through inflammatory pathways.
To date, the mechanisms linking components underlying the metabolic syndrome remain unclear and other investigators have suggested that inflammation plays a primary pathogenic role (24). To explore this possibility, we examined whether associations between core metabolic components and the common factor were diminished if inflammation was statistically controlled. When component factors of the metabolic syndrome were permitted to both correlate with the inflammation factor and to load on the common, latent factor, significant associations were observed between inflammation and all metabolic components, with the strongest relationships for insulin resistance and adiposity. In addition, the strength of all relationships between metabolic components and the common, latent factor diminished with the inflammatory factor in the model, suggesting partial mediation of the correlation of components of the metabolic syndrome by low-grade inflammation. This was particularly notable for the adiposity factor, which accounted for 55% of the common factor when the inflammatory factor was not included and only 17% of the variance when the inflammation factor was included. These findings are generally consistent with the results of a recent principal components factor analysis, which showed that BMI, waist circumference, insulin sensitivity, fibrinogen, and CRP loaded on a single factor among 1,087 non-diabetic mid-life adults (25). Although the current results suggest that inflammation accounts for some of the associations between the metabolic syndrome component risk factors and the common latent factor, the data best fit a partial, rather than full, mediation model, suggesting that inflammation only partially accounts for the correlation among components of the metabolic syndrome and that additional etiologic factors also contribute to the syndrome.
Complex interrelationships exist between inflammation, adiposity, insulin resistance, and the metabolic syndrome, making it difficult to delineate clear causal pathways. However, it is widely suggested that adiposity and insulin resistance drive the clustering of metabolic risk factors (25, 26). Indeed, recent evidence shows that a moderate decrease in weight (8 kg) results in significant reductions in the prevalence of the metabolic syndrome among the obese (27). Obesity is considered a proinflammatory state, with adipocytes producing 10–35% of circulating IL-6 (28, 29) and mononuclear cells of the obese being primed to express proinflammatory cytokines (30). Proinflammatory cytokines, in particular IL-6, stimulate the peripheral production of CRP by hepatocytes (31). Thus, adiposity results in increased systemic levels of both IL-6 and CRP.
Animal models show that inflammation plays a primary pathogenic role in the development of obesity-induced insulin resistance by downregulating components of the insulin signaling cascade, and by inducing suppressors of cytokine signaling (SOCS) known to interfere with insulin signal transduction (32). Consistent with these models, recent human evidence shows that acute adipose inflammation, induced by the experimental administration of low-dose endotoxins, results in the expression of SOCS proteins in adipose tissue and the subsequent induction of systemic insulin resistance (33). Furthermore, the expression of SOCS-3 proteins is increased among the obese and is inversely related to insulin signal transduction and insulin resistance (34). Taken together, these findings suggest that inflammatory processes may result in systemic resistance to insulin.
Independently of obesity, recent evidence suggests that diet may also induce inflammatory processes that contribute to insulin resistance. Ghanim and colleagues (35) randomly assigned lean individuals to receive either a 910 calorie meal high in fat and carbohydrates or an isocaloric meal rich in fruit and fiber. Over the 3 hours following the meal, only the individuals who received the high fat, high carbohydrate meal showed increases in plasma endotoxin concentrations, the expression of toll-like receptors (TLR) -2 and -4 and suppressor of cytokine signaling-3 (SOCS-3) proteins, the generation of reactive oxygen species, and increased nuclear factor (NF)- kappa B binding activity. These findings are consistent with animal models examining the impact of high fat diets (32, 36) and support an up-regulation of inflammatory processes that are known to interfere with insulin signal transduction, including endotoxemia and the expression of SOCS-3, TLR-2, and TLR-4. In contrast, the high fruit and fiber diet was not associated with activation of these inflammatory processes. This is consistent with prospective epidemiologic evidence that diets high in fiber are associated with decreased risk for incident Type 2 diabetes in older men, an association that is partly explained by lower circulating levels of CRP and IL-6 (37). Thus, converging evidence supports the possibility that inflammation plays a primary pathogenic role in the development of insulin resistance and the metabolic syndrome (38).
In addition to diet and obesity, other environmental factors and genetics contribute to systemic inflammation. For example, systemic levels of CRP have been shown to be highly heritable and to share genetic determinants with multiple components of the metabolic syndrome, including BMI, insulin, insulin resistance, BP, and triglycerides (39). Furthermore, a growing literature shows that psychosocial characteristics (e.g., lower socioeconomic status) are associated with increased circulating levels of IL-6 and CRP and with vulnerability to the metabolic syndrome (40, 41). Thus, it is likely that multiple factors contribute to the systemic inflammation that is associated with adiposity, insulin resistance, and the metabolic syndrome.
The current findings have potential implications for the early detection and treatment of systemic inflammation. Evidence that overeating and adiposity contribute to systemic inflammation and development of the metabolic syndrome raises the possibility that lifestyle interventions may provide effective means of reducing risk. In this regard, converging evidence shows that weight reduction and exercise interventions are associated with decreases in markers of systemic inflammation, including IL-6 and CRP (42). Furthermore, lifestyle interventions decreased risk of developing type 2 diabetes in a high-risk, prediabetic population (43). Future longitudinal studies can explore whether the decrease in risk associated with lifestyle changes is mediated by decreases in systemic inflammation.
The present findings should be interpreted in the context of a number of limitations. As noted above, our findings are cross-sectional, which precludes any causal interpretations of relationships between factors that contribute to cumulative risk. Another limitation of the study is the single assessment of risk factors, including the inflammatory markers. Although evidence suggests that both IL-6 and CRP are relatively stable over extended periods (44), a more reliable indicator of chronic interindividual variability would be derived from multiple assessments over time. In addition, our study was limited to European- and African-Americans. Finally, all of the measured variables tended to load strongly on their respective factors, with the potential exception of the loading of glucose on the insulin resistance factor. Future studies may benefit from the inclusion of a more direct measure of insulin resistance.
Despite these shortcomings, our findings show a strong association between peripheral markers of systemic inflammation, IL-6 and CRP and the metabolic syndrome, providing support for the hypothesis that subclinical systemic inflammation comprises a component of the syndrome (10, 45). The current findings also show that inflammation partially mediates the statistical aggregation of the component factors (insulin resistance, central adiposity, dyslipidemia, BP) comprising the metabolic syndrome. The results were particularly strong regarding adiposity, raising the possibility that inflammatory mediators derived from adipose tissue contribute to metabolic risk. Given the relative ease of measuring peripheral markers of systemic inflammation, such as CRP, the inclusion of inflammatory markers in the clinical definition of metabolic syndrome seems warranted and may be tested for its ability to improve prognostic assessment of risk of type 2 diabetes and CVD.
Acknowledgements
The expert technical assistance of Ramasri Saathanoori, MS and Cyndi Kravitz is gratefully acknowledged.
Financial support: This study was supported by grants P01HL40962 from the National Heart Lung and Blood Institute (SBM), and NR008237 from the National Institute of Nursing Research (ALM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement: No competing financial interests or other conflicts of interest exist.
Institutional Approval: Informed consent was obtained in accordance with approved protocol guidelines of the University of Pittsburgh Institutional Review Board.
References
- 1.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. 2005. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47:1093–1100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt MI, Watson RL, Duncan BB, et al. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Atherosclerosis Risk in Communities Study Investigators. Metabolism. 1996;45:699–706. doi: 10.1016/s0026-0495(96)90134-1. [DOI] [PubMed] [Google Scholar]
- 4.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004;24:e13–e18. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Definition, diagnosis and classification of Diabetes Mellitus and its complications: report of a WHO consultation. Geneva, Switzerland: World Health Organization; Part 1: diagnosis and classification of diabetes mellitus. 1999:1–65.
- 7.National Cholesterol Education Program Expert Panel on Detection. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 8.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Brown NJ, Vaughan DE, et al. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109:IV6–IV19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14,719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 11.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 12.Yudkin JS, Stehouwer CD, Emeis JJ, et al. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 13.Han TS, Sattar N, Williams K, et al. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25:2016–2021. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]
- 14.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Current Diabetes Reports. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 15.Rana JS, Nieuwdorp M, Jukema JW, et al. Cardiovascular metabolic syndrome - an interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes Metab. 2007;9:218–232. doi: 10.1111/j.1463-1326.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 16.McCaffery JM, Shen BJ, Muldoon MF, et al. Ambulatory blood pressure and the metabolic syndrome in normotensive and untreated hypertensive men. Metab Syndr Relat Disord. 2007;5:34–44. doi: 10.1089/met.2006.0014. [DOI] [PubMed] [Google Scholar]
- 17.Shen BJ, Todaro JF, Niaura R, et al. Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. Am J Epidemiol. 2003;157:701–711. doi: 10.1093/aje/kwg045. [DOI] [PubMed] [Google Scholar]
- 18.Shen BJ, Goldberg RB, Llabre MM, et al. Is the factor structure of the metabolic syndrome comparable between men and women and across three ethnic groups: the Miami Community Health Study. Ann Epidemiol. 2006;16:131–137. doi: 10.1016/j.annepidem.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 19.Bentler PM, Weeks DG. Linear structural equations with latent variables. Psychometrika. 1980;45:289–308. [Google Scholar]
- 20.Bentler PM. EQS structural equations program manual. Encino, CA: Multivariate Software; 1995. [Google Scholar]
- 21.Piche ME, Lemieux S, Weisnagel SJ, et al. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–97. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 22.Marques-Vidal P, Mazoyer E, Bongard V, et al. Prevalence of insulin resistance syndrome in southwestern France and its relationship with inflammatory and hemostatic markers. Diabetes Care. 2002;25:1371–1377. doi: 10.2337/diacare.25.8.1371. [DOI] [PubMed] [Google Scholar]
- 23.Wannamethee SG, Lowe GD, Shaper AG, et al. The metabolic syndrome and insulin resistance: relationship to haemostatic and inflammatory markers in older non-diabetic men. Atherosclerosis. 2005;181:101–108. doi: 10.1016/j.atherosclerosis.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 25.Hanley AJ, Festa A, D'Agostino RB, Jr, et al. Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes. 2004;53:1773–1781. doi: 10.2337/diabetes.53.7.1773. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32 Suppl 3:24–34. doi: 10.1046/j.1365-2362.32.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 27.Phelan S, Wadden TA, Berkowitz RI, et al. Impact of weight loss on the metabolic syndrome. Int J Obes. 2007;31:1442–1448. doi: 10.1038/sj.ijo.0803606. [DOI] [PubMed] [Google Scholar]
- 28.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta NN, McGillicuddy FC, Anderson PD, et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghanim h, Alijada A, Daoud N, et al. role of inflammatory markers in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia. 2007;50:278–285. doi: 10.1007/s00125-006-0508-9. [DOI] [PubMed] [Google Scholar]
- 35.Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of Toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in dient-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 37.Wannamethee SG, Whincup PH, Thomas MC, Sattar N. Assocaitions between dietary fiber and inflammation, hepatic function, and risk of type 2 diabetes in older men. Diabetes Care. 2009;32:1823–1825. doi: 10.2337/dc09-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends in Immunology. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Wessel J, Moratorio G, Rao F, et al. C-reactive protein, an 'intermediate phenotype' for inflammation: human twin studies reveal heritability, association with blood pressure and the metabolic syndrome, and the influence of common polymorphism at catecholaminergic/beta-adrenergic pathway loci. J Hypertens. 2007;25:329–343. doi: 10.1097/HJH.0b013e328011753e. [DOI] [PubMed] [Google Scholar]
- 40.Rathmann W, Haastert B, Giani G, et al. Is inflammation a causal chain between low socioeconomic status and Type II diabetes? Results from the KORA survey 2000. Eur J Epidemiol. 2006;21:55–60. doi: 10.1007/s10654-005-5085-6. [DOI] [PubMed] [Google Scholar]
- 41.Loucks EB, Magnusson KT, Cook S, et al. Socioeconomic position and the metabolic syndrome in early, middle, and late life: evidence from NHANES 1999–2002. Ann Epidemiol. 2007;17:782–790. doi: 10.1016/j.annepidem.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 43.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao KM, Pieper CS, Currie MS, et al. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol. 1994;102:802–805. doi: 10.1093/ajcp/102.6.802. [DOI] [PubMed] [Google Scholar]
- 45.Festa A, D'Agostino R, Jr, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]