Abstract
Liver disease is responsible for more than 42,000 deaths yearly. Elevated hepatic iron levels have been shown to play a role in chronic liver diseases including hereditary hemochromatosis, thalassemia, and chronic hepatitis C, whereas acetaminophen (APAP) is the leading cause of acute liver failure. The goal of this study was to determine whether increased hepatic iron affects APAP-induced cytotoxicity, reactive oxygen species (ROS) production, and/or mitochondrial dysfunction in primary mouse hepatocytes (PMHs) that are differentiated and have gap junctional intracellular integrity, properties associated with hepatocytes in vivo and important for conducting toxicant studies. Treatment of PMHs with the iron donor 3,5,5-trimethyl-hexanoyl ferrocene (TMHF) caused an elevation in ferritin, reduction in transferrin receptor 1, and accumulation of hemosiderin, but TMHF treatment alone did not induce ROS or cause mitochondrial dysfunction. The threshold APAP dose that induced PMH cell death after TMHF treatment of PMHs was lower than in the absence of TMHF. In addition, treatment with the iron chelator deferoxamine (DFO) protected from APAP and resulted in a higher threshold dose being needed to induce cell death. We also showed that after TMHF treatment, APAP induced ROS and mitochondrial dysfunction at earlier time points than treatment with APAP alone; treatment with DFO increased the length of time required for APAP to induce ROS and mitochondrial dysfunction; and treatment with DFO, subsequent to TMHF, partially protected against TMHF-potentiated APAP injury. We conclude that iron potentiates the effects of APAP on cytotoxicity, ROS production, and mitochondrial dysfunction in PMHs.
Keywords: iron, acetaminophen, hepatocyte, cell death, reactive oxygen species, mitochondrial injury
Acetaminophen (APAP) is a safe analgesic at therapeutic doses, but an overdose can cause severe centrilobular hepatic necrosis in experimental animals and humans. Although extensively studied, the mechanism of APAP-induced liver injury has not been completely elucidated. It is widely accepted that APAP toxicity is initiated by cytochrome P450–mediated metabolism of APAP to produce the reactive metabolite, N-acetyl-p-benzoquinoneimine, which in turn depletes cellular glutathione (GSH). When the GSH is exhausted, N-acetyl-p-benzoquinoneimine covalently binds to cellular proteins including a number of mitochondrial proteins. Previously, covalent binding of APAP to cellular macromolecules was considered as a major cause of liver necrosis. More recent data suggest that reactive oxygen species (ROS) and mitochondrial permeability transition (MPT) play a role in APAP-induced hepatotoxicity.
In this study, we investigated the role of iron in APAP-induced hepatotoxicity in cultured primary mouse hepatocytes (PMHs). Previous in vitro studies examining oxidative stress and MPT associated with APAP toxicity have been carried out using PMHs either freshly isolated or cultured for short periods of time (Bajt et al., 2004; Reid et al., 2005). Hepatocytes in short-term culture are either recovering from cellular isolation procedures or in the process of dedifferentiating. Importantly, gap junctional intercellular communication (GJIC) is not present in short-term hepatocyte culture (Ruch and Klaunig, 1988). Thus, a long-term PMH culture system in which properties associated with hepatocytes in vivo is needed to conduct toxicant studies without confounding results because of dedifferentiation.
We previously reported a method for long-term culture of PMHs (Stoehr and Isom, 2003). This long-term culture system is advantageous for several reasons. First, the hepatocytes remain viable and differentiated for 30 days. Second, cellular morphology is easily observed, as the cells are not in a matrix or a gel. Third, the cells demonstrate increased paracellular junction integrity and decreased membrane permeability over time in culture. Indeed, the susceptibility of PMHs to a toxicant may be influenced by the increased membrane permeability that is observed in short-term mouse hepatocytes, a phenomenon that likely results from the collagenase-based cell isolation procedure. Fourth, PMHs in long-term culture acquire GJIC over time in culture. GJIC has been shown to be crucial for several integral cellular processes essential for normal cellular function, including differentiation and growth control (El-Fouly et al., 1987; Kojima et al., 1996). Previous reports have shown that disruption of hepatocellular gap junctions is associated with defective glycogen mobilization and neoplastic transformation in vivo (Feder et al., 1998; Nelles et al., 1996; Temme et al., 1997). An in vitro cellular system for studying APAP toxicity needs to be one in which GJIC is maintained.
To study the role of iron in APAP-induced hepatotoxicity, it is also necessary to have a cell system in which iron overload can be generated. Using primary rat hepatocytes in long-term culture, we evaluated various iron donors for in vitro studies and reported that treating hepatocytes with 3,5,5-trimethyl-hexanoyl ferrocene (TMHF) most accurately simulated the morphological features of iron-loaded hepatocytes in patients in vivo (Cable et al., 1998). The bioavailability of iron from TMHF is twice as high as from ferrocene and six times higher than from ferrous sulfate or 1,1′-bis (3,5,5-trimethylhexanoyl)ferrocene (Nielsen and Heinrich, 1993). We report here that PMHs in long-term culture can be iron loaded using TMHF as an iron donor.
Elevated hepatic iron levels have been shown to be directly or indirectly involved in liver injury in multiple different chronic liver diseases including hereditary hemochromatosis, thalassemia, and chronic hepatitis C (Isom et al., 2009). ROS can be produced from both endogenous and exogenous sources. One exogenous source is iron. A well-studied physiological biochemical iron redox reaction is the Fenton reaction. In this reaction, ferrous (Fe2+) iron reacts with hydrogen peroxide (H2O2) to produce ferric (Fe3+) iron and highly reactive hydroxyl radicals. The Fenton reaction is of particular importance in the liver, which has high steady-state production of O2 and H2O2 from abundant mitochondrial activity (Eaton and Qian, 2002). In addition to reacting with H2O2, ferrous iron may react with O2 to produce ferric iron and a superoxide radical. The generated radicals can then nonselectively attack proteins, nucleic acids, polysaccharides, and lipids.
Because elevated hepatic iron levels have been shown to be involved in liver injury in chronic liver diseases, we wanted to examine the effect of elevated iron in hepatocytes on acute APAP hepatotoxicity. We selected PMHs in long-term culture as the in vitro model system, TMHF as the iron donor, and focused on effects on ROS and mitochondrial function.
MATERIALS AND METHODS
Mouse hepatocyte isolation.
Male C57BL/6 (8–12 weeks of age) were purchased from Jackson Laboratory (Bar Harbor, ME). All animals used for perfusion received care in accordance with the guidelines for the Care and Use of Laboratory of Animals as adapted by the National Institutes of Health. Primary hepatocytes were isolated from mice by collagenase perfusion as previously described (Stoehr and Isom, 2003). Cell viability was approximately 90%, as determined by trypan blue exclusion test. Cells were plated on collagen I–coated 60-mm dishes (106 cells per dish; BD Sciences, Bedford, MA) and fed Dulbecco's medium (Invitrogen, Eugene, OR) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 1% L-glutamine and refed the same medium at 4 h after plating. At 24 h after plating, cells were fed hepatocyte maintenance medium (HMM; Lonza, Basel, Switzerland) supplemented with 2.25% dimethyl sulfoxide and 25 ng/ml epidermal growth factor (Sigma, St Louis, MO). Cells were fed every 2 or 3 days and remained viable for up to 30 days.
Cell viability assay.
To determine viability of cells, a two-color assay that determines cell viability on the basis of cell membrane integrity and intracellular esterase activity was used. Membrane-permeable calcein cleaved by esterases in live cells is retained in the cells, emitting green fluorescence, whereas membrane-impermeable ethidium homodimer-1 labels nucleic acids in membrane-damaged cells emitting red fluorescence. Experimental hepatocytes incubated for 14 days in HMM were treated with APAP (0, 5, 10, 20, 30, or 50mM). At 20 h following treatment, cells were washed with PBS twice to remove excess APAP and were treated with 1.5μM calcein acetoxymethyl (calcein AM) and 2μM ethidium homodimer (Invitrogen) mix. At 30 min of incubation, the cultures were washed and visualized using fluorescence microscopy. For the time course study, 30mM APAP was added to 14-day-old hepatocytes for 2, 4, 6, 8, 10, and 12 h. Then, cells were treated as described above and evaluated by microscopy to determine cell viability.
GSH detection.
Aliquots of cells (∼4 × 105) were washed in PBS and lysed in 200 ml of 20% (wt/vol) metaphosphoric acid, and precipitated proteins were removed by centrifugation at 14,000 × g for 3 min. Free GSH in cellular extracts was determined using the previously described enzymatic recycling method using Ellman's reagent (Richie et al., 1996).
ROS detection assay.
The production of ROS was determined using 2′7′-dichlorodihydrofluorescein diacetate (H2DCF-DA; Invitrogen) dye. Removal of the acetate groups by intracellular esterases results in retention of the H2DCF-DA in the cells. Oxidation by ROS converts nonfluorescent H2DCF-DA to the impermeable fluorescent form of H2DCF-DA. PMHs were incubated in 30mM APAP for various time points and washed with PBS at each time point. Freshly prepared dye in PBS was added to the cells for 30 min. Following incubation, the cells were washed and observed under the fluorescence microscope.
Mitochondrial membrane potential assay.
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanine iodide (JC-1; Invitrogen), a cationic dye exhibiting potential-dependent accumulation in mitochondria, was used to determine changes in the relative mitochondrial membrane potential (MMP) following APAP treatment. In mitochondria with a low membrane potential, JC-1 exists as a monomer and emits green fluorescence. In mitochondria with a high membrane potential, JC-1 exists as an aggregate and emits red fluorescence. Briefly, APAP (30mM)–treated PMHs were washed in PBS at 2, 4, 6, 8, 12, and 24 h following treatment. Cells were then treated with 6.5μM JC-1 dye and incubated for 25 min. Following incubation, cells were washed with PBS twice and evaluated by fluorescence microscope.
MPT assay.
MPT is an abrupt increase in permeability of the mitochondrial inner membrane to small molecular weight solutes and ions. It leads to altered mitochondrial potential (Δψ), uncoupling of oxidative phosphorylation, and mitochondrial swelling, eventually leading to cell death. MPT is stimulated by various compounds and conditions, and prompted by accumulation of excessive Ca2+. MPT can be determined using tetramethylrhodamine methyl ester (TMRM; Invitrogen), a fluorescent cationic dye accumulating in mitochondria in response to their negative membrane potential. TMRM-labeled mitochondria emit red fluorescence, whereas calcein AM labels the cytosol green. Briefly, cells were washed with PBS to remove excess APAP and then incubated in 500nM TMRM for 15 min, followed by TMRM plus 1μM of calcein AM for 15 min (Nieminen et al., 1997). Cells were rinsed in PBS twice and placed in fresh media. Fluorescence microscopy was used to evaluate MPT.
Perls’ Prussian blue stain.
Cells were fixed in ethanol:acetic acid (99:1) solution for 20 min at −20°C, washed, and fixed with 100% EtOH for 20 min at −20°C. Fixed cells were stored in 100% ethanol at 4°C. Cells were then rinsed twice in PBS, treated with 2% neutral-buffered formalin for 15 min, dehydrated in ethanol, and rehydrated in dH2O. Cells were incubated in 10% potassium ferrocyanide : 20% HCl (1:1) for 15 min and then washed in water. Nuclear fast red (Sigma) was added to the cells for 5 min as a counterstain and then cells were rinsed with water. Coverslips were mounted on the culture dish and cells photographed by light microscopy.
Western blot analysis.
Cells were lysed in radioimmunoprecipitation assay buffer and centrifuged at 6800 × g for 10 min at 4°C. Supernatants were used for protein quantitation as determined by the bicinchoninic acid assay. Twenty-microgram protein samples were subjected to SDS polyacrylamide gel electrophoresis. Blots were blocked in 5% milk in Tris-buffered saline-Tween for 1 h at room temperature and then incubated in primary antibody against transferrin receptor 1 (TfR1; Alpha Diagnostics International, San Antonio, TX) or ferritin (DAKO, Carpinteria, CA). Following incubation with primary antibody, blots were incubated with a horseradish peroxidase–conjugated secondary antibody. Bound antibodies were visualized by enhanced chemiluminescence (PerkinElmer, Shelton, CT).
RESULTS
APAP Cytotoxicity in PMHs in Long-term Culture
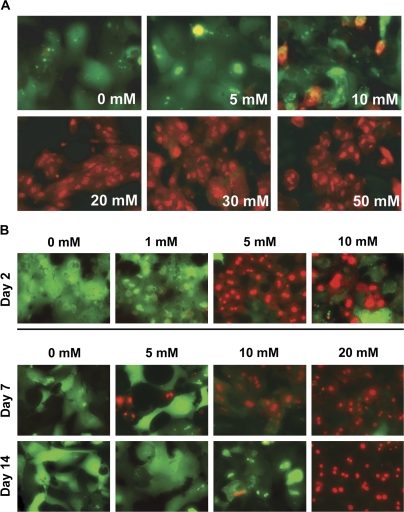
Primary hepatocytes isolated from wild-type C57BL/6 mice were cultured in HMM for 14 days. At this time in culture, the hepatocytes have intact membranes and demonstrate GJIC (Stoehr and Isom, 2003). Cells were exposed to media alone or to 5, 10, 20, 30, or 50mM APAP for 20 h. After washing with PBS, cells were subsequently incubated with two components, 1.5μM calcein AM and 2μM ethidium homodimer for 30 min. Calcein AM (green) is retained in live cells, whereas ethidium homodimer (red) is not able to enter intact cell membranes. In contrast, the damaged membranes of dead cells allow the ethidium homodimer to enter cells and intercalate with DNA. Live cells fluoresce green, whereas dead cells fluoresce red. The hepatocytes exhibited substantial toxicity at 20 h when treated with 20mM and higher (30 or 50mM) APAP (Fig. 1A), whereas cells incubated with 5 or 10mM APAP showed no or minimal cell damage at 20 h following APAP treatment.
FIG. 1.
Concentration curve of APAP-induced cytotoxicity in long-term cultured PMHs. (A) Cytotoxicity of APAP in PMHs in culture for 14 days. PMHs in culture incubated for 14 days were treated with media alone or with 0, 5, 10, 20, 30, or 50mM APAP. At 20 h following APAP treatment, cells were washed to remove excess APAP and incubated with 1.5μM calcein AM (green) and 2μM ethidium homodimer (red) for 30 min. Cells were subsequently washed and visualized by fluorescence microscopy. (B) Cytotoxicity of APAP in PMHs in culture for 2, 7, or 14 days. Two-day-old PMHs were exposed to 0, 1, 5, or 10mM APAP. Higher concentrations of APAP (0, 5, 10, or 20mM) were used for 7- or 14-day-old hepatocytes. At 20 h after APAP treatment, cell viability was measured using two fluorescent dyes, calcein AM (1.5μM) and ethidium homodimer (2μM). At 30-min incubation with dyes, cells were washed in PBS and photographed by fluorescence microscopy. Original magnification is ×200.
In previous studies using PMHs plated for 4 h prior to exposure to APAP, to examine APAP-induced oxidative stress and cell injury, the concentration of APAP used was 5mM (Bajt et al., 2004, 2006) or 10mM (Kon et al., 2004). As shown in Figure 1A, PMHs in long-term culture for 14 days with 5 or 10mM APAP showed no or minimal cell damage. From our previous studies establishing the long-term PMH system (Stoehr and Isom, 2003), we know that paracellular junction integrity (measured by the ability of the cells to retain the fluorescein metabolic product of fluorescein diacetate) did not begin to become intact until day 5 in culture, indicating that before day 5, plasma membranes on the hepatocytes are permeable. In addition, although the gap junction proteins connexin32 and connexin26 were detectable by day 7, expression of these proteins increased between days 7 and 14 at which time the levels remained constant until day 28. Similarly GJIC was minimally detectable before day 3 and increased between days 7 and 14. To evaluate the effects of time in culture in the long-term culture system on response to APAP, PMHs were cultured for 2, 7, and 14 days at which time the cells were exposed to APAP concentrations ranging from 1 to 20mM (Fig. 1B). Hepatocytes in culture for 2 days exhibited substantial toxicity when treated with 5mM APAP or higher, whereas hepatocytes in culture for 7 days exhibited some toxicity after treatment with 5mM but marked toxicity when treated 10mM APAP or higher. Hepatocytes in culture for 14 days were included as a control and the same results were seen as reported in Figure 1A.
To determine the time course of APAP-induced cell damage, mouse hepatocytes in long-term culture were exposed to 30mM APAP for 2, 4, 6, 8, 10, and 12 h (Fig. 2). At the designated times, cells were washed with PBS to remove excess APAP and cell viability was determined using the fluorescence dyes. Exposure to 30mM APAP for 2–6 h did not cause significant cell damage relative to control cells. Cell death became apparent at 8 h and reached approximately 60% by 10 h following APAP treatment. Cell death approached 100% in mouse hepatocytes in long-term culture when exposure to 30mM APAP exceeded 12 h.
FIG. 2.
Time course of APAP-induced cytotoxicity in long-term cultured PMHs. PMHs in culture incubated for 14 days were exposed to 30mM APAP or culture media for 0, 2, 4, 6, 8, 10, 12, or 24 h. After removal of the cell culture medium, cells were exposed to calcein AM and ethidium homodimer to measure cell viability.
APAP-Induced GSH Depletion in PMHs in Long-term Culture
APAP is metabolized in hepatocytes to produce the reactive metabolite, N-acetyl-p-benzoquinoneimine, which in turn depletes cellular GSH. The time course of GSH depletion in APAP-treated PMHs was determined. PMHs in long-term culture for 14 days were exposed to 30mM APAP for 2, 4, and 6 h (Fig. 3). GSH depletion was first observed at the 4-h time point and continued through 6 h.
FIG. 3.
APAP-induced GSH depletion. PMHs in culture incubated for 14 days were exposed to 30mM of APAP for 0, 2, 4, or 6 h. At the indicated time points, cells were extracted and GSH levels measured. Values are expressed as percent of control (%).
APAP-Induced ROS Production and Mitochondrial Changes in PMHs in Long-term Culture
Previous studies have shown that APAP treatment of PMHs either freshly isolated or in short-term culture induces oxidative stress leading to mitochondrial damage (Kon et al., 2004; Reid et al., 2005). To determine the role of oxidative stress in APAP-induced toxicity in the long-term cultured PMHs, we examined ROS production, and generation of both MMP and MPT in 2-week-old hepatocytes. Cells were treated with 30mM APAP for 2, 4, 6, 8, 10, 12, or 24 h. Cells were assayed for ROS production using H2DCF-DA, a compound that is converted to a fluorescent derivative by ROS (Fig. 4A). It is important to note that no background H2DCF-DA fluorescence was observed in control mouse hepatocytes in long-term culture for 14 days (0 h). Incubation of hepatocytes with 30mM APAP led to a dramatic increase of green fluorescence at 4 h following APAP treatment, indicating that treatment of PMHs in long-term culture with APAP is accompanied by the production of ROS.
FIG. 4.
Effects of APAP on oxidative stress generation in long-term cultured PMHs. To determine whether oxidative stress occurs in the course of APAP toxicity, 14-day-old PMHs were incubated with media alone or 30mM APAP for 0, 2, 4, and 6 h. At the indicated times, cells were washed in PBS and incubated with 100μM H2DCF-DA (DCF) for 30 min for detection of ROS (A). To determine MMP (B) and MPT (C), cells were exposed to JC-1 dye or TMRM and calcein AM for 30 min, respectively, and observed using the fluorescence microscope. Original magnification is ×200.
The effects of APAP treatment on mitochondria in these cells were examined using the dyes JC-1 and TMRM with calcein AM. JC-1 is a dye that exhibits potential-dependent accumulation in mitochondria. An aggregate (red) indicates a high mitochondrial membrane potential, whereas a monomer (green) indicates a low mitochondrial membrane potential. Mitochondrial membrane depolarization is indicated by a shift of fluorescence emission from red to green. At 4 h following APAP treatment, a significant mitochondrial depolarization occurred (Fig. 4B); by 6 h of APAP treatment, cells lost their MMP completely. Cells were also evaluated for the effect of 30mM APAP treatment on hepatocyte mitochondria using TMRM and calcein AM (Fig. 4C). TMRM measures mitochondrial depolarization related to cytoplasmic Ca2+ transients (Loew et al., 1994). The mitochondria in untreated cells or cells treated with APAP for 2 h emitted a red fluorescence. At 4 h following APAP treatment, the fluorescence changed from red to an orange/yellow indicating a change in the mitochondrial membranes. By 6 h following APAP treatment, the mitochondria lost all red/orange/yellow color indicating the cells had undergone MPT. Because there was no change after 6 h following APAP treatment in ROS or mitochondrial damage, data are only provided for these time points (Fig. 4).
Iron Is Associated with APAP Toxicity in PMHs
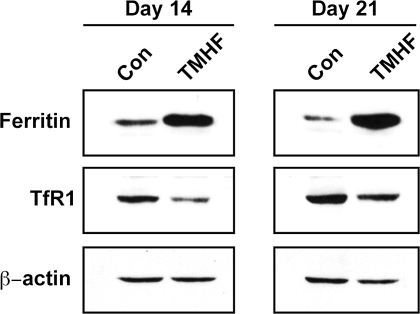
Before measuring the impact of hepatic iron on APAP-induced cell injury in long-term cultured PMHs, it was necessary to determine whether TMHF treatment would result in iron loading of mouse hepatocytes in long-term culture as we had reported previously for rat hepatocytes (Cable et al., 1998). TMHF is a chemically stable, nonionic form of iron that can passively diffuse across hepatocellular membranes without prior metabolism (Cable et al., 1998). Once within the cell, TMHF is metabolized by a cytochrome P450–dependent reaction (Cable and Isom, 1999) that results in the release of iron from the TMHF molecule and the iron can then be incorporated into the iron-storage protein ferritin (Dullmann et al., 1992; Nielsen and Heinrich, 1993). As expected, TMHF treatment of PMHs in long-term culture resulted in increased ferritin protein levels and decreased TfR1 protein levels (Fig. 5). We observed no further increase in ferritin levels or decrease in TfR1 levels between 7 and 14 days of TMHF treatment; therefore, a 7-day TMHF treatment protocol was selected for the APAP experiments.
FIG. 5.
Detection of the level of iron regulatory gene expression in iron-loaded PMHs. PMHs were maintained for 7 days in control medium, after which cultures were maintained in either control medium or medium supplemented with 10μM TMHF for 7 or 14 days. Protein samples were harvested at days 14 and 21 after plating and subjected to immunoblot analysis for ferritin, TfR1, and β-actin (as a loading control).
To determine the effect of hepatic iron on APAP-induced cytotoxicity, PMHs in long-term culture conditions for 1 week were treated with 0, 2, 5, and 10μM TMHF for an additional 7 days. At 7 days following initiation of TMHF treatment (14 days post hepatocyte seeding), the cells were treated with APAP for 24 h to determine the effect of increased iron on APAP cytotoxicity (Fig. 6A). Hepatocytes treated with 5 or 10μM TMHF alone for 7 days did not demonstrate cell death in the live/dead assay; however, hepatocytes treated with 5 or 10μM TMHF and then exposed to APAP demonstrated cell death at earlier time points compared with those treated with APAP alone. A concentration of 20mM APAP was needed to cause cell death at 24 h in hepatocytes not treated with TMHF. In contrast, 10mM APAP was sufficient to cause cell death in mouse hepatocytes treated with 5μM TMHF, and only 5mM APAP caused cell death when hepatocytes were treated with 10μM TMHF.
FIG. 6.
Effect of iron on APAP-induced cytotoxicity in PMHs. (A) PMHs were maintained in control medium for 7 days, after which cells were incubated in medium supplemented with 0, 2, 5, or 10μM of TMHF for an additional 7 days. At day 14 after plating, cells were treated with 0, 5, 10, 20, or 30mM APAP for 24 h. After washing the cells with PBS to remove excess APAP, cells were exposed to calcein AM and ethidium homodimer for 30 min to evaluate cell viability. The cells were then visualized by fluorescence microscopy. (B) Cells were incubated in control medium for 14 days prior to 100μM DFO treatment for 2 days. At day 16 after plating, cells were exposed to 0, 10, 20, or 30mM of APAP for 24 h. To estimate cell viability, cells were loaded with the same dyes as described above.
To further investigate the effects of iron on APAP-induced cell death, the effect of treatment of PMHs in long-term culture with a chelator prior to APAP exposure was examined. PMHs were cultured in HMM for 14 days at which time the cultures were fed medium supplemented with 100μM deferoxamine (DFO). Medium was changed daily. After 48 h of DFO treatment, hepatocytes were exposed to 0, 10, 20, or 30mM APAP. In hepatocytes treated with DFO, higher concentrations of APAP were required to cause cell death (Fig. 6B).
Elevated Cellular Iron Accelerates Generation of ROS and Mitochondrial Damage in PMHs Treated with APAP
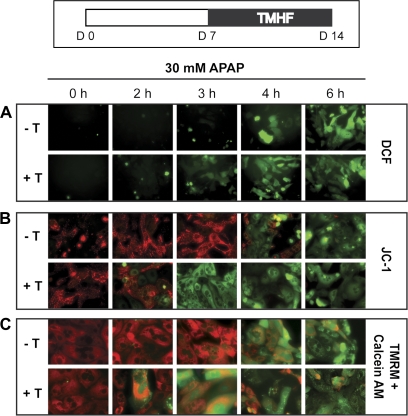
To evaluate the effect of increased hepatocellular iron on oxidative stress and mitochondrial damage caused by APAP, PMHs in culture for 1 week were treated with 10μM TMHF for 7 days. At 7 days following initiation of TMHF treatment (14 post hepatocyte seeding), the cells were treated with 30mM of APAP for various time points (0, 1, 2, 3, 4, and 6 h). At each time point, production of ROS, MMP, and MPT were measured using H2DCF-DA, JC-1, and TMRM with calcein AM, respectively. ROS generation occurred in hepatocytes treated with TMHF and then exposed to APAP at an earlier time point (3 h) compared with those treated with APAP alone (4 h; Fig. 7A). Similarly, loss of MMP as measured using JC-1 (Fig. 7B) and of MPT as measured using TMRM with calcein AM (Fig. 7C) occurred in hepatocytes treated with TMHF and then exposed to APAP at earlier time points (2 to 3 h) compared with those treated with APAP alone (4 h).
FIG. 7.
Effect of iron on oxidative stress induced by APAP in PMHs. PMHs were maintained in control medium for 7 days, after which cells were treated with 10μM TMHF for 7 days. At day 14 after plating, both untreated (−T) and TMHF-treated (+T) cells were exposed to 30mM APAP for 0, 2, 3, 4, and 6 h. (A) Time course of ROS production. After exposure to APAP, cells were washed and incubated with H2DCF-DA dye (DCF) for 30 min and observed by fluorescence microscopy. (B) Time course study of MMP. After exposure to APAP, cells were incubated in control medium supplemented with JC-1 dye for 30 min and observed by fluorescence microscopy. (C) Time course of MPT. After exposure to APAP, TMHF-treated cells were exposed to TMRM and calcein AM for 30 min and observed by fluorescence microscopy. Original magnification is ×200.
Chelation of Cellular Iron Delays Oxidative Stress and Mitochondrial Damage Induced by APAP in PMHs
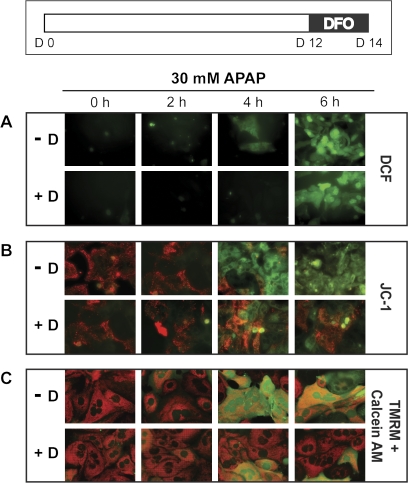
PMHs in long-term culture were untreated or treated with a chelator (100μM DFO) for 48 h. Cells were lysed and protein was examined by western blotting. As expected, chelation resulted in the reverse of what is seen for TMHF treatment; specifically, ferritin protein levels were decreased compared with control cells, and TfR1 protein levels were increased compared with control cells (data not shown). To investigate the effects of decreased cellular iron on APAP-induced oxidative stress and mitochondrial damage, PMHs in culture for 14 days were treated with 100μM DFO for 48 h prior to exposure to 30mM APAP for 0, 2, 4, and 6 h. At each time point, production of ROS, MMP, and MPT were measured using H2DCF-DA, JC-1, and TMRM with calcein AM, respectively. Hepatocytes treated with DFO prior to exposure to 30mM APAP were not positive for ROS production until 6 h after APAP, whereas cells fed control media showed significant ROS production by 4 h following APAP treatment (Fig. 8A). Mitochondrial damage was delayed by treating the hepatocytes with DFO prior to APAP; specifically, many cells within the hepatocyte cultures did not demonstrate loss of MMP and MPT as measured using JC-1 (Fig. 8B) and TMRM with calcein AM (Fig. 8C) at 4 or even 6 h after exposure to APAP. In contrast, hepatocytes fed control media demonstrated loss of MMP and MPT throughout the culture by 4 h following APAP treatment.
FIG. 8.
Effect of DFO, an iron chelator, on oxidative stress in the presence of APAP in PMHs. PMHs were maintained in control medium for 12 days prior to treatment of 100μM DFO for 2 days. At day 14 after plating, control (−D) and DFO-treated (+D) cells were exposed to 30mM of APAP for 0, 2, 4, and 6 h and incubated with H2DCF-DA dye (DCF) to measure ROS (A), JC-1 dye to measure MMP (B), and TMRM and calcein AM dyes to measure MPT (C). Original magnification is ×200.
Partial Reversal of Iron-Enhanced APAP Toxicity in PMHs by Chelation Treatment
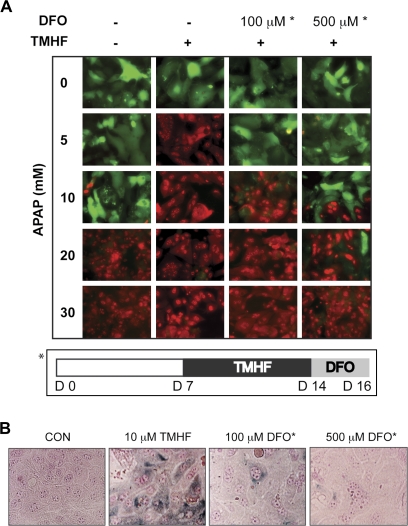
PMHs in long-term culture conditions for 1 week were fed control medium (−/−) or treated with 10μM TMHF for 7 days, followed by treatment with either control medium (−/+) or DFO (100μM/+, 500μM/+) for 2 days. At day 16 after plating, cells were treated with 0, 5, 10, 20, or 30mM of APAP for 24 h. Toxicity was measured using calcein AM and ethidium homodimer (Fig. 9A). Extensive cell death was observed in hepatocytes that had been treated with 10μM TMHF when exposed to only 5mM APAP, whereas a concentration of 20mM APAP was needed to produce similar levels of cell death in hepatocytes incubated in control medium. Either concentration of DFO administered to TMHF-treated cells reduced the extent of iron-enhanced APAP toxicity, with the higher DFO concentration being more effective than the lower concentration.
FIG. 9.
Effect of iron chelation subsequent to TMHF treatment. (A) PMHs treated with TMHF for 7 days were fed HMM containing 100 or 500μM of DFO for 2 days. At day 16 after plating, control cells (−/−), TMHF-treated cells (−/+), and TMHF-treated cells subsequently exposed to DFO (100μM/+, 500μM/+) were washed with PBS, followed by the addition of 0, 5, 10, 20, or 30mM APAP for 24 h. Cells were washed and incubated with calcein AM and ethidium homodimer to determine cell viability and visualized by fluorescence microscopy. The asterisk (*) refers to the protocol used for DFO therapy. Original magnification is ×200. (B) Perls’ Prussian blue staining of control cells (CON), TMHF-treated cells (10μM TMHF), and TMHF-treated cells subsequently exposed to DFO (100 or 500μM DFO). PMHs were maintained for 7 days in control medium, after which cultures were maintained in control medium supplemented with 0 or 10μM TMHF for an additional 7 days. At day 7 of TMHF treatment, control (CON) and TMHF-treated cultures (10μM TMHF) were fixed and subjected to Perls’ Prussian blue staining. At day 7 of TMHF treatment, TMHF-treated cells were incubated with control medium supplemented with 100 or 500μM DFO for an additional 2 days of culture (days 14–16) and subjected to Perls’ Prussian blue staining. Stained cultures were photographed by light microscopy (original magnification: ×400).
A hallmark of iron overload in the liver is the presence of hemosiderin, an insoluble form of tissue storage iron, in hepatocytes that is often visualized using a special stain, Perls’ Prussian blue. In patients with iron overload, for example, individuals with genetic hemochromatosis, hemosiderin is seen in hepatocytes. In parallel PMH cultures, we examined the ability of TMHF-treated hepatocytes to accumulate hemosiderin and the ability of DFO therapy at 100 and 500μM to reduce the levels of hemosiderin (Fig. 9B). Similar to what we observed for the effect of DFO on iron-enhanced APAP toxicity, DFO administered to TMHF-treated cells reduced the amount of hemosiderin, with the higher DFO concentration being more effective than the lower concentration.
DISCUSSION
Liver disease is responsible for more than 42,000 deaths yearly and is the ninth leading disease-related cause of death in the United States. There are many causes of liver disease, but APAP is the leading cause of acute liver failure in the United States (Larson et al., 2005; Ostapowicz et al., 2002). APAP (Tylenol) is the most widely used agent in the United States for pain relief and fever reduction. APAP is readily available and reasonably safe when taken at the recommended dosage by an individual with no underlying liver disease. Deliberate APAP overdose is used in suicide attempts; however, a significant number of individuals experience unintentional APAP overdose resulting in severe liver damage from simultaneous consumption of multiple APAP-containing medications. APAP is not only Tylenol but is also contained in many over-the-counter medications for cold, allergies, arthritis, and so on and in prescription narcotics. The association of APAP with acute liver failure is not restricted to adults but is also seen in children (Squires et al., 2006).
Previous studies have been carried out suggesting that APAP toxicity is decreased by pretreatment with the iron chelator DFO. For example, using a mouse model it has been demonstrated that administering DFO to mice delayed the rate of development of APAP toxicity (Schnellmann et al., 1999). Specifically, alanine transaminase levels were reduced at 2, 4, and 8 h but not at 12 or 24 h in mice treated with APAP and DFO compared with APAP alone. The effects of dietary iron overload in combination with APAP were not evaluated in this mouse study. Studies suggesting that APAP toxicity may be potentiated by increased iron and diminished by chelation to reduce iron have been carried out in cultured hepatocytes (Ito et al., 1994; Kyle et al., 1987). In these studies, the hepatocytes were in short-term culture (2–24 h) and the end point examined was cell death. The effects on ROS and mitochondrial injury were not measured.
The goal of our study was to determine whether hepatic iron affects APAP-induced injury at the levels of cytotoxicity, ROS production, and mitochondrial dysfunction in hepatocytes. We conclude that increasing or decreasing iron within hepatocytes alters APAP toxicity. Specifically, treatment of PMHs with the iron donor TMHF potentiated the effects of APAP such that the threshold dose that induced cell death was lower (5mM for treatment with 10μM TMHF compared with 20mM APAP in the absence of TMHF), whereas treatment with the chelator DFO protected, resulting in a higher threshold dose (30mM instead of 20mM) required to induce cell death. We also showed that at a specified dose of APAP (30mM), treatment with TMHF induced ROS and mitochondrial dysfunction at earlier time points than treatment with APAP alone, whereas treatment with the chelator DFO increased the length of time required for APAP to induce ROS and mitochondrial dysfunction.
We previously reported a method for long-term culture of PMHs (Stoehr and Isom, 2003) in which the mouse hepatocytes retain morphological characteristics of differentiated hepatocytes and continue to produce albumin for 30 days in culture at levels not statistically different from in vivo liver. Expression of liver-specific genes, including albumin, alpha 1-antitrypsin, and cytokeratin 18, are detected through day 30 of culture. The PMHs are both mononucleated and binucleated with round nuclear morphology, exhibit a low nucleus to cytoplasm ratio, and contain multiple distinct nucleoli. At the ultrastructural level, the cells contain large numbers of mitochondria and extensive rough and smooth endoplasmic reticulum. As in the in vivo liver, PMHs demonstrate a low level of DNA synthesis (from 2 to 5%) and do not undergo mitosis. Cell-cell junctions are readily discernible at the ultrastructural level and increased GJIC is observed in the cultures over time concomitantly with increased expression of gap junction proteins, connexin32 and connexin26. Connexin43 is not detected in these cultures, indicating the absence of non-parenchymal cells.
The data presented here clearly demonstrate the advantages of using PMHs in long-term culture for studying APAP-induced toxicity in mouse hepatocytes in vitro. Because mouse hepatocytes reach a steady state with regard to morphology, GJIC, and function by being in culture for 14 days prior to exposure to APAP (Stoehr and Isom, 2003), it is possible to determine at an in situ level temporal effects of APAP on ROS production, mitochondria, and viability. At an APAP concentration of 30mM, ROS and mitochondrial dysfunction were induced by 4 h of treatment and cell death was not detected throughout the culture until 10 h. No cell death was observed at 4 or 6 h and only a few random cells in the culture were dead by 8 h, indicating a substantial delay in time between loss of mitochondrial function and cell death. Temporal responses in short-term hepatocytes are considerably less reliable because the cells have not reestablished GJIC and the effects of the drug are complicated by the fact that the cells are undergoing time-dependent loss of differentiation and viability.
The data in this study extending our previous studies with rat hepatocytes (Cable et al., 1998) demonstrate that PMHs in long-term culture are capable of becoming iron loaded with time and that TMHF is a good iron donor. Treatment of mouse hepatocytes with TMHF caused an elevation in ferritin protein levels and a reduction in TfR1 protein levels indicating that the hepatocytes are undergoing the expected physiological response to iron treatment. In addition, the hepatocytes also accumulate hemosiderin as is seen in hepatocytes in the liver of patients with iron overload, for example, individuals with genetic hemochromatosis. The production of hydroxyl radicals has been demonstrated in rats exhibiting iron overload (Kadiiska et al., 1995). It also has been shown that iron induces oxidative stress and lipid peroxidation in the liver from the results of animal experiments (Bacon et al., 1983; Britton et al., 1987; Houglum et al., 1990) and patient studies (Niemela et al., 1999; Young et al., 1994). It is important to note that PMHs in culture for a total of 14 days and treatment with TMHF (10μM) for the last 7 of those 14 days were not positive for ROS, did not demonstrate mitochondrial dysfunction or MPT, and did not undergo cell death. One possible explanation is that although the intracellular iron concentration is high enough in TMHF-treated PMHs to potentiate the effects of APAP, the levels are not sufficient alone to induce ROS. A second reason is that hepatocytes in culture differ from those in the liver in that they are not surrounded by other cell types including Kupffer and Ito cells and, as such, are not bathed in cytokines and growth factors produced by those cells. As such, the conclusions of this study need to be limited to the realization that the effects of iron alone and in combination with APAP on ROS, mitochondrial function, and viability are limited to what is occurring in isolated mouse hepatocytes.
FUNDING
National Institutes of Health (DK054482 and DK073897) to H.C.I.
Acknowledgments
The authors thank Mike Haaf (Ithaca College, Ithaca, NY) for the gift of TMHF. They also thank Thomas Miller and Emily McDevitt for their excellent technical assistance.
References
- Bacon BR, Tavill AS, Brittenham GM, Park CH, Recknagel RO. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J. Clin. Invest. 1983;71:429–439. doi: 10.1172/JCI110787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Britton RS, Bacon BR, Recknagel RO. Lipid peroxidation and associated hepatic organelle dysfunction in iron overload. Chem. Phys. Lipids. 1987;45:207–239. doi: 10.1016/0009-3084(87)90066-1. [DOI] [PubMed] [Google Scholar]
- Cable EE, Connor JR, Isom HC. Accumulation of iron by primary rat hepatocytes in long-term culture: changes in nuclear shape mediated by non-transferrin-bound forms of iron. Am. J. Pathol. 1998;152:781–792. [PMC free article] [PubMed] [Google Scholar]
- Cable EE, Isom HC. Metabolism of 3,5,5-trimethylhexanoyl-ferrocene by rat liver: release of iron from 3,5,5-trimethylhexanoyl-ferrocene by a microsomal, phenobarbital-inducible cytochrome P-450. Drug Metab. Dispos. 1999;27:255–260. [PubMed] [Google Scholar]
- Dullmann J, Wulfhekel U, Nielsen P, Heinrich HC. Iron overload of the liver by trimethylhexanoylferrocene in rats. Acta Anat. (Basel) 1992;143:96–108. doi: 10.1159/000147235. [DOI] [PubMed] [Google Scholar]
- Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic. Biol. Med. 2002;32:833–840. doi: 10.1016/s0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- El-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Feder JN, Penny DM, Irrinki A, Lee VK, Lebron JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houglum K, Filip M, Witztum JL, Chojkier M. Malondialdehyde and 4-hydroxynonenal protein adducts in plasma and liver of rats with iron overload. J. Clin. Invest. 1990;86:1991–1998. doi: 10.1172/JCI114934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom HC, McDevitt EI, Moon MS. Elevated hepatic iron: a confounding factor in chronic hepatitis C. Biochim. Biophys. Acta. 2009;1790:650–662. doi: 10.1016/j.bbagen.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Ito Y, Suzuki Y, Ogonuki H, Hiraishi H, Razandi M, Terano A, Harada T, Ivey KJ. Role of iron and glutathione redox cycle in acetaminophen-induced cytotoxicity to cultured rat hepatocytes. Dig. Dis. Sci. 1994;39:1257–1264. doi: 10.1007/BF02093791. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Burkitt MJ, Xiang QH, Mason RP. Iron supplementation generates hydroxyl radical in vivo. An ESR spin-trapping investigation. J. Clin. Invest. 1995;96:1653–1657. doi: 10.1172/JCI118205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Yamamoto M, Tobioka H, Mizuguchi T, Mitaka T, Mochizuki Y. Changes in cellular distribution of connexins 32 and 26 during formation of gap junctions in primary cultures of rat hepatocytes. Exp. Cell Res. 1996;223:314–326. doi: 10.1006/excr.1996.0087. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Kyle ME, Miccadei S, Nakae D, Farber JL. Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem. Biophys. Res. Commun. 1987;149:889–896. doi: 10.1016/0006-291x(87)90491-8. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Loew LM, Carrington W, Tuft RA, Fay FS. Physiological cytosolic Ca2+ transients evoke concurrent mitochondrial depolarizations. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12579–12583. doi: 10.1073/pnas.91.26.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles E, Butzler C, Jung D, Temme A, Gabriel HD, Dahl U, Traub O, Stumpel F, Jungermann K, Zielasek J, et al. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9565–9570. doi: 10.1073/pnas.93.18.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P, Heinrich HC. Metabolism of iron from (3,5,5-trimethylhexanoyl)ferrocene in rats. A dietary model for severe iron overload. Biochem. Pharmacol. 1993;45:385–391. doi: 10.1016/0006-2952(93)90074-7. [DOI] [PubMed] [Google Scholar]
- Niemela O, Parkkila S, Britton RS, Brunt E, Janney C, Bacon B. Hepatic lipid peroxidation in hereditary hemochromatosis and alcoholic liver injury. J. Lab. Clin. Med. 1999;133:451–460. doi: 10.1016/s0022-2143(99)90022-7. [DOI] [PubMed] [Google Scholar]
- Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am. J. Physiol. 1997;272:C1286–C1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J. Pharmacol. Exp. Ther. 2005;312:509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Skowronski L, Abraham P, Leutzinger Y. Blood glutathione concentrations in a large-scale human study. Clin. Chem. 1996;42:64–70. [PubMed] [Google Scholar]
- Ruch RJ, Klaunig JE. Kinetics of phenobarbital inhibition of intercellular communication in mouse hepatocytes. Cancer Res. 1988;48:2519–2523. [PubMed] [Google Scholar]
- Schnellmann JG, Pumford NR, Kusewitt DF, Bucci TJ, Hinson JA. Deferoxamine delays the development of the hepatotoxicity of acetaminophen in mice. Toxicol. Lett. 1999;106:79–88. doi: 10.1016/s0378-4274(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Squires RH, Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, Dhawan A, Rosenthal P, Rodriguez-Baez N, Murray KF, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J. Pediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr SA, Isom HC. Gap junction-mediated intercellular communication in a long-term primary mouse hepatocyte culture system. Hepatology. 2003;38:1125–1135. doi: 10.1053/jhep.2003.50418. [DOI] [PubMed] [Google Scholar]
- Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr. Biol. 1997;7:713–716. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- Young IS, Trouton TG, Torney JJ, McMaster D, Callender ME, Trimble ER. Antioxidant status and lipid peroxidation in hereditary haemochromatosis. Free Radic. Biol. Med. 1994;16:393–397. doi: 10.1016/0891-5849(94)90041-8. [DOI] [PubMed] [Google Scholar]