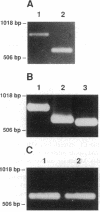
Abstract
It is well established that the terminal renal collecting duct is capable of electrogenic Na+ absorption. The present experiments examined other active ion transport processes in primary cultures of the rat inner medullary collecting duct. When the amiloride analogue benzamil inhibited electrogenic Na+ absorption, cAMP agonists stimulated a transmonolayer short circuit current that was not dependent on the presence of Na+ in the apical solution, but was dependent on the presence of Cl- and HCO3-. This current was not inhibited by the loop diuretic bumetanide, but was inhibited by ouabain, an inhibitor of the Na+/K+ pump. The current was reduced by anion transport inhibitors, with a profile similar to that seen for inhibitors of the cystic fibrosis transmembrane conductance regulator (CFATR) Cl- channel. Using several PCR strategies, we demonstrated fragments of the predicted lengths and sequence identity with the rat CFTR. Using whole-cell patch-clamp analysis, we demonstrated a cAMP-stimulated Cl- current with characteristics of the CFTR. We conclude that the rat inner medullary collecting duct has the capacity to secrete anions. It is highly likely that the CFTR Cl- channel is involved in this process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabantchik Z. I., Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol. 1992 Apr;262(4 Pt 1):C803–C827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Chalfant M. L., Coupaye-Gerard B., Kleyman T. R. Distinct regulation of Na+ reabsorption and Cl- secretion by arginine vasopressin in the amphibian cell line A6. Am J Physiol. 1993 Jun;264(6 Pt 1):C1480–C1488. doi: 10.1152/ajpcell.1993.264.6.C1480. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., George A. M., MacVinish L. Kinin effects on electrogenic ion transport in primary cultures of pig renal papillary collecting tubule cells. Am J Physiol. 1985 Sep;249(3 Pt 2):F439–F447. doi: 10.1152/ajprenal.1985.249.3.F439. [DOI] [PubMed] [Google Scholar]
- Grantham J. J. 1992 Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol. 1993 Jun;3(12):1841–1857. doi: 10.1681/ASN.V3121841. [DOI] [PubMed] [Google Scholar]
- Grupp C., Pavenstädt-Grupp I., Grunewald R. W., Bevan C., Stokes J. B., 3rd, Kinne R. K. A Na-K-Cl cotransporter in isolated rat papillary collecting duct cells. Kidney Int. 1989 Aug;36(2):201–209. doi: 10.1038/ki.1989.180. [DOI] [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Hamati H. F., Britton E. L., Carey D. J. Inhibition of proteoglycan synthesis alters extracellular matrix deposition, proliferation, and cytoskeletal organization of rat aortic smooth muscle cells in culture. J Cell Biol. 1989 Jun;108(6):2495–2505. doi: 10.1083/jcb.108.6.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted R. F., Laplace J. R., Stokes J. B. Enhancement of electrogenic Na+ transport across rat inner medullary collecting duct by glucocorticoid and by mineralocorticoid hormones. J Clin Invest. 1990 Aug;86(2):498–506. doi: 10.1172/JCI114736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler D. O., Rudigier J. F., Bischoff E., Decker K. F. The trapping of uridine phosphates by D-galactosamine. D-glucosamine, and 2-deoxy-D-galactose. A study on the mechanism of galactosamine hepatitis. Eur J Biochem. 1970 Dec;17(2):246–253. doi: 10.1111/j.1432-1033.1970.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Laplace J. R., Husted R. F., Stokes J. B. Cellular responses to steroids in the enhancement of Na+ transport by rat collecting duct cells in culture. Differences between glucocorticoid and mineralocorticoid hormones. J Clin Invest. 1992 Oct;90(4):1370–1378. doi: 10.1172/JCI116003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. B., Schwiebert E. M., Fejes-Toth G., Naray-Fejes-Toth A., Karlson K. H., McCann F. V., Stanton B. A. Chloride channels in the apical membrane of cortical collecting duct cells. Am J Physiol. 1990 Feb;258(2 Pt 2):F273–F280. doi: 10.1152/ajprenal.1990.258.2.F273. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Kokko K. E., Eaton D. C. Prostaglandin E2 activates clusters of apical Cl- channels in principal cells via a cyclic adenosine monophosphate-dependent pathway. J Clin Invest. 1994 Feb;93(2):829–837. doi: 10.1172/JCI117037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoo-Karim R., Uchic M., Lechene C., Grantham J. J. Renal epithelial cyst formation and enlargement in vitro: dependence on cAMP. Proc Natl Acad Sci U S A. 1989 Aug;86(15):6007–6011. doi: 10.1073/pnas.86.15.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunaka Y., Eaton D. C. Chloride channels in the apical membrane of a distal nephron A6 cell line. Am J Physiol. 1990 Feb;258(2 Pt 1):C352–C368. doi: 10.1152/ajpcell.1990.258.2.C352. [DOI] [PubMed] [Google Scholar]
- Matsuda J. J., Volk K. A., Shibata E. F. Calcium currents in isolated rabbit coronary arterial smooth muscle myocytes. J Physiol. 1990 Aug;427:657–680. doi: 10.1113/jphysiol.1990.sp018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. D., Li M., Welsh M. J. Identification and regulation of whole-cell chloride currents in airway epithelium. J Gen Physiol. 1989 Dec;94(6):1015–1036. doi: 10.1085/jgp.94.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld T. K., Douglass D., Grant M., Ye M., Silva F., Nadasdy T., Grantham J. J. In vitro formation and expansion of cysts derived from human renal cortex epithelial cells. Kidney Int. 1992 May;41(5):1222–1236. doi: 10.1038/ki.1992.184. [DOI] [PubMed] [Google Scholar]
- Poncet V., Tauc M., Bidet M., Poujeol P. Chloride channels in apical membrane of primary cultures of rabbit distal bright convoluted tubule. Am J Physiol. 1994 Apr;266(4 Pt 2):F543–F553. doi: 10.1152/ajprenal.1994.266.4.F543. [DOI] [PubMed] [Google Scholar]
- Romero J. C., Bentley M. D., Vanhoutte P. M., Knox F. G. Intrarenal mechanisms that regulate sodium excretion in relationship to changes in blood pressure. Mayo Clin Proc. 1989 Nov;64(11):1406–1424. doi: 10.1016/s0025-6196(12)65383-x. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Briggs J., Schubert G. In situ studies of the distal convoluted tubule in the rat. I. Evidence for NaCl secretion. Am J Physiol. 1982 Aug;243(2):F160–F166. doi: 10.1152/ajprenal.1982.243.2.F160. [DOI] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated anion transport in rabbit cortical collecting duct. Kinetics, stoichiometry, and conductive pathways. J Clin Invest. 1986 Dec;78(6):1621–1630. doi: 10.1172/JCI112755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. J., Welsh M. J. Fluid and electrolyte transport by cultured human airway epithelia. J Clin Invest. 1993 Apr;91(4):1590–1597. doi: 10.1172/JCI116365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg H. Secretion of salt and water into the medullary collecting duct of Ringer-infused rats. Am J Physiol. 1975 Feb;228(2):565–568. doi: 10.1152/ajplegacy.1975.228.2.565. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Low W., Elie D., Hanrahan J. W. Low-conductance chloride channel activated by cAMP in the epithelial cell line T84. FEBS Lett. 1990 Sep 17;270(1-2):157–164. doi: 10.1016/0014-5793(90)81257-o. [DOI] [PubMed] [Google Scholar]
- Trezise A. E., Szpirer C., Buchwald M. Localization of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) in the rat to chromosome 4 and implications for the evolution of mammalian chromosomes. Genomics. 1992 Dec;14(4):869–874. doi: 10.1016/s0888-7543(05)80107-7. [DOI] [PubMed] [Google Scholar]
- Verrey F. Antidiuretic hormone action in A6 cells: effect on apical Cl and Na conductances and synergism with aldosterone for NaCl reabsorption. J Membr Biol. 1994 Feb;138(1):65–76. doi: 10.1007/BF00211070. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Wingo C. S. Potassium secretion by the cortical collecting tubule: effect of C1 gradients and ouabain. Am J Physiol. 1989 Feb;256(2 Pt 2):F306–F313. doi: 10.1152/ajprenal.1989.256.2.F306. [DOI] [PubMed] [Google Scholar]
- Ye M., Grantham J. J. The secretion of fluid by renal cysts from patients with autosomal dominant polycystic kidney disease. N Engl J Med. 1993 Jul 29;329(5):310–313. doi: 10.1056/NEJM199307293290503. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L. Hormonal regulation of inner medullary collecting duct sodium transport. Am J Physiol. 1993 Aug;265(2 Pt 2):F159–F173. doi: 10.1152/ajprenal.1993.265.2.F159. [DOI] [PubMed] [Google Scholar]