Abstract
AMOP-H-OH (6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol) and some of its sulfur-bearing analogs were tested for their actions in vitro at human α4β2-, α4β4-, α3β4*- and α1*-nicotinic acetylcholine receptors (nAChRs). AMOP-H-OH also was assessed in a model of antidepressant efficacy. AMOP-H-OH and some of its analogs have high potency and selectivity for α4β2-nAChRs over other nAChR subtypes. Effects are manifest as partial agonism, perhaps reflecting selectivity for high sensitivity (α4)3(β2)2-nAChRs. More prolonged exposure to AMOP-H-OH and its analogs produces inhibition of subsequent responses to acute challenges with nicotinic full agonists, again selectively for α4β2-nAChRs over other nAChR subtypes. The inhibition is mediated either via antagonism or desensitization of nAChR function, but the degree of inhibition of α4β2-nAChRs is limited by the drugs’ activities as partial agonists. Certain aspects of the in vitro pharmacology suggest that AMOP-H-OH and some of its analogs have a set of binding sites on α4β2-nAChRs that are distinct from those for full agonists. The in vitro pharmacological profile suggests that peripheral side effects of AMOP-H-OH or its analogs would be minimal and that their behavioral effects would be dominated by central nAChR actions. AMOP-H-OH also has profound and high potency antidepressant-like effects in the forced swim test. The net action of prolonged exposure to AMOP-H-OH or its analogs, as for nicotine, seems to be a selective decrease in α4β2-nAChR function. Inactivation of nAChRs may be a common neurochemical endpoint for nicotine dependence, its treatment, and some of its manifestations, including relief from depression.
Keywords: nicotine, nAChRs, depression, α4β2, Sazetidine-A, AMOP-H-OH
Introduction
Drugs targeting nAChRs have potential for the treatment of disorders, such as Alzheimer’s disease, Parkinson’s disease, dyskinesias, Tourette’s syndrome, schizophrenia, attention deficit disorder, anxiety, pain, smoking cessation, and depression.[1–4] In addition, nAChRs are the biological substrates for nicotine (1), the active ingredient in tobacco products that produce dependence and subsequent morbidity and mortality associated with tobacco related-diseases. The identification of safe nicotine surrogates free of side effects may thus find use for smoking cessation (such as varenicline (2) which is now on the market), thereby limiting smoking-associated disabilities and deaths.
Reports of a high incidence of depression in smokers (e.g., [5]) underscore a wide range of other studies indicating that nicotine administration has antidepressant-like properties.[6–10] These studies suggest that nicotinic ligands manipulated to remove or reduce dependence liability while maintaining the ability to balance mood could provide a new family of antidepressant drugs, perhaps with greater efficacy and fewer side effects than antidepressants currently on the market that work via inhibiting monoamine reuptake.
nAChRs are diverse members of an extended family of chemical signaling molecules.[11–13] nAChRs are composed as pentamers of various combinations of subunits (α1-α10, β1-β4, γ, δ, and ε) encoded from seventeen, different, vertebrate genes. Different subunit combinations define the various nAChR subtypes, and different receptor subtypes have characteristic pharmacological and biophysical properties, as well as different locations within the nervous system.[14] The subunits that comprise the receptor have a common structure consisting of a large extracellular N-terminal domain that contains the binding sites for acetylcholine, nicotine, and other ligands, followed by three hydrophobic transmembrane domains, a large intracellular loop, and then a fourth hydrophobic transmembrane domain, before ending with a short, extracellular, C-terminal tail.[15]
While a host of nAChR ligands have been identified, there is still a substantial need to discover subtype-selective nAChR ligands that can be used to establish the physiological and pathophysiological significance of each of the specific receptor subtypes, both in vivo and in vitro. As is now apparent from clinical results obtained from a variety of modern day medicines, both the safety and efficacy of therapeutic agents are often dependent upon their subtype selectivity. While a large number of nicotinic agonists and non-competitive antagonists exist, very few of these ligands are subtype selective.[16, 17] Subtype selectivity is difficult to achieve because of the large number of possible subtypes together with the relatively subtle differences in their structures. The design and development of selective agonists or antagonists is therefore particularly challenging, but it is of tremendous societal value in terms of possible disease treatment.
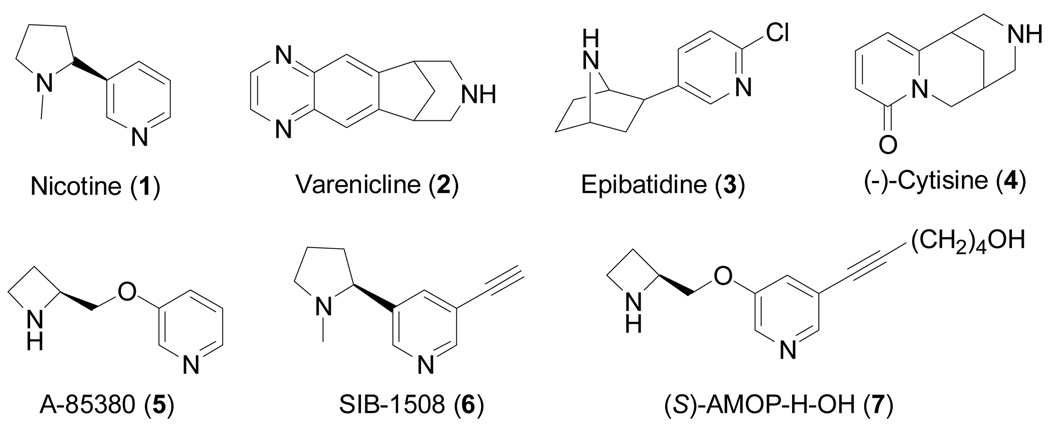
During our own research studies, we have investigated various analogs of epibatidine (3),[18] cytisine (4),[19] and A-85380 (5),[20] and we elaborate on our work on analogs of the later compound here.
Previously, compounds of the 3-pyridyl ether class were reported to show sub-nanomolar affinity for nicotinic acetylcholine receptors.[21] Additionally, the incorporation of groups such as an acetylene into the pyridine ring of nicotine as in SIB-1508 (6) was shown to lead to a compound of potential use in the treatment of Parkinson’s disease.[22] The α4β2 agonist A-85380 showed antidepressant-like effects in the mouse forced swim test.[23] Also, guided by the observation that the 5-iodo analog of A-85380 retains high affinity and displays even higher selectivity for α4β2-nAChRs,[24] Fan and Musachio first introduced other substituents at the 5-position of the pyridine ring to probe the possibility to create high-affinity nAChR ligands that could be used for affinity isolation or labeled with reporter groups to generate fluorescent probes. These early, unpublished studies led us to further explore the chemistry and pharmacology of AMOP-H-OH (6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol; 7; previously called “sazetidine-A” for S-azetidine, but this refers only to part of the molecule;[20]) and several of its analogs.
Results and Discussion
Chemistry
Based upon the lead structure, AMOP-H-OH (7), we decided to explore the activity of a small number of compounds in which the terminal OH group was replaced by a sulfur substituent. We hypothesized that such compounds might serve as useful nicotinic ligands and possibly show therapeutic potential as antidepressants.
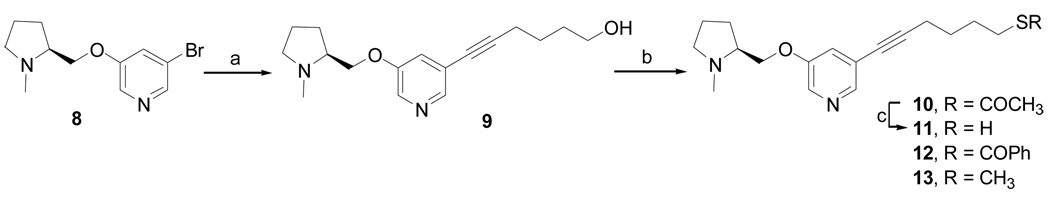
The synthesis of compounds 10–13 is shown in Scheme 1. Compound 8 was coupled with 5-hexyn-1-ol via palladium-catalyzed Sonogashira reaction at 90 °C to afford compound 9. The coupling product was then converted to the thioacetate 10 and thiobenzoate derivatives 12 under Mitsunobu reaction conditions. The thioacetate was deprotected with NaOCH3 (30 wt %) in MeOH to afford 11 in 50% yield.
Scheme 1a.
a Reagents and conditions: (a) [PdCl2(PPh3)2], CuI, Et3N, 5-hexyn-1-ol, 90 °C, 16 h, 95%; (b) for 10 and 11: diisopropyl azodicarboxylate (DIAD) ,PPh3, CH3COSH, THF, 0 °C-rt, 3 h, 79%; for 12: DIAD, PPh3, PhCOSH, THF, 0 °C-rt, 3 h, 87%; for 13: (i) 4-dimethylamino pyridine (DMAP), Et3N, p-toluenesulfonyl chloride (TsCl), CH2Cl2, 0 °C-rt, 16 h, 42%; (ii) NaSMe, EtOH, rt, 12 h, 90%; (c) NaOCH3 solution (30 wt %), CH3OH, 0 °C-rt, 12 h, 50%.
Furthermore, compound 9 was converted to its tosylate derivative, which was reacted with sodium thiomethoxide in EtOH to afford 13 in 90% yield.
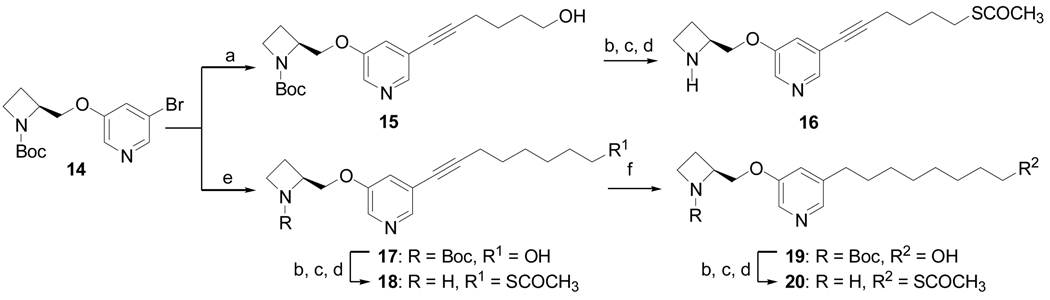
The analogs 16, 18, and 20 were prepared as shown in Scheme 2. Compound 14 was coupled with 5-hexyn-1-ol under Sonogashira conditions to afford compound 15 as reported previously.[20] Intermediate 15 was converted to its thioacetate through the tosylate derivative, and the Boc group was removed using trifluoroacetic acid in CH2Cl2 to afford 16. The Sonogashira coupling reaction of compound 14 and 7-octyn-1-ol provided compound 17, which was further converted to compound 19 by catalytic hydrogenation at atmospheric pressure. The same methodology was used to prepare compounds 18 and 20 from compounds 17 and 19, respectively.
Scheme 2a.
a Reagents and conditions: (a) [PdCl2(PPh3)2], CuI, Et3N, 5-hexyn-1-ol, 90 °C, 16 h, 95%; (b) DMAP, Et3N, TsCl, CH2Cl2, 0 °C-rt, 16 h, 91%; (c) Cs2CO3, CH3COSH, DMF, rt, 91%; (d) TFA, CH2Cl2, 0 °C, 3 h, 87%; (e) [PdCl2(PPh3)2], CuI, Et3N, 7-octyn-1-ol, 90 °C, 16 h, 95% ; (f) Pd/C, H2, CH3OH, 87%.
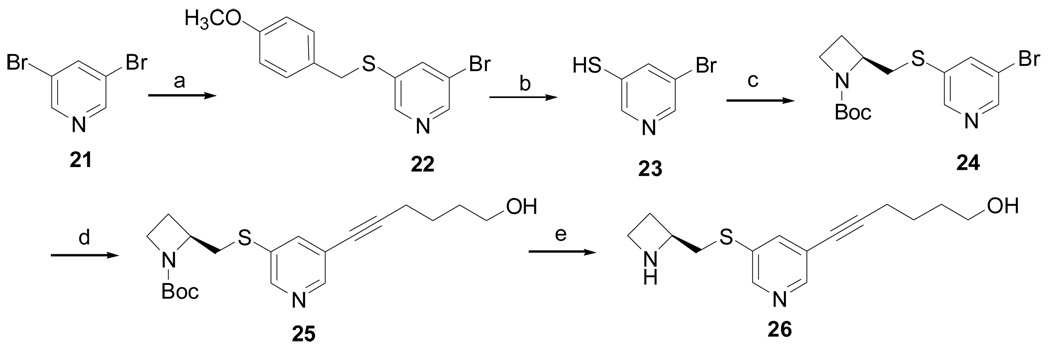
Thio analog 26 was prepared as shown in Scheme 3. Alkylation of (4-methoxy-phenyl)methanethiol with 3,5-dibromopyridine (21) using sodium hydride, followed by deprotection of the methoxy benzyl group, provided compound 23. Compound 24 was obtained from the condensation reaction of compound 23 and 2-(toluene-4-sulfonyloxymethyl)-azetidine-1-carboxylic acid tert-butyl ester, which was further coupled with 5-hexyn-1-ol to afford 25. The Boc group of compound 25 was deprotected with 4 M HCl in dioxane to provide 26.
Scheme 3a.
a Reagents and conditions: (a) NaH (60%), p-MeOC6H4CH2SH, DMF, 10 h, 69%; (b) m-cresol, TFA, reflux, 24 h, 53%; (c) 2-(toluene-4-sulfonyloxymethyl)azetidine-1-carboxylic acid tert-butyl ester, K2CO3, DMF, 12 h, 50%; (d) Pd(PPh3)2Cl2, PPh3, CuI, Et3N, 5-hexyn-1-ol, 12 h, 84%; (e) 4M HCl/dioxane, 4 h, 50%.
In vitro studies
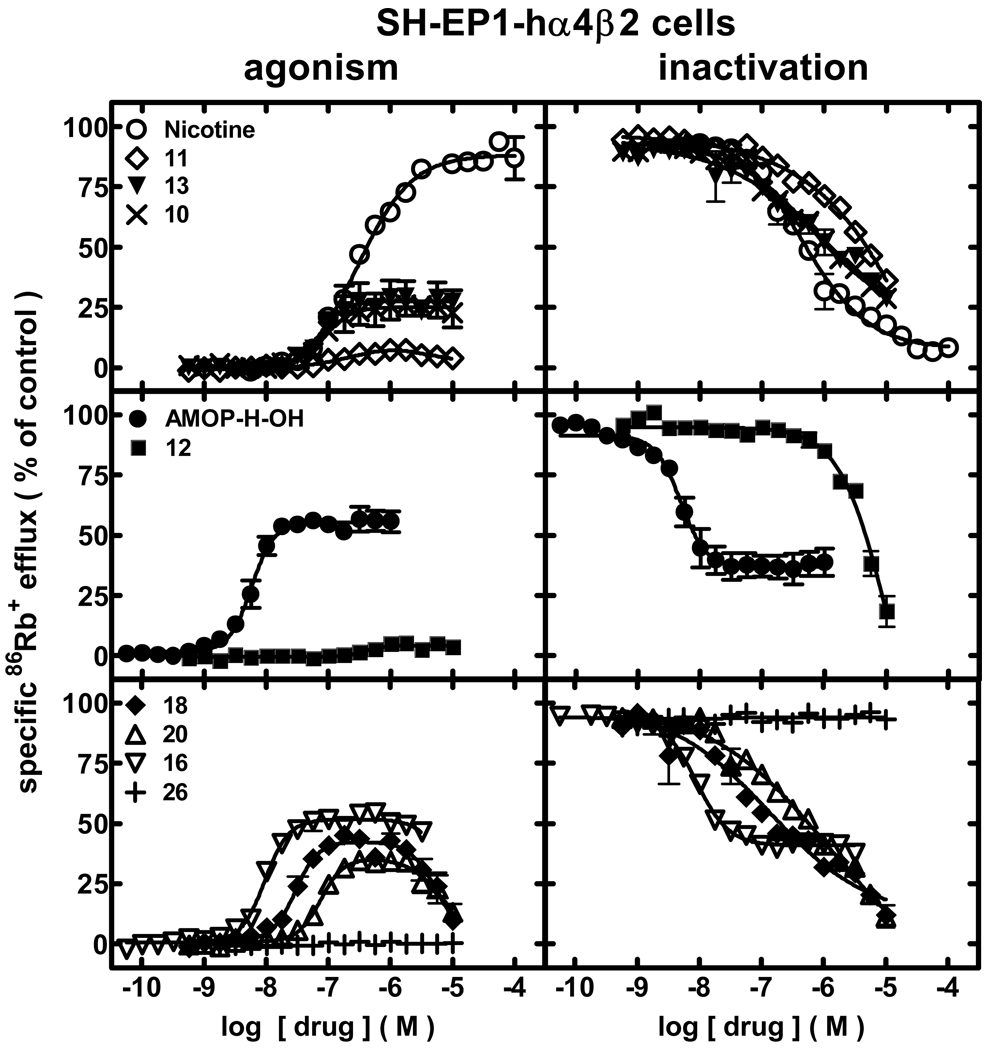
Pharmacological studies were done assessing effects of AMOP-H-OH and its analogs on the function of diverse, human nAChR subtypes naturally or heterologously expressed in human, model cell lines. Perhaps the most relevant effects are those of AMOP-H-OH and analogs on function of human α4β2-nAChRs. This nAChR subtype is the most abundant, high affinity nicotine binding site in the brain and has been implicated in nicotine self-administration. Initial studies assessed specific 86Rb+ efflux from SH-EP1-hα4β2 cells over a 10-min drug exposure period, thus measuring intrinsic agonist activity (Fig. 2, left panels; Table 1; Supplementary Data Table 1). Nicotine itself acts as a full agonist at α4β2-nAChRs, having an EC50 value of 300 nM. AMOP-H-OH acts as a potent, partial agonist, having about one-half of nicotine’s peak efficacy (55%), but reaching that level of efficacy at a > 50-fold lower concentration (5.8 nM) than nicotine’s EC50 value (Fig. 2; Table 1; Supplementary Data Table 1). AMOP-H-OH seems to be a true, partial agonist rather than a self-inhibiting agonist, because levels of α4β2-nAChR function peaked and remained constant over at least two orders of magnitude of ligand concentration. Rivaling AMOP-H-OH for efficacy and potency at human α4β2-nAChRs is 16, also acting as a true, partial agonist (9.8 nM EC50, 52% efficacy). Also clustering as sub-maximally efficacious agonists (here and throughout using EC50 values to make reference to the concentration of ligand giving half of its maximal efficacy, although realizing that the peak efficacies for these ligands are less than that of a full agonist) with EC50 values of between 30 and 100 nM and peak efficacies of between 25–46% are (in rank order potency) 18, 13, 20, and 10. Of these, 18 and 20 show self-inhibition (displayed as a bell-shaped concentration-response curve) at higher concentrations, and ligands with higher potency also appear to be more efficacious. 11 and 12 are only weakly (but not statistically significant) efficacious as agonists, and no agonism was observed for 26 (Fig. 2; Table 1; Supplementary Data Table 1). Concentration-response curves for many of the tested compounds were steep, reflected by Hill coefficients ≥ 2.0 (Supplementary Data Table 1).
Figure 2.
Specific 86Rb+ efflux (ordinate; percentage of control) was determined for human α4β2-nAChRs expressed by SH-EP1-hα4β2 cells in the presence of ligand alone (graphs on the left; normalized to responses to 1 mM carbamylcholine) or in the presence of an ~EC90 concentration of carbamylcholine (200 µM; graphs on the right) as described in Materials and Methods. Symbol key: Nicotine (○); 11 (◇); 13 (▼); 10 (×); AMOP-H-OH (●); 12 (■); 18 (◆); 20 (△); 16 (▽); 26 (+). Log molar EC50 or log molar IC50 values (± S.E.) and agonist efficacies or residual activity in the presence of inactivating drug (± S.E.) are provided in Table 1. Molar EC50 or IC50 values and Hill coefficients are provided inSupplementary Data Table 1.
TABLE 1. Sensitivities and efficacies of ligand action at different nAChR subtypes.
86Rb+ efflux assays were conducted as described in Methods and in the legends to Fig. 2–Fig. 5 for cells expressing the indicated nAChR subtypes and for the ligands listed in column 1. Agonism: Results for agonism were fit to the logistic equation to determine log molar EC50 values (± SE; upper values) and efficacies (% ± SE relative to a fully efficacious concentration of carbamylcholine; lower values). Log molar IC50 values (± SE; in parentheses) also are given for drugs with agonist activity also displaying self-inhibition. Inactivation: Results for inactivation of responses mediated by a standard agonist were fit to the logistic equation to determine log molar IC50 values (± SE; upper values) and degree of function remaining at high ligand concentration (% ± SE relative to a fully efficacious concentration of carbamylcholine; lower values). Where efficacy or degree of inactivation values show no SE, they were constrained for curve fitting. ND – not determined. Please see Supplementary Data Table 1 for mean EC50 or IC50 values and Hill coefficients.
| Drug | Agonism | Inactivation | ||||||
|---|---|---|---|---|---|---|---|---|
| log M EC50 values (± SE) | log M IC50 values (± SE) | |||||||
| (log M IC50 values (± SE) for self inhibition) | ||||||||
| % efficacy | % remaining function at high ligand concentration | |||||||
| α4β2 | α4β4 | α3β4* | α1* | α4β2 | α4β4 | α3β4* | α1* | |
| 7 | −8.23 ± 0.03 | −7.60 ± 0.07 | −6.47 ± 0.06 | −5.65 ± 0.05 | −8.32 ± 0.05 | −7.54 ± 0.01 | −6.22 ± 0.15 | −5.61 ± 0.04 |
| (−4.60 ± 0.29) | ||||||||
| 55 ± 1% | 98 ± 5% | 97 ± 4% | 51 ± 3% | 37± 2 % | −16 ± 1% | 12 ± 11% | 0% | |
| 16 | −8.03 ± 0.02 | −7.40 ± 0.10 | −6.09 ± 0.04 | −5.98 ± 0.02 | −8.05 ± 0.03 | −7.49 ± 0.01 | −5.88 ± 0.07 | −6.06 ± 0.04 |
| (−5.05 ± 0.55) | (−4.14 ± 0.52) | (−5.15 ± 0.03) | (−4.90 ± 0.03) | |||||
| 52 ± 1% | 114 ± 34% | 43% | 50% | 41 ± 1% | −8 ± 1% | −2 ± 7% | 0% | |
| 18 | −7.53 ± 0.04 | 6.62 ± 0.04 | ≫ −5.0 | −6.31 ± 0.05 | −6.74 ± 0.18 | −6.93 ± 0.04 | −6.21 ± 0.09 | −6.43 ± 0.05 |
| (−5.26 ± 0.05) | (−5.46 ± 0.55) | (−5.48 ± 0.07) | ||||||
| 43 ± 1% | 97 ± 7% | 7 ± 1% | 0% | −8 ± 3% | −2 ± 7% | 0% | ||
| 13 | −7.12 ± 0.08 | −6.16 ± 0.07 | ≫ −5.0 | ≫ −5.0 | −6.16 ± 0.24 | −5.98 ± 0.05 | −5.94 ± 0.11 | −5.55 ± 0.08 |
| (−4.83 ± 0.15) | ||||||||
| 28 ± 1% | 52 ± 6% | 20% | −15 ± 5% | 6 ± 9% | 0% | |||
| 20 | −7.13 ± 0.04 | −6.48 ± 0.07 | ≫ −5.0 | ≫ −5.0 | −6.24 ± 0.06 | −6.66 ± 0.06 | −6.10 ± 0.07 | −6.12 ± 0.05 |
| (−5.15 ± 0.06) | (−5.32 ± 0.07) | |||||||
| 36 ± 2% | 100 ± 11% | 0% | −8 ± 4% | 11 ± 5% | 0% | |||
| 10 | −7.06 ± 0.08 | −6.17 ± 0.07 | ≫ −5.0 | ≫ −5.0 | −6.19 ± 0.12 | −6.41 ± 0.05 | −6.05 ± 0.04 | −5.74 ± 0.02 |
| (−5.15 ± 0.05) | ||||||||
| 25 ± 1% | 76 ± 8% | 20% | −7 ± 4% | 5 ± 4% | 0% | |||
| 11 | −6.97 ± 0.13 | −5.72 ± 0.16 | ≫ −5.0 | ≫ −5.0 | −5.26 ± 0.03 | −5.91 ± 0.08 | −5.39 ± 0.07 | −4.83 ± 0.09 |
| 6 ± 1% | 105 ± 17% | 0% | −9± 7% | 0% | 0% | |||
| 1 | −6.53 ± 0.05 | ND | ND | ND | −6.37 ± 0.06 | ND | ND | ND |
| 88 ± 2% | 8 ± 2% | |||||||
| 12 | −6.42 ± 0.12 | −6.50 ± 0.03 | ≫ −5.0 | ≫ −5.0 | −5.34 ± 0.02 | −6.39 ± 0.05 | −6.08 ± 0.03 | −6.07 ± 0.03 |
| (−5.50 ± 0.03) | ||||||||
| 4 ± 1% | 45% | 0% | 0 ± 4% | 7 ± 3% | 0% | |||
| 26 | ≫ −5.0 | ≫ −5.0 | ≫ −5.0 | ≫ −5.0 | ≫ −5.0 | ND | ≫ −5.0 | ≫ −5.0 |
| - | - | - | - | - | - | - | - | |
When cells were then exposed to drugs for 10 min prior to and then during another 5 min period in the presence of an ~EC90 concentration of the full nicotinic agonist, carbamylcholine, assessments could be made of the test drug’s ability to inactivate human α4β2-nAChR function (Fig. 2, right panels; Table 1; Supplementary Data Table 1). In these assays, 10-min prior exposure to nicotine produces a concentration-dependent loss in α4β2-nAChR function measured as an acute response to carbamylcholine challenge in the continued presence of nicotine. This inactivation is nearly full after exposure to 100 µM nicotine and is ~50% after exposure to 430 nM nicotine. There is a trend toward complete inactivation of α4β2-nAChR function after exposure to high concentrations of 10, 11, or 13, although we did not conduct studies at high enough concentration to demonstrate that α4β2-nAChR function in the presence of the ligands plus full agonist fell to levels below those achieved in the presence of ligand alone. This is perhaps a pertinent caveat, because AMOP-H-OH and 16, which had highest potency of the other ligands surveyed and partial efficacy as agonists, also produced potent (IC50 values of 4.8 and 8.9 nM, respectively) concentration-dependent inactivation of α4β2-nAChR functional responses to a full agonist, but the degree of inactivation seems to have a lower limit (37% and 41% of control specific function, respectively) very close to the level of α4β2-nAChR function achieved on exposure to the drugs alone. Concentration-response curves more typical of antagonism were observed for 12, 18, and 20, but 26 displayed no ability to inactivate α4β2-nAChR function (Fig. 2; Table 1; Supplementary Data Table 1).
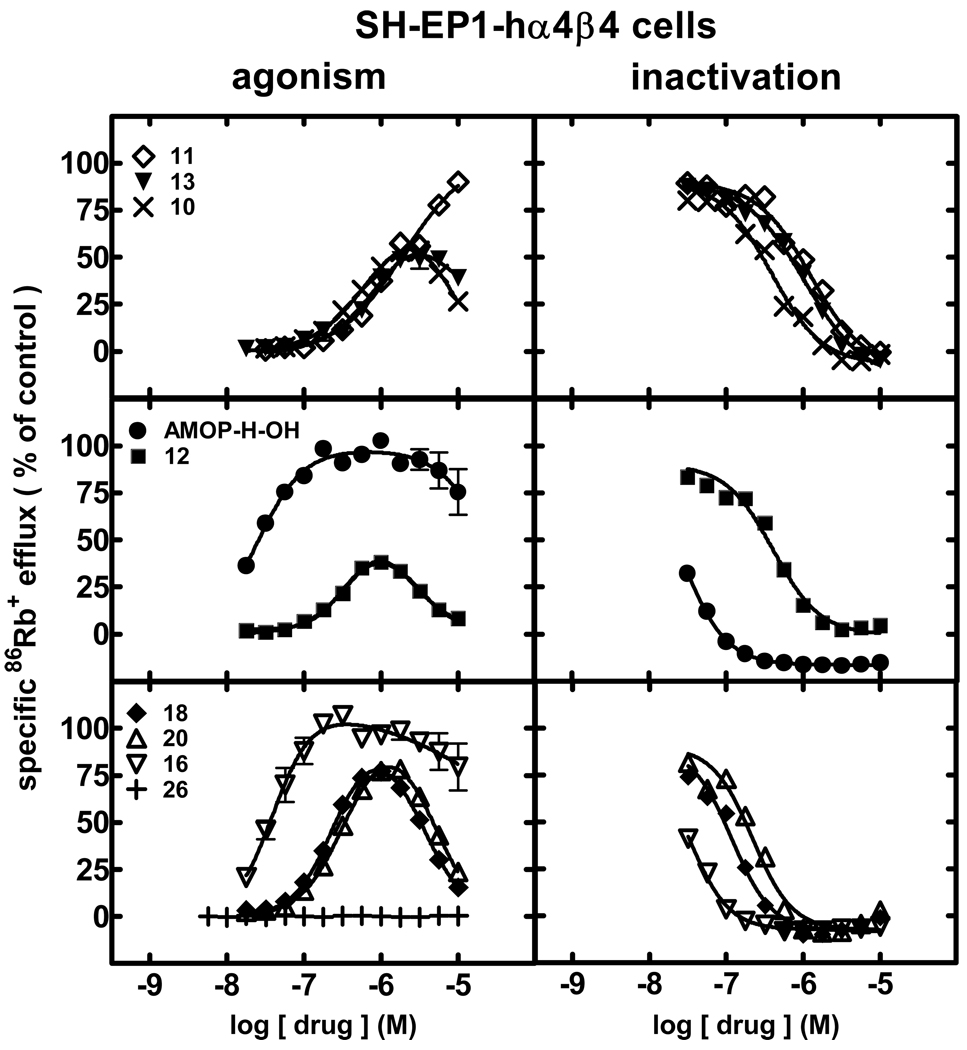
When the same set of experiments was conducted using SH-EP1-hα4β4 cells, effects were distinct from those seen for studies of α4β2-nAChR function. Intrinsic agonist activity assessments (Fig. 3, left panels; Table 1; Supplementary Data Table 1) revealed that AMOP-H-OH (25 nM EC50) and 16 (40 nM EC50) were nearly equally potent, full agonists also showing some self-inhibitory activity at higher concentrations when acting at human α4β4-nAChRs. With potencies about 5- or 10-fold lower, 18 and 20 displayed characteristics of self-inhibiting agonists, as did 10, 12, and 13, but 10, 12 and 13 had 10- 20-fold lower potency than AMOP-H-OH. 11 was a full agonist but had EC50 values of ~1.9 µM, and 26 was inactive at α4β4-nAChRs (Fig. 2; Table 1; Supplementary Data Table 1).
Figure 3.
Specific 86Rb+ efflux (ordinate; percentage of control) was determined for human α4β4-nAChRs expressed by SH-EP1-hα4β4 cells in the presence of ligand alone (graphs on the left; normalized to responses to 1 mM carbamylcholine) or in the presence of an ~EC90 concentration of carbamylcholine (200 µM; graphs on the right) as described in Materials and Methods. Symbol key: 11 (◇); 13 (▼); 10 (×); AMOP-H-OH (●); 12 (■); 18 (◆); 20 (△); 16 (▽); 26 (+). Log molar EC50 or log molar IC50 values (± S.E.) and agonist efficacies or residual activity in the presence of inactivating drug (± S.E.) are provided in Table 1. Molar EC50 or IC50 values and Hill coefficients are provided inSupplementary Data Table 1.
Pre- plus co-exposure to any of the ligands tested led to inactivation of human α4β4-nAChR function in response to an ~EC90 concentration of carbamylcholine (Fig. 3, right panels; Table 1; Supplementary Data Table 1). AMOP-H-OH and 16 were the most potent inactivators (IC50 values of 29 and 32 nM, respectively). Rank order inactivation ability of the other ligands was 18 (120 nM IC50) > 20 > 10 ≈ 12 > 13 ≈ 11 (1.2 µM IC50). Inactivation of α4β4-nAChR function was complete for all drugs tested; there was no limit to the degree of inactivation as seen for some ligands acting at α4β2-nAChRs.
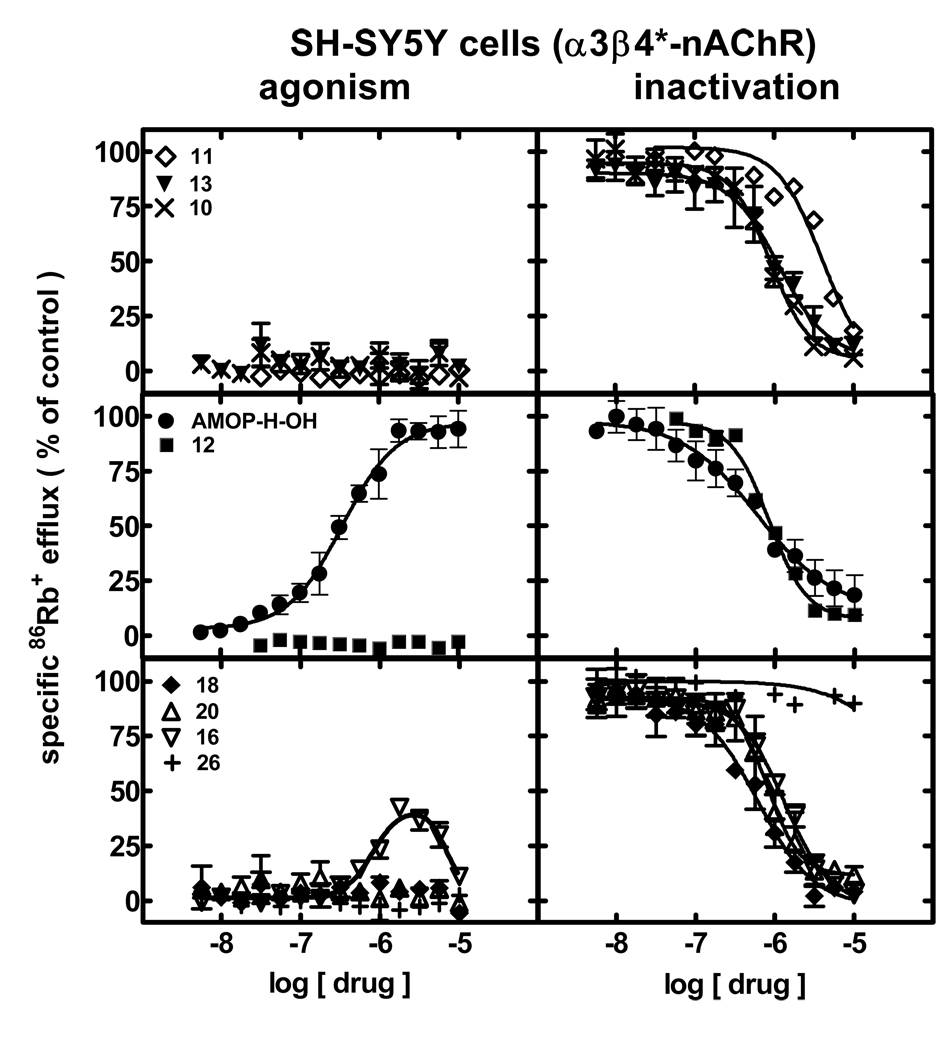
Intrinsic agonist activity assessments for drug interactions with human α3β4*-nAChRs naturally expressed by SH-SY5Y cells (Fig. 4; left panels; Table 1;Supplementary Data Table 1) revealed that only AMOP-H-OH was a full agonist (340 nM EC50). 16 was a self-inhibitory agonist only stimulating a peak response less than 40% of the peak response to AMOP-H-OH. None of the other compounds induced α3β4*-nAChR function.
Figure 4.
Specific 86Rb+ efflux (ordinate; percentage of control) was determined for human α3β4*-nAChRs expressed by SH-SY5Y cells in the presence of ligand alone (graphs on the left; normalized to responses to 3 mM carbamylcholine) or in the presence of an ~EC90 concentration of carbamylcholine (2 mM; graphs on the right) as described in Materials and Methods. Symbol key: 11 (◇); 13 (▼); 10 (×); AMOP-H-OH (●); 12 (■); 18 (◆); 20 (△); 16 (▽); 26 (+). Log molar EC50 or log molar IC50 values (± S.E.) and agonist efficacies or residual activity in the presence of inactivating drug (± S.E.) are provided in Table 1. Molar EC50 or IC50 values and Hill coefficients are provided inSupplementary Data Table 1.
When abilities of drugs to inactivate human α3β4*-nAChR function were ascertained (Fig. 4, right panels; Table 1;Supplementary Data Table 1), AMOP-H-OH produced concentration-dependent, full inactivation (IC50 of 600 nM) of responses to an ~EC90 concentration of carbamylcholine. The ligand showing self-inhibitory agonism, 16, also produced full inactivation of α3β4*-nAChR responses (1.3 µM IC50). 26 was only weakly antagonistic. However, the other compounds (18, 20, 10, 12, 13) all showed comparable ability to fully inhibit function of α3β4*-nAChRs and had IC50 values of ~1 µM (4.1 µM IC50 for 11) like those obtained for AMOP-H-OH or 16.
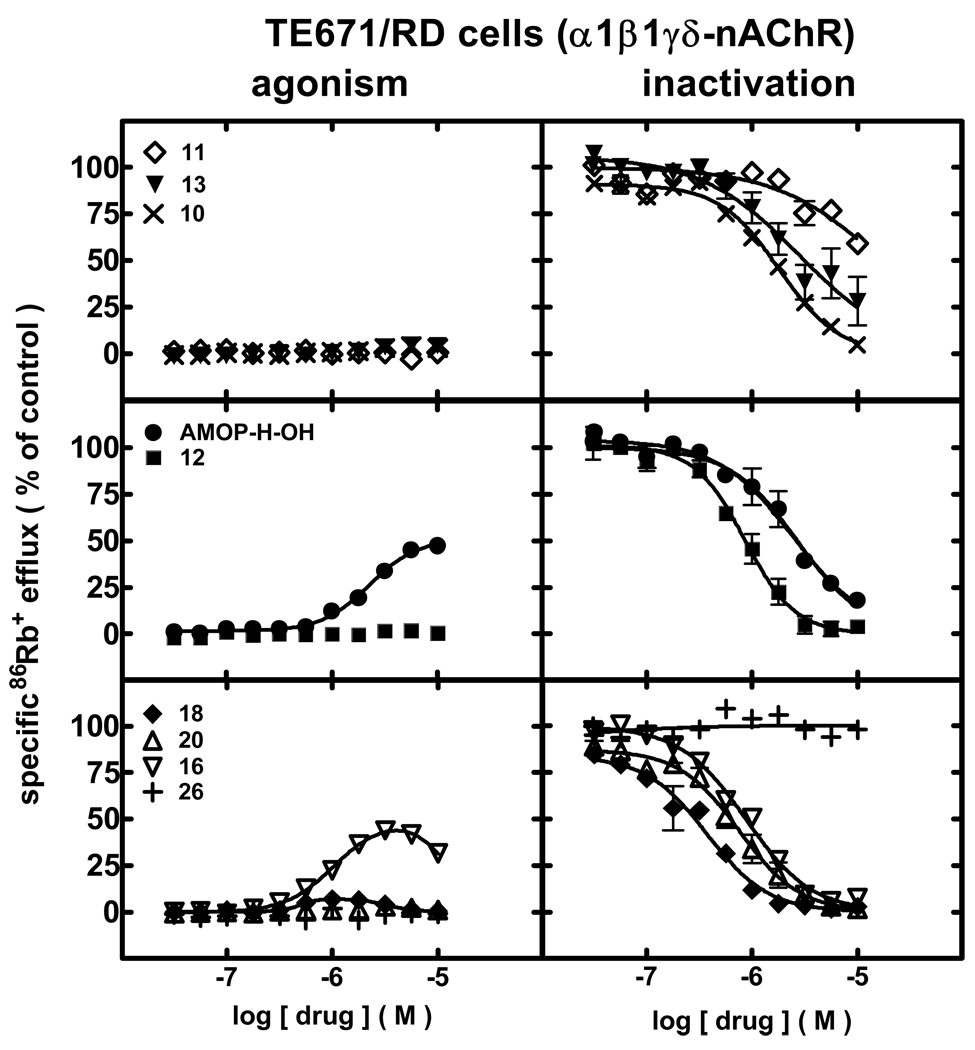
AMOP-H-OH acted as a partial agonist (EC50 value = 2.2 µM; 51% efficacy) at human muscle-type nAChRs naturally expressed by TE671/RD cells (Fig 5, left panels; Table 1; Supplementary Data Table 1). 16 also showed evidence of being a partial agonist (1 µM EC50; ~50% efficacy) but with self-inhibitory activity. None of the other compounds induced α1*-nAChR function.
Figure 5.
Specific86Rb+ efflux (ordinate; percentage of control) was determined for human α1β1γδ-nAChRs expressed by TE671/RD cells in the presence of ligand alone (graphs on the left; normalized to responses to 1 mM carbamylcholine) or in the presence of an ~EC90 concentration of carbamylcholine (464 µM; graphs on the right) as described in Materials and Methods. Symbol key: 11 (◇); 13 (▼); 10 (×); AMOP-H-OH (●); 12 (■); 18 (◆); 20 (△); 16 (▽); 26 (+). Log molar EC50 or log molar IC50 values (± S.E.) and agonist efficacies or residual activity in the presence of inactivating drug (± S.E.) are provided in Table 1. Molar EC50 or IC50 values and Hill coefficients are provided in Supplementary Data Table 1.
Independent of their expression or lack thereof of intrinsic partial agonist activity, pre- plus co-exposure to 18 (370 nM IC50) > 20 ≈ 12 ≈ 16 > 10 > AMOP-H-OH ≈ 13 (2.8 µM IC50) produced concentration-dependent inactivation of human α1*-nAChR function (Fig 5, left panels; Table 1;Supplementary Data Table 1). Inhibition also was evident, although less pronounced, after exposure to 11. 26 did not show appreciable ability to inactivate muscle-type nAChR.
Behavior
Mouse Forced Swim Test
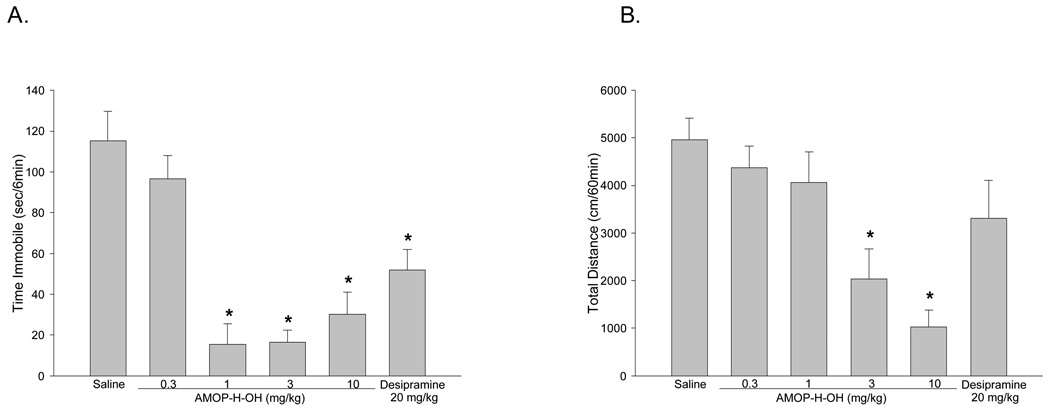
Antidepressant efficacy was assesed with the mouse forced swim test, an assay in which mice are place into a beaker of water and the time the mouse spends passively floating in the water (immobility) is recorded. Most traditional antidepressants decrease the amount of time the mouse spends immobile.[25] Mice were administered AMOP-H-OH (0.3, 1, 3, or 10 mg/kg) or the tricyclic antidepressant desipramine (20 mg/kg), 30 min before forced swim testing. AMOP-H-OH at 1, 3, and 10 mg/kg reduced immobility, suggestive of an antidepressant-like effect. The lowest dose of AMOP-H-OH (0.3mg/kg) was inactive and none of the active doses of AMOP-H-OH were significantly different from each other. Desipramine produced the expected decrease in immobility (Figure 6A).
Figure 6.
AMOP-H-OH dose dependently reduced in immobility in the forced swim test (F(4,45) = 19.1, p < 0.0001). Post hoc Tukey tests showed that AMOP-H-OH produced significant reductions in immobility at 1, 3, and 10 mg/kg, whereas the 0.3 mg/kg dose was inactive. The tricyclic antidepressant, desipramine (20 mg/kg) also significantly reduced immobility (F1,17)=12.3, p < 0.01). AMOP-H-OH reduced locomotor activty (F(4,45)=10.4, p < 0.0001), but only at the highest doses (3 and 10 mg/kg). Post-hoc Tukey tests found no difference between vehicle and the 1 mg/kg dose of AMOP-H-OH, demonstrating that the antidepressant-like effect of AMOP-H-OH was not due to alterations in locomotor activity. Desipramine (20 mg/kg) did not significantly alter locomotor activity. (* significantly different from saline vehicle; n = 9–10/group)
Mouse Locomotor Activity Test
Psychostimulants have been shown to be false positives in the mouse forced swim test.[25] To determine whether the antidepressant-like response of AMOP-H-OH could have resulted from increased locomotor activity, mice were adminstered AMOP-H-OH (0.3, 1, 3, or 10 mg/kg) and 30 min later were tested for locomotor activity for 60 min. Only the highest doses of AMOP-H-OH (3 and 10 mg/kg) produced reductions in locomotor activty. AMOP-H-OH at 1 mg/kg, a dose that was active in forced swim, did not alter locomotor activity (Figure 6B). These results demonstrate that the antidepressant-like activity of AMOP-H-OH in forced swim could not be attributed to alterations in locomotor activity.
Discussion
Based upon the starting structure of AMOP-H-OH, we chose to look at other variations to the terminal substituent of the hexynol appendage in which the hydroxyl group was replaced by thiol or thiolester, together with changes in the length of the side chain appendage.[18] The acetylene group was saturated as in the case of 20. Also, effects of replacement of the azetidine ring by the 5-membered pyrrolidine ring were assessed. While a host of other chemical changes to the starting AMOP-H-OH structure can be imagined, these early-stage modifications provide the guideposts needed to define future studies aimed at generating druggable ligands.
AMOP-H-OH has potent, antidepressant-like activity in the forced swim test, underscoring possible roles of nAChRs in mood and implicating nAChRs as potential molecular targets for novel antidepressant medications. The current results concerning pharmacological fingerprinting of AMOP-H-OH action at several, human nAChR subtypes further illuminates possible molecular bases for AMOP-H-OH’s promising behavioral effects, including previously reported analgesic behaviors[26] and characterizes sites and modes of action of novel AMOP-H-OH analogs.
With regard to intrinsic activity, virtually all of the ligands tested, including AMOP-H-OH, have highest potency (EC50 values ≤ 100 nM or less) at human α4β2-nAChRs. The only exception is 12 (380 nM EC50), which has only 4% efficacy at α4β2-nAChR and is about equipotent (~380 nM EC50) as a self-inhibiting agonist at α4β4-nAChRs or in that low efficacy action at α4β2-nAChR. AMOP-H-OH is selective for α4β2-nAChRs by ~4-fold over α4β4-nAChRs, which are thought to have limited expression in rodents and primates.[15] AMOP-H-OH also is selective for α4β2-nAChRs by > 50-fold over α3β4*-nAChRs, which would be expected to mediate effects of drugs on autonomic functions, and by > 350-fold over muscle-type (α1*-) nAChRs. This profile suggests that AMOP-H-OH is not likely to have peripheral side effects on autonomic or muscle function and places it among the most selective ligands identified for actions at α4β2-nAChRs. AMOP-H-OH also has highest potency of all the agents tested at α4β2-, α4β4-, and α3β4*-nAChRs. All of the other ligands tested (except for 12 and inactive 26) show > 4-fold selectivity for α4β2-nAChRs over α4β4-nAChRs, and all of the ligands tested (except for inactive 26) show a minimum of 80-fold selectivity for α4β2-nAChRs over α3β4*- or α1*-nAChRs, except for 18, which has the highest absolute potency of all ligands at the latter, muscle-type nAChRs. Although the present results do not address ligand interactions with α7-nAChRs, it is clear that the series of compounds is very promising for selectivity of action at α4β2-nAChRs and for behavioral consequences that would be dominated by central actions with a wide peripheral side-effect safety margin.
The observation that AMOP-H-OH acts as a true (no evidence of self inhibition) partial agonist at human α4β2-nAChRs is particularly germane because 16, 13 and 10 also act as true partial agonists at α4β2-nAChRs. 18 and 20 also have sub-100% efficacy at α4β2-nAChRs, but display self inhibition at higher concentrations, and thus do not behave exclusively as true partial agonists. In a previous report, it was suggested that AMOP-H-OH desensitized α4β2-nAChRs without activating them, leading to application of the term “silent desensitization”.[20] This is in apparent conflict with the current finding, especially given the use by Xiao et al. and in this report of the same cell line, SH-EP1-hα4β2.[15] However, we noted that the apparent potency of other nicotinic agonists studied along with AMOP-H-OH by Xiao et al. was lower than we initially reported for SH-EP1-hα4β2 cells [15] and for the cells as examined in this study. From time-to-time, we also have noted that apparent potencies of nicotinic agonists in these cells can become lower (Eaton and Lukas, unpublished observations). However, further insight into these observations comes from studies where ratios of nAChR α4 and β2 subunits are manipulated during heterologous expression in Xenopus oocytes to create cells that express a predominance of α4β2-nAChR with α4:β2 subunit ratios presumably of 3:2 or 2:3.[15] (α4)3(β2)2-nAChRs have lower sensitivity to nicotinic agonists than do (α4)2(β2)3-nAChRs, and the low sensitivity form when expressed in oocytes has much less responsiveness to AMOP-H-OH than the high sensitivity form.[15] Our interpretation of the results is that the studies by Xiao et al. also demonstrated low functional responses to AMOP-H-OH in SH-EP1-hα4β2 cells that were expressing the low sensitivity form of α4β2-nAChRs. However, SH-EP1-hα4β2 cells in the more stable form expressing high sensitivity, presumably (α4)2(β2)3-nAChRs and as studied in work reported here have a higher level of responsiveness to AMOP-H-OH. Relative to responses to acetylcholine, AMOP-H-OH showed nearly full efficacy when acting at high sensitivity, (α4)2(β2)3-nAChRs expressed in oocytes,[15] in contrast to the partial agonist effects observed in our studies of SH-EP1-hα4β2 cells, so further studies are needed to determine factors that influence levels of efficacy, such as subunit assembly partners and the presence of admixtures of high and low sensitivity α4β2-nAChRs in each expression system. Nevertheless, the results are very intriguing, because AMOP-H-OH and its analogs are not only selective for α4β2-nAChRs over other nAChR subtypes, they also seem to be selective for high over low sensitivity forms of α4β2-nAChRs.
Conclusion
Studies examining effects of pretreatment with AMOP-H-OH and analogs on human nAChR function revealed that pre-exposure to many of the agents tested produced ligand concentration-dependent inhibition of responses of each of the nAChR subtypes. All of the ligands tested except for 11 and 12 and inactive 26 have sub-µM IC50 values in these assays at α4β2-nAChRs. For actions at α4β2-nAChRs, IC50 values are comparable to EC50 values for intrinsic activity as full, self-inhibiting, or partial agonists for AMOP-H-OH and 16, although ligands such as 18, 13, 20, 10, 11 and 12 have lower inhibitory “potency” than intrinsic agonist potency when acting at α4β2-nAChRs. AMOP-H-OH and 16 show ~5-fold selectivity as inactivators at α4β2-nAChRs over α4β4-nAChRs and even more selectivity over α3β4*- and α1*-nAChR. However, the other ligands have lower or comparable IC50 values for inhibition of α4β4-nAChR function than for inhibition of α4β2-nAChR function. Inactivation would appear to be due to true, competitive antagonism for drugs that have no or negligible intrinsic activity, such as 18, 13, 20, 10, 11, or 12 acting at α3β4*-nAChR or 13, 20, 10, 11, or 12 acting at α1*-nAChR. It is likely that inactivation reflects desensitization of nAChR function (e.g., AMOP-H-OH and 16 across all nAChR subtypes tested; 18, 13, 20, 10, 11, or 12 at α4β2- and α4β4-nAChRs), which warrants further examination of the effects of longer term exposure of AMOP-H-OH and its analogs.
Interestingly, for drugs acting as partial agonists, but not for drugs acting as self-inhibiting or full agonists, at human α4β2-nAChRs, the extent of inhibition of function in response to a challenge with a full agonist was limited and did not reach 100% (i.e., did not fall to 0% of specific ion flux relative to that in control samples). Partial agonism is largely if not exclusively a phenomenon occurring at α4β2-nAChRs. In the only other case where an agent (AMOP-H-OH) showed signs of partial agonism at another nAChR subtype (α1*-nAChRs; although self inhibition might be manifest at higher concentrations than tested), there was no evidence for a limit in the degree of inactivation after pre-exposure to the drug. The absolute levels of α4β2-nAChR function achieved are comparable after pre-exposure and in the continuing presence of test drug plus full agonist or on initial exposure at the highest concentrations of the test drug for partial agonists AMOP-H-OH, 16, 13, and 12. There are several possible interpretations of these results that merit continued investigation. One is that responses to the full agonist are completely inhibited by these ligands, which in addition exert persisting, partial agonist activity at α4β2-nAChRs. This may indicate that true partial agonists have binding sites on α4β2-nAChRs that are at least partially distinct from those of full agonists, one site being where they exert partial agonism, and another where they act as competitive inhibitors of full agonist function. Another interpretation consistent with the collective interpretation of the current results and those of Zwart et al. and Xiao et al. already discussed above revolves around the apparent selectivity of AMOP-H-OH for high- as opposed to low-sensitivity forms of α4β2-nAChR, having agonist-like efficacy at high-sensitivity and no intrinsic efficacy at low-sensitivity α4β2-nAChR. However, it is not entirely clear why this would occur when there presumably are two, identical, α4 subunit-β2 subunit interfaces in either receptor form unless the nature of the fifth subunit affects agonist efficacy for some ligands. More work is required to develop a molecular description of true partial agonism at α4β2-nAChRs.
It has been hypothesized that a selective decrease in function of α4β2-nAChRs is a common neurochemical endpoint for chronic exposure to nicotine or successful smoking cessation aids, such as bupropion (or, more accurately, its 2S, 3S-hydroxy metabolite).[15] Our observation that AMOP-H-OH produces a reduction in immobility in the mouse forced swim test supports the hypothesis that activation and/or desensitization of α4β2-nAChRs induces antidepressant-like effects in rodents.[27, 28] By acting at α4β2-nAChRs as partial agonists, to desensitize receptors, and/or to inhibit responses to full agonists, AMOP-H-OH and analogs have promise as aids to smoking cessation and some of its manifestations such as relief from depression.
Experimental Section
Chemistry
Analaytical Techniques and Materials
1H and 13C NMR spectra were obtained with a Bruker Avance spectrometer at 300 and 75 MHz, or a Bruker Avance spectrometer at 400 and 100 MHz, respectively. 1H chemical shifts (δ) were reported in ppm downfield from internal Me4Si. Mass spectra were measured in positive mode electrospray ionization (ESI). The HRMS data were obtained on a Micromass Q-TOF-2TM, ThermoFinnigan LTQFT and Shimadzu LCMSITTOF instruments. Optical rotations were measured with an AUTOPOL IV (Rudolph Research Analytical) instrument. TLC was performed on silica gel 60F254 glass plates; column chromatography was performed using Merck silica gel (230–400 mesh). Analytical HPLC was performed using a Shimadzu LC-10AD system equipped with the following columns: Column 1: ACE 5 AQ C18 UltraInert column (4.6 × 250 mm; 5 µm). Column 2: ACE 3 AQ C18 UltraInert column (4.6 × 100 mm; 3.5 µm). HPLC data were recorded using following methods. Method A: H2O/MeCN (0.1% TFA), 90/10 → 0/100 in 18 min, + 2 min isocratic, flow rate of 1.6 mL/min, λ = 254, 280 nm. Method B: H2O/MeCN (0.1% TFA), 100/0 → 0/100 in 20 min, + 2 min isocratic, flow rate of 1.6 mL/min, λ = 254, 280 nm. Method C: H2O/MeCN (0.1% TFA), 70/30 → 0/100 in 21 min, + 7 min isocratic, flow rate of 1.3 mL/min, λ = 254, 280 nm. Method D: H2O/MeCN (0.1% TFA), 90/10 → 0/100 in 20 min, + 7 min isocratic, flow rate of 1mL/min, λ = 254, 280 nm. Method E: H2O/MeCN (0.1% TFA), 100/0 → 0/100 in 30 min, + 3 min isocratic, flow rate of 1.3 mL/min, λ = 254, 280 nm. Starting materials were obtained from Aldrich, Alfa Aesar, or Acros. Solvents were obtained from Fisher Scientific or Aldrich and were used without further purification unless noted otherwise.
3-Bromo-5-(1-methylpyrrolidin-2-ylmethoxy)pyridine (8)
To a stirred solution of (S)-1-metyl-2-pyrrolidinylmethanol (2.5 g, 21.7 mmol) in anhydrous DMF (40 mL) was added in small portions NaH (60% in mineral oil, 955 mg, 23.8 mmol). The mixture was stirred at room temperature for 30 min, and then 3, 5-dibromopyridine (7.71 g, 32.5 mmol) was added. The mixture was stirred for 40 min at room temperature and then stirred overnight at 50 °C. The reaction mixture was poured into ice-cold water (200 mL) and extracted with EtOAc (100 mL × 3). The organic layers were combined, washed with brine, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by chromatography (silica gel, CH2Cl2/MeOH = 5:1) to give an oil (2 g, 34%). [α]D −42.4 (c 1.8, CHCl3). 1H NMR (CDCl3, 400 MHz) δ 8.28 (d, 1H, J = 2.1 Hz), 8.25 (d, 1H, J = 2.7 Hz), 7.38 (t, 1H, J = 2.4 Hz), 4.00 (dd, 1H, J = 9.3, 5.4 Hz), 3.93 (dd, 1H, J = 9.3, 5.4 Hz), 3.16−3.08 (m, 1H), 2.72−2.63 (m, 1H), 2.48 (s, 3H), 2.32 (td, 1H, J = 9.3, 7.2 Hz), 2.10−1.97 (m, 1H), 1.92−1.66 (m, 3H). 13C NMR (CDCl3, 100 MHz) δ 155.3, 142.7, 136.3, 123.8, 120.2, 71.4, 64.0, 57.7, 41.7, 28.5, 23.1. Anal. Calcd for C11H15BrN2O: C, 48.72; H, 5.58; N, 10.33. Found: C, 48.55; H, 5.64; N, 10.12.
6-[5-(1-Methylpyrrolidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (9)
To a solution of 3-bromo-5-(1-methylpyrrolidin-2-ylmethoxy)pyridine (2.0 g, 7.4 mmol), PPh3 (155 mg, 0.59 mol) and CuI (112 mg, 0.59 mmol) in triethyl amine (25 mL) was added PdCl2(PPh3)2 (207 mg, 0.29 mmol). The mixture was stirred at room temperature for 20 min under argon and then 5-hexyn-1-ol (1.6 mL, 14.8 mmol) was added. The reaction mixture was kept at reflux overnight. The mixture was diluted with water, extracted with EtOAc, washed with brine, dried over anhydrous Na2SO4, and filtered through Celite. The Filtrate was concentrated under reduced pressure and purified by chromatography (silica gel, with CH2Cl2/MeOH = 9:1) to give an oil 9 (2.02 g, 95%). [α]D −35.5 (c 0.81, CHCl3). 1H NMR (CD3OD, 400 MHz) δ 8.28 (d, 1H, J = 2.4 Hz), 8.2 (s, 1H), 7.57 (dd, 1H, J =1.5, 1.0 Hz), 4.50 (dd, 1H, J = 8.0, 3.1 Hz), 4.35 (dd, 1H, J = 6.8, 4.2 Hz), 3.91−3.90 (m, 1H), 3.77−3.73 (m, 1H), 3.61 (t, 2H, J = 6.0 Hz), 3.35 (s, 1H), 3.28−3.21 (m, 1H), 3.07 (s, 3H), 2.517−2.48 (m, 2H), 2.43−2.36 (m, 1H), 2.23−2.03 (m, 3H), 1.74−1.67 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ 154.4, 144.4, 136.84, 123.1, 121.1, 93.4, 77.4, 71.0, 64.0, 62.0, 57.6, 41.7, 31.8, 28.4, 24.8, 22.9, 19.2. MS (ESI) m/z 289 [MH+]. HRMS (ESI) calculated for C17H24N2O2+ [MH+] 289.1910, found 289.1919.
Thioacetic acid (S)-{6-[5-(1-methylpyrrolidin-2-ylmethoxy)pyridin-3-yl]hex-5-ynyl} ester (10)
To a solution of PPh3 (545 mg, 2.08 mmol) in dry THF (17 mL), DIAD (0.53 mL, 2.77 mmol) was added at 0 °C. The mixture was stirred for 30 min until a white solid precipitated. A solution of the alcohol 9 (400 mg, 1.38 mol) in dry THF (4 mL) was added drop wise to the mixture, followed by the addition of AcSH (0.20 mL, 2.77 mol). The mixture was stirred for 1 h at 0 °C, slowly warmed to room temperature, and stirred at room temperature for 3 h. The mixture was diluted with water, extracted with EtOAc, washed with brine and dried. The crude product was further purified by HPLC to get 380 mg (79%) of thioacetate 10. HPLC purity: 8.2 min, 97.7% (column 1, method D). 1H NMR (400 MHz, CDCl3) δ 8.12 (d, 1H, J = 2.7 Hz), 8.07 (d, 1H, J = 1.3 Hz), 7.47 (d, 1H, J = 0.7 Hz), 4.34 (dd, 1H, J = 8.3, 2.8 Hz), 4.18 (dd, 1H, J = 5.9, 5.2 Hz), 3.76−3.74 (m, 1H), 3.62−3.57 (m, 1H), 3.13−3.06 (m, 1H), 2.87 (s, 3H), 2.74 (t, 2H, J = 7.0 Hz), 2.32 (t, 2H, J = 6.6 Hz), 2.27−2.19 (m, 1H), 2.17 (s, 3H), 2.09−2.04 (m, 1H), 1.96−1.86 (m, 2H), 1.59−1.45 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 201.1, 154.3, 142.1, 133.6, 127.0, 122.5, 96.2, 76.0, 67.4, 65.7, 57.1, 40.5, 29.9, 28.3, 27.9, 26.6, 25.9, 22.1, 18.3. MS (ESI) m/z 347 [MH+]. HRMS (ESI) calculated for C19H26N2O2S+ [MH+] 347.1787, found 347.1789.
6-[5-(1-Methylpyrrolidin-2-ylmethoxy)pyridin-3-yl]-hex-5-yne-1-thiol (11)
The S-acyl group was removed by methanolysis in the presence of sodium methoxide under an hydrogen atmosphere. To a mixture of the above thioacetate 10 (50 mg, 0.144 mmol) in dry MeOH (2 mL) at 0 °C under H2 (1 atm) was added NaOMe solution (4 mL, 30 wt %). The reaction mixture was allowed to warm to room temperature and stirred overnight which was then adjusted to PH 7−8. The resulting mixture was extracted with dichloromethane, dried, and separated by using semipreparative HPLC to get the more polar monomer 11 (22 mg) and the less polar dimer (25 mg). HPLC purity: 6.1 min, 99.4% (column 1, method D). 1H NMR (400 MHz, CDCl3) δ 8.42 (s, 1H), 8.19 (d, 1H, J = 2.4 Hz), 8.12 (s, 1H), 6.43 (s, 1H), 4.50 (dd, 1H, J = 8.1, 2.9 Hz), 4.35 (dd, 1H, J = 5.9, 5.2 Hz), 3.82−3.80 (m, 1H), 3.62−3.60 (m, 1H), 3.13−3.10 (m, 1H), 2.89 (s, 3H), 2.70 (t, 2H, J = 5.8 Hz), 2.52 (t, 2H, J = 6.2 Hz), 2.28−2.26 (m, 1H), 2.08−2.07 (m, 1H), 1.98−1.94 (m, 2H), 1.84−1.79 (m, 2H), 1.66−1.63 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 162.8 (d, J = 35.1 Hz), 155.8, 146.2, 137.8, 134.7, 130.5, 125.6, 118.2, 117.7, 114.8, 67.3, 66.4, 57.2, 40.5, 35.9, 29.0, 25.8, 24.8, 24.7, 22.0. MS (ESI) m/z 305.16 [MH+]. HRMS (ESI) calculated for C17H24N2OS+ [MH+] 305.4514, found 305.4518.
Thiobenzoic acid (S)-{6-[5-(1-methylpyrrolidin-2-ylmethoxy)pyridin-3-yl]-hex-5-ynyl} ester (12)
To a solution of PPh3 (690 mg, 2.60 mol) in dry THF (17 mL), DIAD (0.5 mL, 2.60 mmol) was added at 0 °C. The mixture was stirred for 30 min until a white solid precipitated. A solution of the alcohol 9 (500 mg, 1.73 mol) in dry THF (5 mL) was added dropwise to the resulting mixture, followed by the addition of PhSH (0.4 mL, 3.46 mmol). The mixture was stirred for 1 h at 0 °C, slowly warmed to room temperature, and then stirred for 3 h. The mixture was diluted with water, extracted with EtOAc, washed with brine and dried. The crude product was further purified by HPLC to get 602 mg (85%) of thiobenzoate 12. HPLC purity: 12.0 min, 98.7% (column 1, method D). 1H NMR (300 MHz, CDCl3) δ 8.31 (d, 2H, J = 2.5 Hz), 8.02−7.97 (m, 2H), 7.69−7.58 (m, 3H), 4.71 (dd, 1H, J = 8.1, 2.6 Hz), 4.58 (dd, 1H, J = 5.7, 4.9 Hz), 3.96−3.84 (m, 1H), 3.81−3.69 (m, 1H), 3.29−3.16 (m, 3H), 3.01 (s, 3H), 2.52 (t, 2H, J = 6.7 Hz), 2.47−1.89 (m, 4H), 1.88−1.76 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 201.1, 153.2, 143.5, 134.9, 124.4, 121.7, 117.9, 114.09, 94.4, 76.1, 67.1, 66.3, 56.7, 53.9, 49.3, 40.9, 40.1, 27.5, 27.4, 26.7, 21.9, 18.4, 14.7. MS (ESI) m/z 409 [MH+]. HRMS (ESI) calculated for C24H28N2O2S+ [MH+] 409.1946, found 409.1946.
5-(6-Tosyloxy-1-hexynyl)-3-(1-methyl-2(S)-pyrrolidinyl methoxy)pyridine
To a stirred solution of 5-(6-hydroxy-1-hexynyl)-3-(1-methyl-2(S)-pyrrolidinylmethoxy) pyridine 9 (207 mg, 0.72 mmol) in dry CH2Cl2 (10 mL) at 0 °C, DMAP (8.7 mg, 0.07 mmol), Et3N (0.131 mL, 0.93 mmol), and pTsCl (177 mg, 0.93 mmol) were added. The reaction mixture was stirred overnight at room temperature and diluted with CH2Cl2 (50 mL). The organic phase was washed with aqueous NaHCO3, brine, dried over Na2SO4, and concentrated. The residue was purified by chromatography (silica gel, hexane/EtOAc = 2:1) to give tosylate compound as a viscous oil (133 mg, 42%). 1H NMR (CDCl3, 400 MHz) δ 8.21 (d, 1H, J = 2.3 Hz), 8.18 (br s, 1H), 7.80 (d, 2H, J = 8.1 Hz), 7.34 (d, 2H, J = 7.8 Hz), 7.17 (br s,1H), 4.09 (t, 2 H, J = 6.2 Hz), 3.99 (dd, 1H, J = 5.4, 3.8 Hz), 3.92 (dd, 1H, J = 5.4, 3.8 Hz), 3.12 (t, 1H, J = 7.4 Hz), 2.60−2.64 (m, 1H), 2.47 (s, 3H), 2.44−2.40 (m, 6H), 2.32 (dd, 1H, J = 7.4, 9.4 Hz), 2.06−2.01 (m, 1H), 1.85−1.64 (m, 7H). MS (ESI) m/z 443 [MH+].
3-((1-Methylpyrrolidin-2-yl)methoxy)-5-(6-(methylthio)hex-1-ynyl)pyridine (13)
To a solution of above-mentioned tosylated compound (133 mg, 0.30 mmol) in EtOH (7 mL) was added methylmercaptan sodium salt (42.1 mg, 0.60 mmol). The solution was stirred at room temperature for 16 h. The reaction mixture was diluted with AcOEt, washed with water and brine, and dried over MgSO4, and filtered through Celite. The Filtrate was concentrated under reduced pressure and purified by flash chromatography (silica gel, hexane/EtOAc = 2:1) to give 13 (76.5 mg, 85%) as a colorless oil. HPLC purity: 11.4 min, 98.8% (column 1, method D). 1H NMR (300 MHz, CDCl3) δ 8.31 (d, 1H, J = 2.2 Hz), 7.31 (br s, 1H), 4.78 (dd, 1H, J = 7.9, 2.5 Hz), 4.56 (dd, 1H, J = 5.5, 4.7 Hz), 3.88−3.83 (m, 1H), 3.81−3.71 (m, 1H), 3.08−3.06 (m, 1H), 3.02 (s, 3H), 2.72 (t, 2H, J = 6.9 Hz), 2.54 (t, 2H, J = 6.4 Hz), 2.51−2.47 (m, 1H), 2.31−2.27 (m, 3H), 2.21 (s, 3H), 1.88−1.71 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 191.7, 153.3, 143.8, 136.7, 135.1, 133.1, 128.3, 126.7, 124.6, 121.8, 94.3, 67.2, 49.6, 28.4, 28.0, 27.1, 21.9, 18.6. MS (ESI) m/z 319 [MH+]. HRMS (ESI) calculated for C18H26N2OS+ [MH+] 319.1838, found 319.1834.
2-(5-Bromopyridin-3-yloxymethyl)azetidine-1-carboxylic acid tert-butyl ester (14)
To a stirred solution of (S)-1-(tert-butoxycarbonyl)-2-azetidinemethanol (10.0 g, 53.8 mmol), 3-bromo-5-hydroxypyridine (10.0 g, 57.5 mmol), and PPh3 (21.1 g, 80.6 mmol) in THF (250 mL) was slowly added diethyl azodicarboxylate (DEAD, 12.8 mL, 80.6 mmol). The reaction mixture was stirred at room temperature for 48 h, and concentrated in vacuo. The residue was purified by column chromatography (silica gel, hexane/EtOAc = 4:1) to give the title compound 14 as a light yellow oil (15.6 g, 85%). 1H NMR (CDCl3, 400 MHz) δ 8.29 (d, 1H, J = 2.1 Hz), 8.28 (d, 1H, J = 2.7 Hz), 7.43 (t, 1H, J = 2.4 Hz), 4.51 (m, 1H), 4.34 (m, 1H), 4.13 (dd, 1H, J = 10.2, 3.0 Hz), 3.89 (t, 2H, J = 7.5 Hz), 2.42−2.22 (m, 2H), 1.43 (s, 9H). 13C NMR (CDCl3, 75 MHz) δ 156.1, 155.4, 143.1, 136.6, 124.0, 120.3, 79.8, 68.9, 59.8, 47.0, 28.3, 18.9. MS (ESI) m/z 343 [M+H+].
2-[5-(6-Hydroxyhex-1-ynyl)pyridin-3-yloxymethyl]azetidine-1-carboxylic acid tert-butyl ester (15)
Prepared by the same procedure as described for 9 above. [α]D −37.1 (c 1.9, CHCl3). 1H NMR (CDCl3) δ 8.22 (d, 2H, J = 2.1 Hz), 7.24 (t, 1H, J = 2.1 Hz), 4.50 (m, 1H), 4.32 (m, 1H), 4.11 (dd, 1H, J = 10.2, 3.0 Hz), 3.88 (t, 2H, J = 7.5 Hz), 3.75−3.65 (m, 2H), 2.47 (t, 2H, J = 6.9 Hz), 2.41−2.20 (m, 2H), 1.95 (s, 1H), 1.80−1.64 (m, 4H), 1.42 (s, 9H). 13C NMR (CDCl3, 75 MHz) δ 156.1, 154.4, 144.6, 132.6, 131.9, 128.4, 93.6, 79.7, 77.2, 68.6, 61.8, 59.9, 31.7, 28.3, 24.9, 19.1, 18.9. MS (ESI) m/z 361 [M+H+].
2-[5-(6-Acetylsulfanyl-hex-1-ynyl)pyridin-3-yloxymethyl] azetidine-1-carboxylic acid tert-butyl ester
To a stirred solution of compound 15 (500 mg, 1.39 mol) in dry CH2Cl2 (10 mL) at 0 °C, DMAP (17 mg, 0.14 mol), Et3N (0.58 mL, 4.17 mol), and pTsCl (397.5 mg, 2.08 mmol) were added. The reaction mixture was stirred overnight at room temperature, and diluted with CH2Cl2 (50 mL). The organic phase was washed with aqueous NaHCO3, brine, dried over Na2SO4, and concentrated. The residue was purified by chromatography with hexane-EtOAc (2:1) to give tosylate compound as a viscous oil (656.7 mg, 92%).
The above tosylated compound was (656 mg, 1.27 mmol) dissolved in 5 mL of DMF followed by the addition of CsSAc (1.32 g, 6.35 mol) at room temperature and continued stirring for 12 h and diluted with EtOAc, The organic phase was washed with aqueous NaHCO3, brine, dried over Na2SO4, and concentrated. The residue was purified by chromatography (silica gel, hexane/EtOAc = 1:1) to give the titled compound as a viscous oil (464 mg, 87%). 1H NMR (CDCl3, 300 MHz) δ 8.25 (d, 2H, J = 2.1 Hz), 7.29−7.24 (br s, 1H), 4.51−4.46 (m, 1H), 4.39−4.28 (m, 1H), 4.21 (dd, 1H, J = 10.1, 2.9 Hz), 3.89 (t, 2H, J = 7.4 Hz), 2.89 (t, 2H, J = 6.8 Hz), 2.46−2.44 (m, 2H), 2.43 (s, 3H), 2.42−2.40 (m, 1H), 1.82−1.64 (m, 4H), 1.43 (s, 9H). MS (ESI) m/z 419 [M+H+].
(Thioacetic acid S-{6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]-hex-5-ynyl} ester (16)
To a stirred solution of above compound (200 mg, 0.47 mmol) in dry CH2Cl2 (4.0 mL) at 0 °C was added trifluoroacetic acid (1.0 mL). The mixture was stirred at room temperature for 3 h. The above solution was concentrated in vacuo and purified by HPLC to obtain pure 16 (129.7 mg, 85%). HPLC purity: 8.2 min, 96.4% (column 1, method D). 1H NMR (CDCl3, 300 MHz) δ 10.12−9.89 (br s, 1H), 8.35 (d, 2H, J = 2.1 Hz), 7.65−7.61 (br s, 1H), 4.89−4.79 (m, 1H), 4.51−4.42 (m, 2H), 4.22−4.04 (m, 2H), 2.92 (t, 2H, J = 6.9 Hz), 2.51−2.42 (m, 2H), 2.41 (t, J = 7.0 Hz, 3H), 2.31 (s, 3H), 1.86−1.69 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ 196.3, 154.7, 142.4, 133.5, 127.2, 124.6, 96.4, 63.0, 57.7, 41.5, 31.8, 31.3, 28.8, 28.1, 27.6, 21.7, 19.3. MS (ESI) m/z 319 [MH+]. HRMS (ESI) calculated for C17H22N2O2S+ [MH+] 319.1474, found 319.1479.
2-[5-(8-Hydroxyoct-1-ynyl) pyridin-3-yloxymethyl]azetidine-1-carboxylic acid tert-butyl ester (17)
Prepared by the same procedure as described for 9 above. 1H NMR (CDCl3) δ 8.25 (d, 2H, J = 2.1 Hz), 7.32−7.27 (br s, 1H), 4.51 (m, 1H), 4.33 (m, 1H), 4.16 (dd, 1H, J = 10.2, 3.0 Hz), 3.91 (t, 2H, J = 7.5 Hz), 3.79−3.67 (m, 2H), 2.47 (t, 2H, J = 6.9 Hz), 2.45−2.18 (m, 3H), 2.15 (s, 1H), 1.65−1.58 (m, 4H), 1.44 (s, 9H). 13C NMR (CDCl3, 75 MHz) δ 156.2, 155.2, 144.5, 131.4, 132.9, 128.4, 92.7, 79.7, 77.1, 68.0, 61.3, 58.9, 31.7, 28.3, 23.7, 19.6, 19.2, 18.9. 17.3. MS (ESI) m/z 389 [M+H+].
2-[5-(8-Acetylsulfanyloct-1-ynyl)pyridin-3-yloxymethyl] azetidine-1-carboxylic acid tert-butyl ester
This compound was prepared in 86% yield following the procedure described above. 1H NMR (CDCl3, 300 MHz) δ 8.25 (d, 2H, J = 2.1 Hz), 7.28−7.24 (br s, 1H), 4.52−4.45 (m, 1H), 4.38−4.26 (m, 1H), 4.22 (dd, 1H, J = 10.1, 2.9 Hz), 3.89 (t, 2H, J = 7.4 Hz), 2.89 (t, 2H, J = 6.7 Hz), 2.46−2.44 (m, 2H), 2.41 (s, 3H), 2.39−2.36 (m, 2H), 1.78−1.67 (m, 4H), 1.47−1.42 ((m, 2H), 1.43 (s, 9H). MS (ESI) m/z 447.2 [MH+].
Thioacetic acid S-{8-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]oct-7-ynyl} ester (18)
This compound was prepared in 87% yield following the procedure described for 16. HPLC purity: 10.1 min, 95% (column 1, method D). 1H NMR (CDCl3, 300 MHz) δ 10.32−9.88 (br s, 1H), 8.34 (d, 2H, J = 2.1 Hz), 7.65−7.61 (br s, 1H), 4.87−4.79 (m, 1H), 4.51−4.42 (m, 2H), 4.21−4.04 (m, 2H), 2.92 (t, 2H, J = 6.9 Hz), 2.51−2.42 (m, 2H), 2.41 (t, J = 6.9 Hz, 3H), 2.32 (s, 3H), 1.86−1.67 (m, 4H), 1.43−1.32 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ 195.7, 153.4, 143.9, 135.1, 127.3, 124.6, 95.0, 79.8, 66.7, 57.7, 42.6, 33.4, 30.2, 28.9, 28.5, 27.9, 27.4, 20.5, 19.0. MS (ESI) m/z 347 [MH+]. HRMS (ESI) calculated for C19H26N2O2S+ [MH+] 347.1787, found 347.1783.
2-[5-(8-Hydroxyoctyl)pyridin-3-yloxymethyl]azetidine-1-carboxylic Acid tert-Butyl Ester (19)
To a solution of compound 17 (1 g, 11.5 mmol) in anhydrous MeOH (10 mL) was added a catalytic amount of 10% Pd/C and the mixture was stirred at room temperature under a H2 atmosphere for 3 h. Then the catalyst was filtered and washed with EtOAc. The filtrate was concentrated under reduced pressure and purified by column chromatography (silica gel, hexane/EtOAc = 1:1) to afford 19 as a colorless liquid (878 mg, 87%). 1H NMR (CDCl3, 300 MHz) δ 8.13 (d, 1H, J = 2.0 Hz), 8.06 (d, 1H, J = 2.1 Hz), 7.32 (br s, 1H), 4.78−4.67 (m, 1H), 4.54−4.38 (m, 1H), 4.18 (dd, 1H, J = 10.1, 2.8 Hz), 3.93 (t, 2H, J = 7.2 Hz), 3.66 (t, 2H, J = 6.5 Hz), 2.65 (t, 2H, J = 7.1 Hz), 2.41−2.36 (m, 2H), 2.32−2.64 (m, 2H), 1.64−1.51 (m, 4H), 1.58 (s, 9H), 1.44−1.35 (m, 8H). MS (ESI) m/z 393 [MH+].
2-[5-(8-Acetylsulfanyloctyl)pyridin-3-yloxymethyl]azetidine-1-carboxylic acid tert-butyl ester
This compound was prepared in 86% yield following the procedure described above. 1H NMR (CDCl3, 300 MHz) δ 8.18 (d, 1H, J = 2.0 Hz), 8.16 (d, 1H, J = 2.1 Hz), 7.07 (br s, 1H), 4.76−4.67 (m, 1H), 4.44−4.32 (m, 1H), 4.21 (dd, 1H, J = 10.1, 2.8 Hz), 3.91 (t, 2H, J = 7.2 Hz), 2.87 (t, 2H, J = 6.6 Hz), 2.49 (t, 2H, J = 7.1 Hz), 2.38−2.31 (m, 2H), 2.30 (s, 3H), 1.71−1.61 (m, 4H), 1.57 (s, 9H), 1.55−1.43 (m, 8H).
Thioacetic acid S-{8-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]octyl} ester (20)
This compound 20 was prepared in 87% yield following the procedure described for 16. HPLC purity: 12.8 min, 95.2% (column 1, method D).1H NMR (CDCl3, 300 MHz) δ 8.49 (d, 1H, J = 2.1 Hz), 8.27 (d, 1H, J = 2.1 Hz), 7.88 (br s, 1H), 5.08−4.95 (m, 1H), 4.47−4.39 (m, 2H), 4.11−3.97 (m, 2H), 2.96 (t, 2H, J = 6.8 Hz), 2.89−2.78 (m, 4H), 2.33 (s, 3H), 1.66−1.52 (m, 4H), 1.47−1.22 (m, 6H). 13C NMR (CDCl3, 100 MHz) δ 195.8, 156.0, 143.5, 133.8, 130.7, 127.1, 67.5, 57.7, 42.8, 38.8, 32.2, 30.2, 29.8, 29.0, 28.6, 28.4, 27.9, 27.8, 20.1. MS (ESI) m/z 351 [MH+]. HRMS (ESI) calculated for C19H30N2O2S+ [MH+] 351.2100, found 351.2091.
3-(4-Methoxybenzylthio)-5-bromopyridine (22)
To a solution of 4-methoxy-α-toluenethiol (2.50 mL, 18.00 mmol) in 60 mL of anhydrous DMF, Sodium hydride was added (60%, 740 mg, 18.50 mmol) at 0 °C. The mixture was stirred for 30 min and then warmed to room temperature for another 1 h. The solution of 3,5-dibromopyridine (4.26 g, 18.00 mmol) in 60 mL of anhydrous DMF was added to the mixture. The resulting mixture was continually stirred for 8 h and then partitioned between Et2O (200 mL) and H2O (100 mL). The organic layer was washed with H2O (80 mL), brine (80 mL), dried over Na2SO4, and evaporated at reduced pressure to afford the crude product. The residue was purified with chromatography (silica gel, hexane/EtOAc = 20:1) to obtain 22 (3.86 g, 69% yield). 1H NMR (CDCl3, 400 MHz) δ 8.64 (s, 1H), 8.59 (s, 1H), 7.62 (s, 1H), 7.25 (d, J = 8.0 Hz, 2H), 7.13 (d, J = 8.0 Hz, 2H), 4.00 (s, 2H), 3.68 (s, 3H). 13C NMR (CDCl3, 100 MHz) δ 158.7, 147.9, 147.8, 138.6, 135.0, 129.7, 127.5, 120.2, 113.8, 54.9, 37.7.
(S)-tert-Butyl 2-((5-bromopyridin-3-ylthio)methyl)azetidine-1-carboxylate (24)
Compound 22 (1.67 g, 5.38 mmol) was added to the mixture of m-cresol (6.20 mL) and TFA (5.0 mL). The resulting mixture was heated to reflux for 24 h. After then, the mixture was concentrated at reduced pressure and purified with short Silica gel column to afford crude 23, which was used for the following reaction without further purification.
Potassium carbonate (1.16 g, 8.43mmol) was added to a solution of 23 (541 mg, 2.85 mmol) in anhydrous DMF (8 mL) at 0 °C. The resulting mixture was stirred for 1 h and then added dropwise with the solution of 2-(toluene-4-sulfonyloxymethyl)azetidine-1-carboxylic acid tert-butyl ester (885 mg, 2.59 mmol) in 5 mL of anhydrous DMF. The resulting mixture was stirred overnight, poured into ice water, extracted with EtOAc (100 mL), washed with H2O (50 mL), brine (50 mL), dried over Na2SO4 and filtered through Celite. The Filtrate was concentrated under reduced pressure and purified by flash chromatography (silica gel, hexane/EtOAc = 10:1) to afford 24 (508 mg, 50% yield). 1H NMR (CDCl3, 400 MHz) δ 8.49 (d, J = 1.6 Hz, 1H), 8.46 (J = 1.6 Hz, 1H), 7.84 (s, 1H), 4.38 (br s, 1H), 3.85 −3.79 (m, 2H), 3.45 (dd, J = 3.2, 13.6 Hz, 1H), 3.17 (br s, 1H), 2.34 −2.30 (m, 1H), 2.04 −1.99 (m, 1H), 1.44 (s, 9H).
(S)-tert-Butyl-(((5-(6-hydroxyhex-1-ynyl)pyridin-3-yl)sulfanyl) methyl)azetidine-1-carboxylate (25)
Prepared by the same procedure as described for 9 above. 1H NMR (CDCl3, 400 MHz) δ 8.45 (d, J = 1.6 Hz, 1H), 8.40 (d, J = 1.6 Hz, 1H), 7.68 (s, 1H), 4.35 (br s, 1H), 3.84−3.79 (m, 2H), 3.71 (t, J = 6.0 Hz, 2H), 3.45 (dd, J = 3.2, 13.6 Hz, 2H), 3.15 (br s, 1H), 2.47 (t, J = 6.0 Hz, 2H), 2.31−2.29 (m, 1H), 2.19 (s, 1H), 2.08−1.99 (m, 1H), 1.73−1.69 (m, 4H), 1.43 (s, 9H). 13C NMR (CDCl3, 100 MHz) δ 156.1, 149.5, 148.2, 138.7, 132.9, 121.3, 94.4, 77.2, 62.2, 60.1, 37.8, 31.9, 28.4, 21.5, 19.3. MS (ESI) m/z: 377 [M + H]+.
6-(5-(((S)-Azetidin-2-yl)methylthio)pyridin-3-yl)hex-5-yn-1-ol (26)
Compound 25 (130 mg, 0.0345 mmol) was dissolved in 4 mL of HCl/dioxane solution (4 M) at 0 °C. The resulting mixture was stirred for 4 h and concentrated at reduced pressure to afford 26 (60 mg, 50% yield). 1H NMR (CDCl3, 400 MHz) δ 8.49 (s, 2H), 7.68 (s, 1H), 4.31 (s, 1H), 3.72 (t, J = 6.0 Hz, 2H), 2.49 (t, J = 6.0 Hz, 2H), 2.37−2.29 (m, 3H), 1.78−1.71 (m, 5H), 1.43 (t, J = 8.0 Hz, 2H). 13C NMR (D2O, 100 MHz) δ 145.4, 144.5, 143.8, 117.9, 115.0, 98.4, 75.1, 61.2, 58.5, 42.7, 35.1, 30.6, 24.1, 23.9, 18.5. MS (ESI) m/z: 277. HRMS (ESI) calculated for C15H21N2OS+ [MH+] 277.1369, found 277.1374. HPLC purity: 97.8% (column 1, method E).
In vitro studies
Cell lines and culture
Cell lines naturally or heterologously expressing specific, functional, human nAChR subtypes were used. The human clonal cell line TE671/RD naturally expresses human muscle-type, α1*-nAChRs containing α1, β1, γ, and δ subunits with function detectable using 86Rb+ flux assays.[29] The human neuroblastoma cell line SH-SY5Y naturally expresses autonomic α3β4*-nAChRs containing α3, β4, probably α5, and sometimes β2 subunits that also display function detectable using 86Rb+ flux assays.[30] SH-SY5Y cells also express homopentameric α7-nAChR, but these receptors do not contribute detectably to 86Rb+ flux functional responses under the conditions used. Whole-cell current recording-based studies of drug interactions with α7-nAChR will be reported elsewhere. SH-EP1 human epithelial cells stably transfected with human α4 and β2 subunits (SH-EP1-hα4β2 cells) or with human α4 and β4 subunits (SH-EP1-hα4β4 cells) also have been established and characterized.[31, 32] Both α4β2-nAChRs and α4β4-nAChRs show functional responses in ion flux assays.
TE671/RD, SH-SY5Y, or transfected SH-EP1 cell lines were maintained as low passage number (1–26 from our frozen stocks) cultures to ensure stable expression of native or heterologously-expressed nAChR as previously described.[29] Cells were passaged once weekly by splitting just-confluent cultures 1/300 (TE671/RD), 1/5 (SH-SY5Y), or 1/20 (transfected SH-EP1) in serum-supplemented medium to maintain log-phase growth.
86Rb+ efflux assays
Function of nAChR subtypes investigated was established based on a proven, routine, 86Rb+ efflux assay protocol.[29] The assay is specific for nAChR function under the conditions used, for example, giving identical results in the presence of 100 nM atropine to exclude possible contributions of muscarinic acetylcholine receptors Cells harvested at confluence from 100-mm plates under a stream of fresh medium only (SH-SY5Y) or after mild trypsinization (Irvine Scientific, Santa Ana, CA; for TE671/RD or transfected SH-EP1 cells) were then suspended in complete medium and evenly seeded at a density of 1.25 to 2 confluent 100-mm plates per 24-well plate (Falcon; ~ 100–125 µg of total cell protein per well in a 500 µL volume; poly-L-lysine-coated for SH-SY5Y cells). After cells had adhered (generally overnight, but no sooner than 4 h later), the medium was removed and replaced with 250 µL per well of complete medium supplemented with ~350,000 cpm of 86Rb+ (NEN; counted at 40% efficiency using Cerenkov counting and the Packard TriCarb 1900 Liquid Scintillation Analyzer). After at least 4 h and typically overnight, 86Rb+ efflux was measured using the “flip-plate” technique.[29] Briefly, after aspiration of the bulk of 86Rb+ loading medium from each well of the “cell plate,” each well containing cells was rinsed with 2 mL of fresh 86Rb+ efflux buffer (130 mM NaCl, 5.4 mM KCl, 2 mM CaCl2, 5 mM glucose, 50 mM HEPES, pH 7.4) to remove extracellular 86Rb+. Following removal of residual rinse buffer by aspiration, the flip-plate technique was used again to simultaneously introduce 1.5 mL of fresh efflux buffer containing drugs of choice at indicated final concentrations from a 24-well “efflux/drug plate” into the wells of the cell plate. After a 10-min incubation, the solution was “flipped” back into the efflux/drug plate, and any remaining buffer in the cell plate was removed by aspiration. A second efflux/drug plate was then used to reintroduce the same concentrations of drugs of choice with the addition of an ~EC90 concentration of the full agonist carbamylcholine for 5 min (~EC90 concentrations were 200 µM for SH-EP1-hα4β2 and SH-EP1-hα4β4 cells, 2 mM for SH-SY5Y cells, and 464 µM for TE671/RD cells). The second drug treatment was then flipped back into its drug plate, and the remaining cells in the cell plate were lysed and suspended by addition of 1.5 mL of 0.1 M NaOH, 0.1% sodium dodecyl sulfate to each well. Suspensions in each well were then subjected to Cerenkov counting (Wallac Micobeta Trilux 1450; 25% efficiency) after placement of inserts (Wallac 1450-109) into each well to minimize cross-talk between wells.
For quality control and normalization purposes, the sum of 86Rb+ in cell plates and efflux/drug plates was defined to confirm material balance (i.e., that the sum of 86Rb+ released into the efflux/drug plates and 86Rb+ remaining in the cell plate were the same for each well). Similarly, the sum of 86Rb+ in cell plates and efflux/drug plates also determined the efficiency of 86Rb+ loading (the percentage of applied 86Rb+ actually loaded into cells). Furthermore, the sum of 86Rb+ in cell plates and the second efflux/drug plates defined the amount of intracellular 86Rb+ available at the start of the second, 5-min assay and was used to normalize nAChR function thus assessed.
For each experiment, in one set of control samples, total 86Rb+ efflux was assessed in the presence of only a fully efficacious concentration of carbamylcholine (1 mM for SH-EP1-hα4β2 or -hα4β4 cells, or for TE671/RD cells, or 3 mM for SH-SY5Y cells). Non-specific 86Rb+ efflux in another set of control samples was measured either in the presence of the fully efficacious concentration of carbamylcholine plus 100 µM mecamylamine, which gave full block of agonist-induced and spontaneous nAChR-mediated ion flux, or in the presence of efflux buffer alone. Both determinations of non-specific efflux were equivalent. Specific efflux was then taken as the difference in control samples between total and non-specific 86Rb+ efflux. The same approaches were used to define total, non-specific, and specific ion flux responses in samples subjected to the second, 5-min exposure to test drug when applicable plus either carbamylcholine at its EC90 concentration or efflux buffer. Intrinsic agonist activity of test drugs was ascertained during the initial, 10-min exposure period using samples containing test drug only at different concentrations and was normalized, after subtraction of non-specific efflux, to specific efflux in test drug-free control samples. Specific 86Rb+ efflux elicited by test drug as a percentage of specific efflux in the absence of test drug was the same in these samples whether measured in absolute terms or as a percentage of loaded 86Rb+. Even in samples previously giving an efflux response during the initial, 10-min exposure to a partial or full agonist, residual intracellular 86Rb+ typically was adequate to allow assessment of nAChR function in the secondary, 5-min assay. However, care was needed to ensure that data were normalized to the amount of intracellular 86Rb+ available at the time of the assay, as absolute levels of total, non-specific, or specific efflux varied in cells depleted of intracellular 86Rb+ due to action of any agonist present during the 10-min drug exposure period. That is, calculations of specific efflux as a percentage of loaded 86Rb+ typically corrected for any variation in the electrochemical gradient of 86Rb+ created by intracellular ion depletion after the first (agonism/pretreatment) drug treatment. The electrochemical gradient variation was largest in SH-EP1-hα4β4 cells after initial exposure to high concentrations of full agonists, but effects on apparent IC50 values are small as judged from measures of apparent IC50 values obtained using other techniques.
Ion flux assays (n = 3 separate studies for each drug and cell line combination) were fit to the Hill equation, F = Fmax / (1 + (X/EC50)n), with F as a percentage of control, Fmax, for EC50 (n > 0 for agonists) or IC50 (n < 0 for antagonists using Prism 4 (GraphPad, San Diego, CA). In some cases, biphasic concentration-ion flux response curves were evident and were fit to a two-phase Hill equation from which EC50 and Hill coefficients for the rising, agonist phase, and IC50 and Hill coefficients for the falling, self-inhibitory phase could be determined (Prism). Most ion flux data were fit allowing maximum and minimum ion flux values to be determined by curve fitting, but in some cases where antagonists or agonists had weak functional potency, minimum ion flux was set at 0% of control or maximum ion flux was set at 100% of control, respectively.
Behavior
Animals
C57BL/6J male mice (9–12 weeks of age at testing) were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were housed 4 to a cage in a colony room maintained at 22 °C on a 12 h light-dark cycle. All animal experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the PsychoGenics Animal Care and Use Committee.
Mouse Forced Swim Test
Procedures were based on those previously described.[25] Mice were individually placed into clear glass cylinders (i.e., 15 cm tall × 10 cm wide, 1 L beakers) containing 23 ± 1 °C water 12 cm deep (approximately 800 mL). Mice were administered AMOP-H-OH or desipramine by intraperitoneal (IP) injection and 30 min later, the time the animal spent immobile was recorded over a 6 min trial. Immobility was defined as the postural position of floating in the water. After testing, mice were dried and returned to their home cage.
Mouse Locomotor Activity Test
General locomotor activity was measured in clear Plexiglas square chambers (27.3 × 27.3 × 20.3 cm; Med Associates Inc., St Albans, VT) surrounded by infrared photobeam sources. Mice were administered AMOP-H-OH or desipramine, and 30 min later, locomotor activity (distance traveled in cm) was measured from beam breaks for 60 min. This measure of total distance traveled was used as an index of activity. At the end of each test session the chambers were thoroughly cleaned.
Drugs
AMOP-H-OH was synthesized as previously reported[20] and desipramine was purchased from a commercial vendor (Sigma, St. Louis, MO). All compounds were dissolved in sterile saline.
Supplementary Material
Figure 1.
Structures are shown for nicotine (1), varenicline (2), epibatidine (3), cytisine (4), A-85380 (5), SIB-1508 (6) and AMOP-H-OH (7).
Acknowledgements
This work was supported by National Institutes of Health grant R01DA017980 (to APK). Other effort was supported by grants (to RJL) from the National Institutes of Health (R01DA015389) and the Barrow Neurological Foundation.
References
- 1.D′Hoedt D, Bertrand D. Expert Opin. Ther. Targets. 2009;13:395. doi: 10.1517/14728220902841045. [DOI] [PubMed] [Google Scholar]
- 2.Buccafusco JJ, Beach JW, Terry AVJ. J. Pharmacol. Exp. Ther. 2009;328:364. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karlin A. Nat. Rev. Neurosci. 2002;3:102. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- 4.Jensen AA, Frφlund B, Liljefors T, Krogsgaard-Larsen P. J. Med. Chem. 2005;48:4705. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- 5.Lugoboni F, Quaglio G, Pajusco B, Mezzelani P, Lechi A. Intern Emerg Med. 2007;2:196. doi: 10.1007/s11739-007-0057-3. [DOI] [PubMed] [Google Scholar]
- 6.Semba J, Mataki C, Yamada S, Nankai M, Toru M. Biol. Psychiatry. 1998;43:389. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- 7.Djuriâc VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M. Physiol. Behav. 1999;67:533. doi: 10.1016/s0031-9384(99)00091-8. [DOI] [PubMed] [Google Scholar]
- 8.Suemaru K, Yasuda K, Cui R, Li B, Umeda K, Amano M, Mitsuhashi H, Takeuchi N, Inoue T, Gomita Y, Araki H. Physiol. Behav. 2006;88:545. doi: 10.1016/j.physbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Jr, Janowsky DS, Kling MA. Psychopharmacology (Berl) 1999;142:193. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- 10.Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J. Pharmacol. Biochem. Behav. 2004;78:165. doi: 10.1016/j.pbb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Lindstrom J. Mol. Neurobiol. 1997;15:193. doi: 10.1007/BF02740634. [DOI] [PubMed] [Google Scholar]
- 12.Paterson D, Nordberg A. Prog. Neurobiol. 2000;61:75. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd GK, Williams M. J. Pharmacol. Exp. Ther. 2000;292:461. [PubMed] [Google Scholar]
- 14.Millar NS. Biochem. Soc. Trans. 2003;31:869. doi: 10.1042/bst0310869. [DOI] [PubMed] [Google Scholar]
- 15.Gotti C, Clementi F. Prog Neurobiol. 2004;74:363. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Holladay MW, Dart MJ, Lynch JK. J. Med. Chem. 1997;40:4169. doi: 10.1021/jm970377o. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd GK, Williams M. J. Pharmacol. Exp. Ther. 2000;292:461. [PubMed] [Google Scholar]
- 18.Wei ZL, Xiao Y, Yuan H, Baydyuk M, Petukhov PA, Musachio JL, Kellar KJ, Kozikowski AP. J. Med. Chem. 2005;48:1721. doi: 10.1021/jm0492406. [DOI] [PubMed] [Google Scholar]
- 19.Chellappan SK, Xiao Y, Tueckmantel W, Kellar KJ, Kozikowski AP. J. Med. Chem. 2006;49:2673. doi: 10.1021/jm051196m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Mol. Pharmacol. 2006;70:1454. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- 21.Abreo MA, Lin NH, Garvey DS, Gunn DE, Hettinger AM, Wasicak JT, Pavlik PA, Martin YC, Donnelly-roberts DL, Anderson DJ, Sullivan JP, Williams M, Arneric SP, Holladay MW. J. Med. Chem. 1996;39:817. doi: 10.1021/jm9506884. [DOI] [PubMed] [Google Scholar]
- 22.Schneider JS, Tinker JP, Menzaghi F, Lloyd GK. J. Pharmacol. Exp. Ther. 2003;306:401. doi: 10.1124/jpet.103.051912. [DOI] [PubMed] [Google Scholar]
- 23.Buckley MJ, Surowy C, Meyer M, Curzon P. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28:723. doi: 10.1016/j.pnpbp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Mukhin AG, Gèundisch D, Horti AG, Koren AO, Tamagnan G, Kimes AS, Chambers J, Vaupel DB, King SL, Picciotto MR, Innis RB, London ED. Mol. Pharmacol. 2000;57:642. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- 25.Porsolt RD, Bertin A, Jalfre M. Arch. Int. Pharmacodyn Ther. 1977;229:327. [PubMed] [Google Scholar]
- 26.Cucchiaro G, Xiao Y, Gonzalez-Sulser A, Kellar KJ. Anesthesiology. 2008;109:512. doi: 10.1097/ALN.0b013e3181834490. [DOI] [PubMed] [Google Scholar]
- 27.Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Mol Psychiatry. 2002;7:525. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 28.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. Prog Neurobiol. 2008;84:329. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukas RJ, Fryer JD, Eaton JB, Gentry CL. e. C. P. in Nicotinic Receptors and the Nervous System (ED Levin, Boca Raton. 2002:3–27. [Google Scholar]
- 30.Lukas RJ, Norman SA, Lucero L. Molec. Cell Neurosci. 1993;4:1. doi: 10.1006/mcne.1993.1001. [DOI] [PubMed] [Google Scholar]
- 31.Eaton JB, Peng JH, Schroeder KM, George AA, Fryer JD, Krishnan C, Buhlman L, Kuo YP, Steinlein O, Lukas RJ. Mol. Pharmacol. 2003;64:1283. doi: 10.1124/mol.64.6.1283. [DOI] [PubMed] [Google Scholar]
- 32.Gentry CL, Lukas RJ. J. Pharmacol. Expe. Ther. 2001;299:1038. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.