Abstract
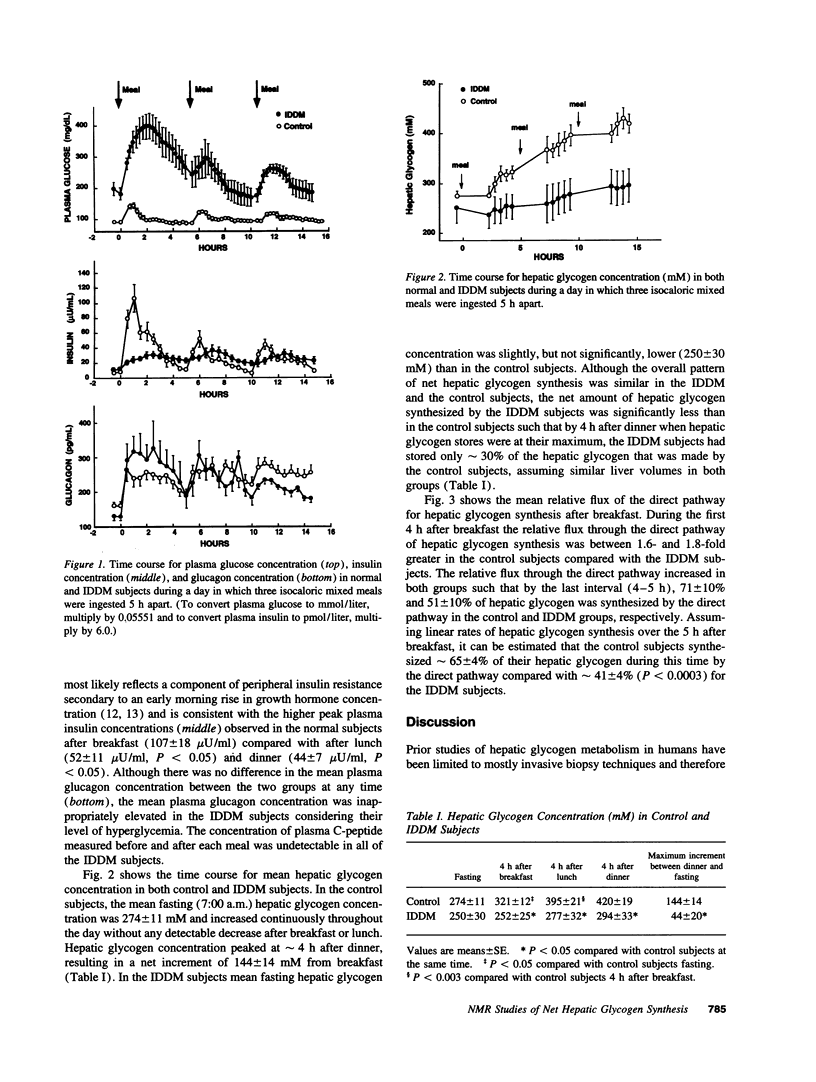
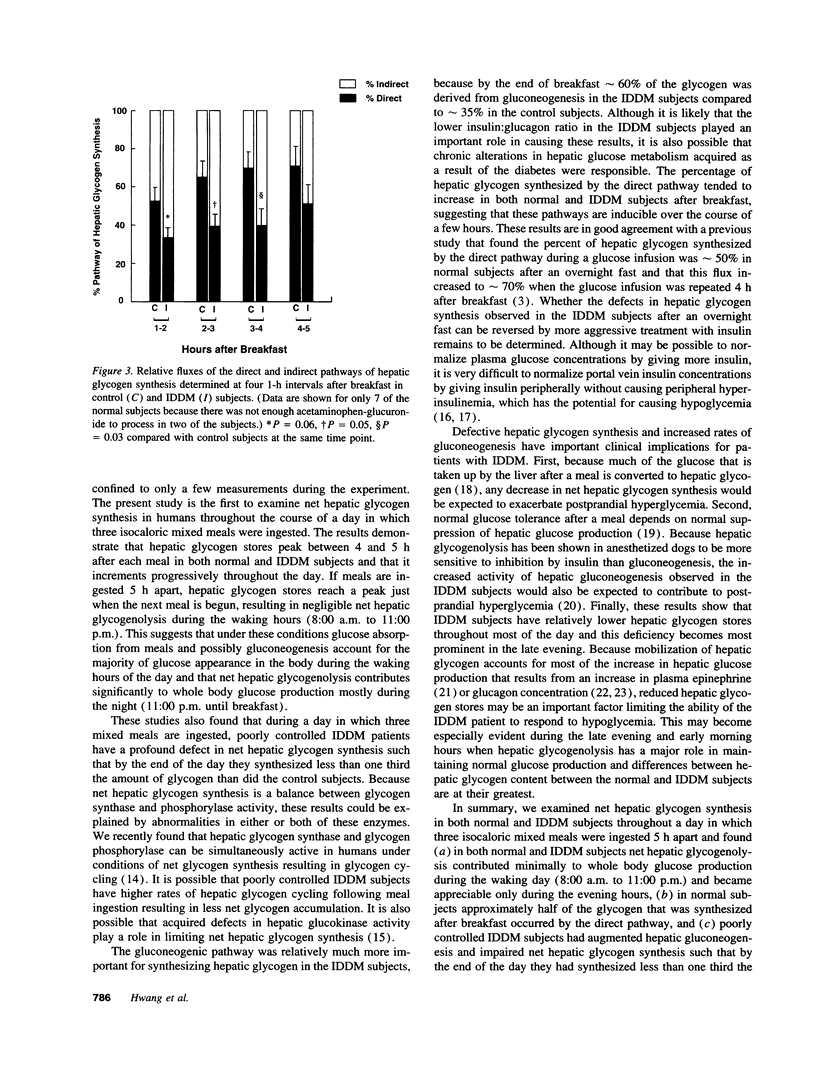
Hepatic glycogen concentration was measured in six subjects with insulin-dependent diabetes mellitus (IDDM) and nine weight-matched control subjects using 13C nuclear magnetic resonance spectroscopy during a day in which three isocaloric mixed meals were ingested. The relative fluxes of the direct and indirect (3 carbon units-->-->glycogen) pathways of hepatic glycogen synthesis were also assessed using [1-13C]glucose in combination with acetaminophen to noninvasively sample the hepatic UDP-glucose pool. Mean fasting hepatic glycogen content was similar in the two groups. After each meal, hepatic glycogen content increased, peaking 4-5 h after the meal in both groups. By 11:00 p.m. the IDDM subjects had synthesized only 30% of the glycogen that was synthesized by the control group [IDDM subjects, net increment = 44 +/- 20 (mean +/- SE) mM; control subjects, net increment = 144 +/- 14 mM; P < 0.05]. After breakfast the flux through the gluconeogenic pathway relative to the direct pathway of hepatic glycogen synthesis was 1.7-fold greater in the IDDM subjects (59 +/- 4%) than in the control subjects (35 +/- 4%, P < 0.0003). In conclusion, under mixed meal conditions, subjects with poorly controlled IDDM have a major defect in net hepatic glycogen synthesis and augmented hepatic gluconeogenesis. The former abnormality may result in an impaired glycemic response to counterregulatory hormones, whereas both abnormalities may contribute to postprandial hyperglycemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzilai N., Rossetti L. Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem. 1993 Nov 25;268(33):25019–25025. [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C., Andrews S. S. Portal and peripheral vein immunoreactive glucagon concentrations after arginine or glucose infusions. Diabetes. 1974 Mar;23(3):199–202. doi: 10.2337/diab.23.3.199. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Shulman G. I., Lacy W. W. Differential time course of glucagon's effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes. 1981 Mar;30(3):180–187. doi: 10.2337/diab.30.3.180. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Finger F. E., Lacy W. W. Differential sensitivity of glycogenolysis and gluconeogenesis to insulin infusions in dogs. Diabetes. 1976 Apr;25(4):283–291. doi: 10.2337/diab.25.4.283. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Binder C., Bolli G. B., Cherrington A. D., Gale E. A., Gerich J. E., Sherwin R. S. Hypoglycemia in IDDM. Diabetes. 1989 Sep;38(9):1193–1199. doi: 10.2337/diab.38.9.1193. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Harris M. D., Ziel F. H., Rosenberg C. S. Suppression of sleep-induced growth hormone secretion by anticholinergic agent abolishes dawn phenomenon. Diabetes. 1988 Feb;37(2):166–171. doi: 10.2337/diab.37.2.166. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Bonadonna R. C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Gerard D. P., Katz L. D., Shulman G. I. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes. 1995 Feb;44(2):185–189. doi: 10.2337/diab.44.2.185. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Jucker B., Cline G. W., Shulman R. G., Shulman G. I. Liver glycogen turnover in fed and fasted humans. Am J Physiol. 1994 May;266(5 Pt 1):E796–E803. doi: 10.1152/ajpendo.1994.266.5.E796. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992 Oct;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. C., Cherrington A. D., Cline G., Pagliassotti M. J., Jones E. M., Neal D. W., Badet C., Shulman G. I. Sources of carbon for hepatic glycogen synthesis in the conscious dog. J Clin Invest. 1991 Aug;88(2):578–587. doi: 10.1172/JCI115342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Rossetti L., Rothman D. L., DeFronzo R. A., Shulman G. I. Effect of dietary protein on in vivo insulin action and liver glycogen repletion. Am J Physiol. 1989 Aug;257(2 Pt 1):E212–E219. doi: 10.1152/ajpendo.1989.257.2.E212. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Magnusson I., Katz L. D., Shulman R. G., Shulman G. I. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991 Oct 25;254(5031):573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- Saccà L., Vigorito C., Cicala M., Corso G., Sherwin R. S. Role of gluconeogenesis in epinephrine-stimulated hepatic glucose production in humans. Am J Physiol. 1983 Sep;245(3):E294–E302. doi: 10.1152/ajpendo.1983.245.3.E294. [DOI] [PubMed] [Google Scholar]
- Shishko P. I., Kovalev P. A., Goncharov V. G., Zajarny I. U. Comparison of peripheral and portal (via the umbilical vein) routes of insulin infusion in IDDM patients. Diabetes. 1992 Sep;41(9):1042–1049. doi: 10.2337/diab.41.9.1042. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Cline G., Schumann W. C., Chandramouli V., Kumaran K., Landau B. R. Quantitative comparison of pathways of hepatic glycogen repletion in fed and fasted humans. Am J Physiol. 1990 Sep;259(3 Pt 1):E335–E341. doi: 10.1152/ajpendo.1990.259.3.E335. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., DeFronzo R. A., Rossetti L. Differential effect of hyperglycemia and hyperinsulinemia on pathways of hepatic glycogen repletion. Am J Physiol. 1991 May;260(5 Pt 1):E731–E735. doi: 10.1152/ajpendo.1991.260.5.E731. [DOI] [PubMed] [Google Scholar]
- Shulman G. I., Rossetti L., Rothman D. L., Blair J. B., Smith D. Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest. 1987 Aug;80(2):387–393. doi: 10.1172/JCI113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. S., Joseph R. I., Chen C. N., Sank V. J., Hoult D. I. Selective population inversion in NMR. Nature. 1984 Aug 23;310(5979):681–683. doi: 10.1038/310681a0. [DOI] [PubMed] [Google Scholar]


