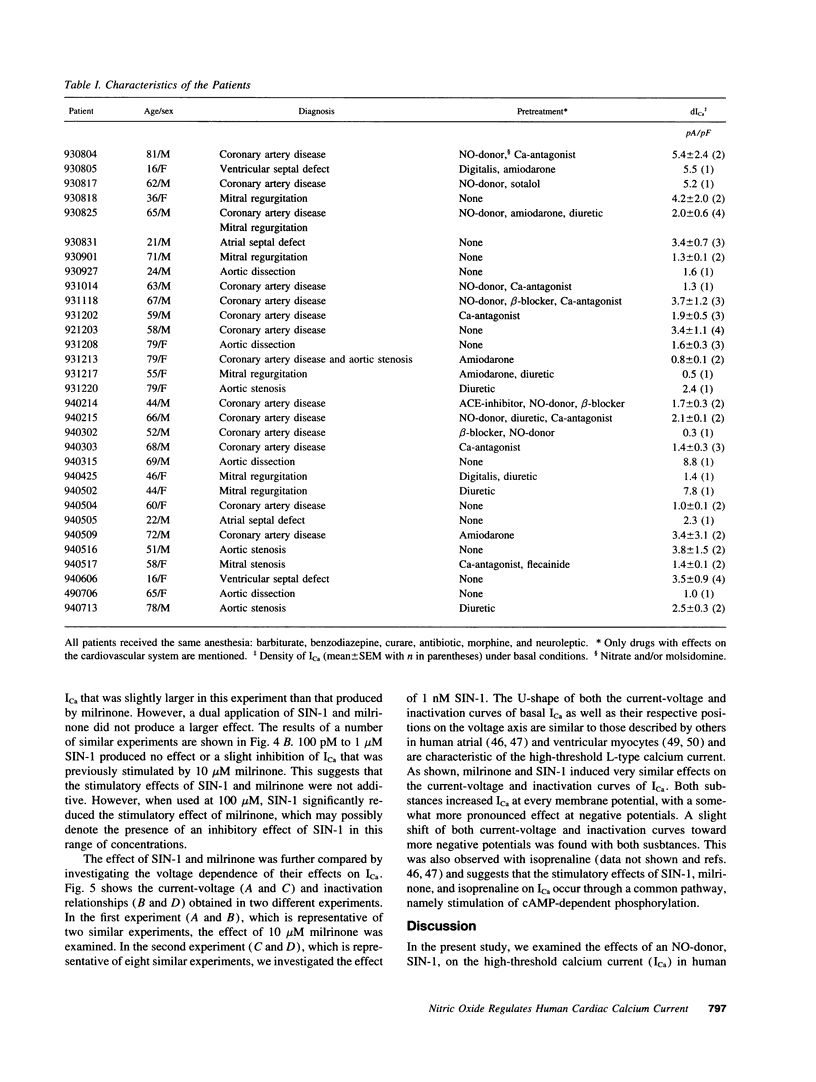
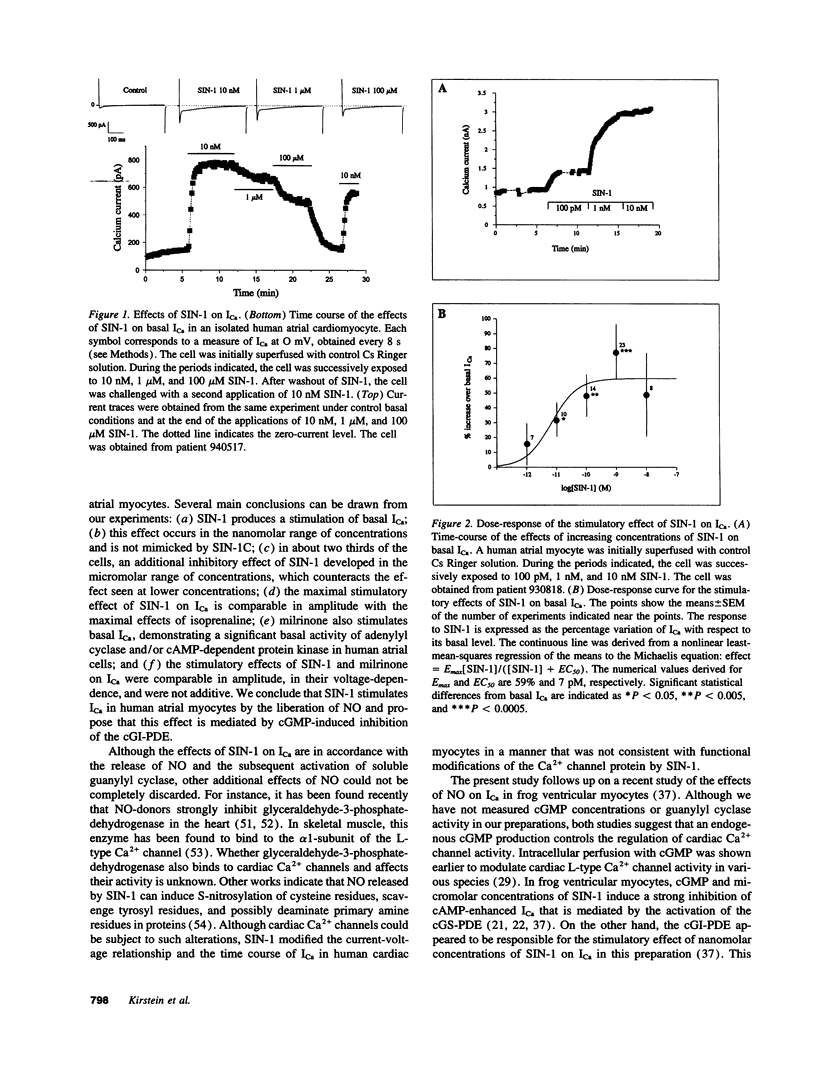
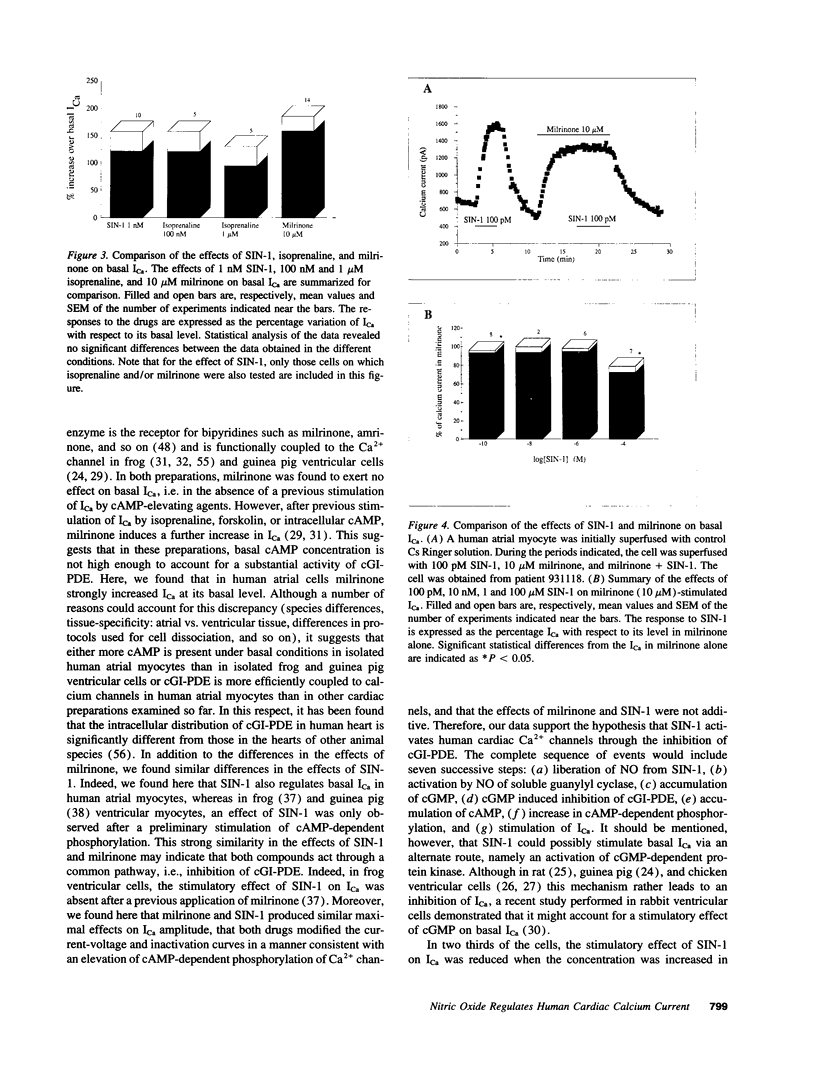
Abstract
Cardiac Ca2+ current (ICa) was shown to be regulated by cGMP in a number of different species. Recently, we found that the NO-donor SIN-1 (3-morpholino-sydnonimine) exerts a dual regulation of ICa in frog ventricular myocytes via an accumulation of cGMP. To examine whether NO also regulates Ca2+ channels in human heart, we investigated the effects of SIN-1 on ICa in isolated human atrial myocytes. An extracellular application of SIN-1 produced a profound stimulatory effect on basal ICa at concentrations > 1 pM. Indeed, 10 pM SIN-1 induced a approximately 35% increase in ICa. The stimulatory effect of SIN-1 was maximal at 1 nM (approximately 2-fold increase in ICa) and was comparable with the effect of a saturating concentration (1 microM) of isoprenaline, a beta-adrenergic agonist. Increasing the concentration of SIN-1 to 1-100 microM reduced the stimulatory effect in two thirds of the cells. The stimulatory effect of SIN-1 was not mimicked by SIN-1C, the cleavage product of SIN-1 produced after liberation of NO. This suggests that NO mediates the effects of SIN-1 on ICa. Because, in frog heart, the stimulatory effect of SIN-1 on ICa was found to be due to cGMP-induced inhibition of cGMP-inhibited phosphodiesterase (cGI-PDE), we compared the effects of SIN-1 and milrinone, a cGI-PDE selective inhibitor, on ICa in human. Milrinone (10 microM) induced a strong stimulation of ICa (approximately 150%), demonstrating that cGI-PDE controls the amplitude of basal ICa in this tissue. In the presence of milrinone, SIN-1 (0.1-1 nM) had no stimulatory effect on ICa, suggesting that the effects of SIN-1 and MIL were not additive. We conclude that NO may stimulate ICa in human atrial myocytes via inhibition of the cGI-PDE.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argibay J. A., Fischmeister R., Hartzell H. C. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988 Jul;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand J. L., Ungureanu D., Kelly R. A., Kobzik L., Pimental D., Michel T., Smith T. W. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. J Clin Invest. 1993 May;91(5):2314–2319. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuckelmann D. J., Näbauer M., Erdmann E. Characteristics of calcium-current in isolated human ventricular myocytes from patients with terminal heart failure. J Mol Cell Cardiol. 1991 Aug;23(8):929–937. doi: 10.1016/0022-2828(91)90135-9. [DOI] [PubMed] [Google Scholar]
- Bkaily G., Perron N., Wang S., Sculptoreanu A., Jacques D., Ménard D. Atrial natriuretic factor blocks the high-threshold Ca2+ current and increases K+ current in fetal single ventricular cells. J Mol Cell Cardiol. 1993 Nov;25(11):1305–1316. doi: 10.1006/jmcc.1993.1143. [DOI] [PubMed] [Google Scholar]
- Bkaily G., Sperelakis N. Injection of guanosine 5'-cyclic monophosphate into heart cells blocks calcium slow channels. Am J Physiol. 1985 May;248(5 Pt 2):H745–H749. doi: 10.1152/ajpheart.1985.248.5.H745. [DOI] [PubMed] [Google Scholar]
- Bénitah J. P., Bailly P., D'Agrosa M. C., Da Ponte J. P., Delgado C., Lorente P. Slow inward current in single cells isolated from adult human ventricles. Pflugers Arch. 1992 Jun;421(2-3):176–187. doi: 10.1007/BF00374825. [DOI] [PubMed] [Google Scholar]
- Cramb G., Banks R., Rugg E. L., Aiton J. F. Actions of atrial natriuretic peptide (ANP) on cyclic nucleotide concentrations and phosphatidylinositol turnover in ventricular myocytes. Biochem Biophys Res Commun. 1987 Nov 13;148(3):962–970. doi: 10.1016/s0006-291x(87)80226-7. [DOI] [PubMed] [Google Scholar]
- Diamond J., Ten Eick R. E., Trapani A. J. Are increases in cyclic GMP levels responsible for the negative inotropic effects of acetylcholine in the heart? Biochem Biophys Res Commun. 1977 Dec 7;79(3):912–918. doi: 10.1016/0006-291x(77)91197-4. [DOI] [PubMed] [Google Scholar]
- Escande D., Coulombe A., Faivre J. F., Deroubaix E., Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987 Jan;252(1 Pt 2):H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- Fach W. A., Becker H. J. Wirkdauer und Dosis-Wirkungs-Beziehung von Molsidomin bei Patienten mit koronarer Herzkrankheit. Z Kardiol. 1984 Oct;73(10):613–622. [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992 Jul 17;257(5068):387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic AMP phosphodiesterases and Ca2+ current regulation in cardiac cells. Life Sci. 1991;48(25):2365–2376. doi: 10.1016/0024-3205(91)90369-m. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic guanosine 3',5'-monophosphate regulates the calcium current in single cells from frog ventricle. J Physiol. 1987 Jun;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Regulation of calcium current by low-Km cyclic AMP phosphodiesterases in cardiac cells. Mol Pharmacol. 1990 Sep;38(3):426–433. [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Direct regulation of cardiac Ca2+ channels by G proteins: neither proven nor necessary? Trends Pharmacol Sci. 1992 Oct;13(10):380–385. doi: 10.1016/0165-6147(92)90117-o. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Irisawa H. Electrophysiology of single cardiac cells. Jpn J Physiol. 1984;34(3):375–388. doi: 10.2170/jjphysiol.34.375. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Hamaguchi M., Kato K., Kawada T., Ohta H., Sasage H., Imai S. Relationship between myoglobin contents and increases in cyclic GMP produced by glyceryl trinitrate and nitric oxide in rabbit aorta, right atrium and papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1993 May;347(5):553–561. doi: 10.1007/BF00166750. [DOI] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Nitric oxide release from the isolated guinea pig heart. Eur J Pharmacol. 1988 Oct 18;155(3):317–321. doi: 10.1016/0014-2999(88)90522-5. [DOI] [PubMed] [Google Scholar]
- Kim K. C., Caswell A. H., Talvenheimo J. A., Brandt N. R. Isolation of a terminal cisterna protein which may link the dihydropyridine receptor to the junctional foot protein in skeletal muscle. Biochemistry. 1990 Oct 2;29(39):9281–9289. doi: 10.1021/bi00491a025. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L., Kummer W., Mayer B., Couraud J. Y., Preissler U., Philippin B., Heym C. Nitric oxide synthase in cardiac nerve fibers and neurons of rat and guinea pig heart. Circ Res. 1992 Dec;71(6):1533–1537. doi: 10.1161/01.res.71.6.1533. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Haap K. 8-bromo-guanosine-3',5' -monophosphate mimics the effect of acetylcholine on slow response action potential and contractile force in mammalian atrial myocardium. J Mol Cell Cardiol. 1978 Jun;10(6):573–586. doi: 10.1016/0022-2828(78)90015-9. [DOI] [PubMed] [Google Scholar]
- Lamontagne D., Pohl U., Busse R. NG-nitro-L-arginine antagonizes endothelium-dependent dilator responses by inhibiting endothelium-derived relaxing factor release in the isolated rabbit heart. Pflugers Arch. 1991 Apr;418(3):266–270. doi: 10.1007/BF00370525. [DOI] [PubMed] [Google Scholar]
- Le Grand B., Deroubaix E., Couétil J. P., Coraboeuf E. Effects of atrionatriuretic factor on Ca2+ current and Cai-independent transient outward K+ current in human atrial cells. Pflugers Arch. 1992 Aug;421(5):486–491. doi: 10.1007/BF00370260. [DOI] [PubMed] [Google Scholar]
- Le Grand B., Hatem S., Deroubaix E., Couetil J. P., Coraboeuf E. Calcium current depression in isolated human atrial myocytes after cessation of chronic treatment with calcium antagonists. Circ Res. 1991 Aug;69(2):292–300. doi: 10.1161/01.res.69.2.292. [DOI] [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989 Apr;413(6):685–687. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Fischmeister R., Walter U. Signal transduction by cGMP in heart. Basic Res Cardiol. 1991 Nov-Dec;86(6):503–514. doi: 10.1007/BF02190700. [DOI] [PubMed] [Google Scholar]
- Masuoka H., Ito M., Nakano T., Naka M., Tanaka T. Effects of amrinone and enoximone on the subclasses of cyclic AMP phosphodiesterase from human heart and kidney. J Cardiovasc Pharmacol. 1990 Feb;15(2):302–307. doi: 10.1097/00005344-199002000-00018. [DOI] [PubMed] [Google Scholar]
- McDonald L. J., Moss J. Stimulation by nitric oxide of an NAD linkage to glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6238–6241. doi: 10.1073/pnas.90.13.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Pelzer S., Trautwein W., Pelzer D. J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994 Apr;74(2):365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Mehegan J. P., Muir W. W., Unverferth D. V., Fertel R. H., McGuirk S. M. Electrophysiological effects of cyclic GMP on canine cardiac Purkinje fibers. J Cardiovasc Pharmacol. 1985 Jan-Feb;7(1):30–35. doi: 10.1097/00005344-198501000-00006. [DOI] [PubMed] [Google Scholar]
- Méry P. F., Brechler V., Pavoine C., Pecker F., Fischmeister R. Glucagon stimulates the cardiac Ca2+ current by activation of adenylyl cyclase and inhibition of phosphodiesterase. Nature. 1990 May 10;345(6271):158–161. doi: 10.1038/345158a0. [DOI] [PubMed] [Google Scholar]
- Méry P. F., Lohmann S. M., Walter U., Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méry P. F., Pavoine C., Belhassen L., Pecker F., Fischmeister R. Nitric oxide regulates cardiac Ca2+ current. Involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J Biol Chem. 1993 Dec 15;268(35):26286–26295. [PubMed] [Google Scholar]
- Nargeot J., Nerbonne J. M., Engels J., Lester H. A. Time course of the increase in the myocardial slow inward current after a photochemically generated concentration jump of intracellular cAMP. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2395–2399. doi: 10.1073/pnas.80.8.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath H. Does cyclic GMP mediate the negative inotropic effect of acetylcholine in the heart? Nature. 1977 May 5;267(5606):72–74. doi: 10.1038/267072a0. [DOI] [PubMed] [Google Scholar]
- Nicholson C. D., Challiss R. A., Shahid M. Differential modulation of tissue function and therapeutic potential of selective inhibitors of cyclic nucleotide phosphodiesterase isoenzymes. Trends Pharmacol Sci. 1991 Jan;12(1):19–27. doi: 10.1016/0165-6147(91)90484-a. [DOI] [PubMed] [Google Scholar]
- Ono K., Trautwein W. Potentiation by cyclic GMP of beta-adrenergic effect on Ca2+ current in guinea-pig ventricular cells. J Physiol. 1991 Nov;443:387–404. doi: 10.1113/jphysiol.1991.sp018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid H., Séguin J., Richard S., Chaptal P. A., Nargeot J. Properties and Modulation of Ca channels in adult human atrial cells. J Mol Cell Cardiol. 1991 Jan;23(1):41–54. doi: 10.1016/0022-2828(91)90037-m. [DOI] [PubMed] [Google Scholar]
- Ramaciotti C., Sharkey A., McClellan G., Winegrad S. Endothelial cells regulate cardiac contractility. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4033–4036. doi: 10.1073/pnas.89.9.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reden J. Molsidomine. Blood Vessels. 1990;27(2-5):282–294. doi: 10.1159/000158820. [DOI] [PubMed] [Google Scholar]
- Richard S., Nerbonne J. M., Nargeot J., Lester H. A., Garnier D. Photochemically produced intracellular concentration jumps of cAMP mimic the effects of catecholamines on excitation-contraction coupling in frog atrial fibers. Pflugers Arch. 1985 Mar;403(3):312–317. doi: 10.1007/BF00583606. [DOI] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Gagne G. D., Nakane M., Pollock J. S., Miller M. F., Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992 Oct;40(10):1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Schulz R., Nava E., Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992 Mar;105(3):575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R., Smith J. A., Lewis M. J., Moncada S. Nitric oxide synthase in cultured endocardial cells of the pig. Br J Pharmacol. 1991 Sep;104(1):21–24. doi: 10.1111/j.1476-5381.1991.tb12378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A. M., Lewis M. J., Henderson A. H. Effects of 8-bromo-cyclic GMP on contraction and on inotropic response of ferret cardiac muscle. J Mol Cell Cardiol. 1991 Jan;23(1):55–64. doi: 10.1016/0022-2828(91)90038-n. [DOI] [PubMed] [Google Scholar]
- Singh J., Flitney F. W. Inotropic responses of the frog ventricle to dibutyryl cyclic AMP and 8-bromo cyclic GMP and related changes in endogenous cyclic nucleotide levels. Biochem Pharmacol. 1981 Jun 15;30(12):1475–1481. doi: 10.1016/0006-2952(81)90370-1. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Shah A. M., Fort S., Lewis M. J. The influence of endocardial endothelium on myocardial contraction. Trends Pharmacol Sci. 1992 Mar;13(3):113–116. doi: 10.1016/0165-6147(92)90040-d. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992 Jul 24;257(5069):494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Tohse N., Sperelakis N. cGMP inhibits the activity of single calcium channels in embryonic chick heart cells. Circ Res. 1991 Aug;69(2):325–331. doi: 10.1161/01.res.69.2.325. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Taniguchi J., Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982 Feb;392(4):307–314. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Trube G. Negative inotropic effect of cyclic GMP in cardiac fiber fragments. Pflugers Arch. 1976 Nov 5;366(2-3):293–295. doi: 10.1007/BF00585895. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Tuganowski W., Kopeć P. The effect of cGMP in rabbit auricle as studied by a cut-end method. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;304(3):211–213. doi: 10.1007/BF00507960. [DOI] [PubMed] [Google Scholar]
- Wahler G. M., Rusch N. J., Sperelakis N. 8-Bromo-cyclic GMP inhibits the calcium channel current in embryonic chick ventricular myocytes. Can J Physiol Pharmacol. 1990 Apr;68(4):531–534. doi: 10.1139/y90-076. [DOI] [PubMed] [Google Scholar]
- Wahler G. M., Sperelakis N. Intracellular injection of cyclic GMP depresses cardiac slow action potentials. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(1):83–95. [PubMed] [Google Scholar]
- Walter U. Cyclic-GMP-regulated enzymes and their possible physiological functions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:249–258. [PubMed] [Google Scholar]
- Walter U. Physiological role of cGMP and cGMP-dependent protein kinase in the cardiovascular system. Rev Physiol Biochem Pharmacol. 1989;113:41–88. doi: 10.1007/BFb0032675. [DOI] [PubMed] [Google Scholar]
- Wilkerson R. D., Paddock R. J., George W. J. Effects of derivatives of cyclic amp and cyclic gmp on contraction force of cat papillary muscles. Eur J Pharmacol. 1976 Mar;36(1):247–251. doi: 10.1016/0014-2999(76)90280-6. [DOI] [PubMed] [Google Scholar]
- de Belder A. J., Radomski M. W., Why H. J., Richardson P. J., Bucknall C. A., Salas E., Martin J. F., Moncada S. Nitric oxide synthase activities in human myocardium. Lancet. 1993 Jan 9;341(8837):84–85. doi: 10.1016/0140-6736(93)92559-c. [DOI] [PubMed] [Google Scholar]