Abstract
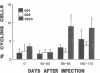
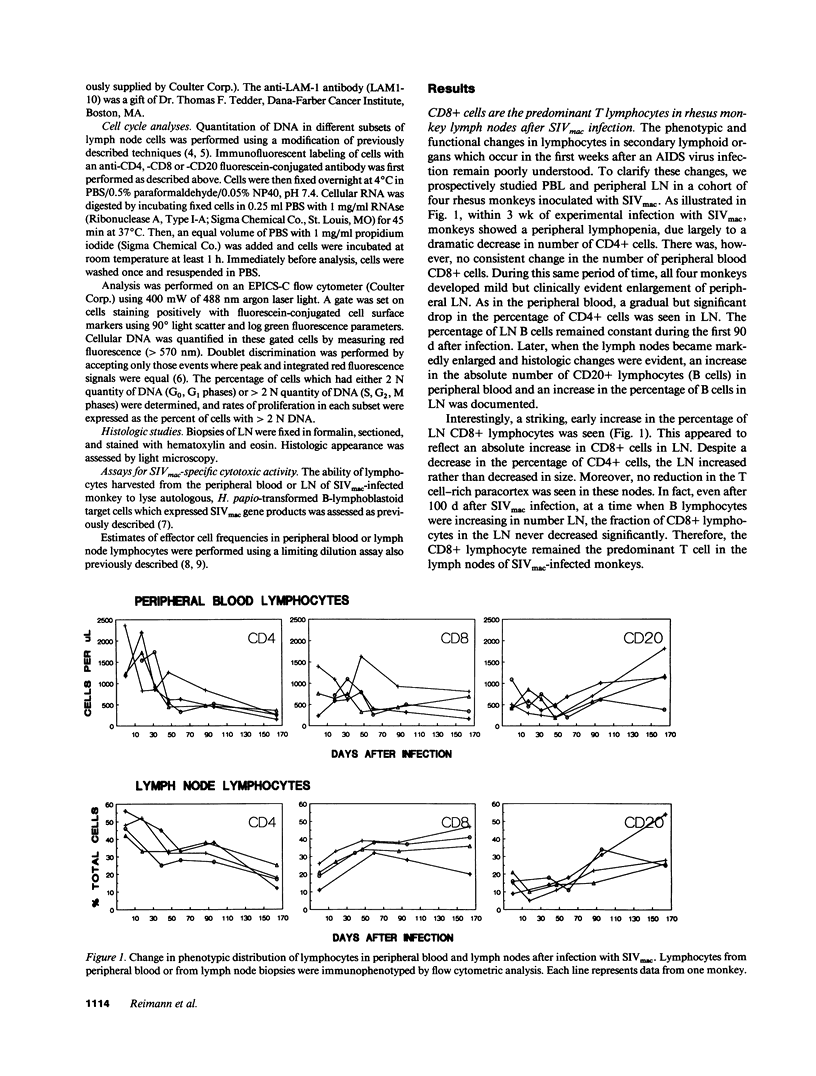
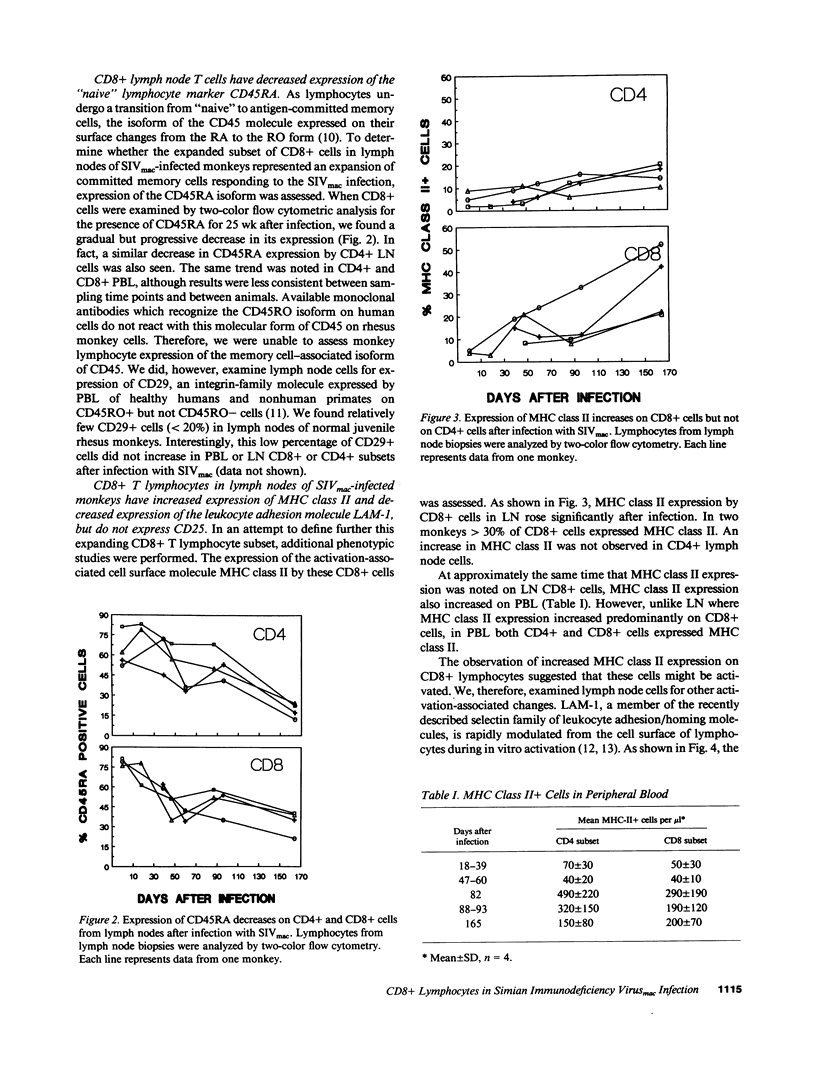
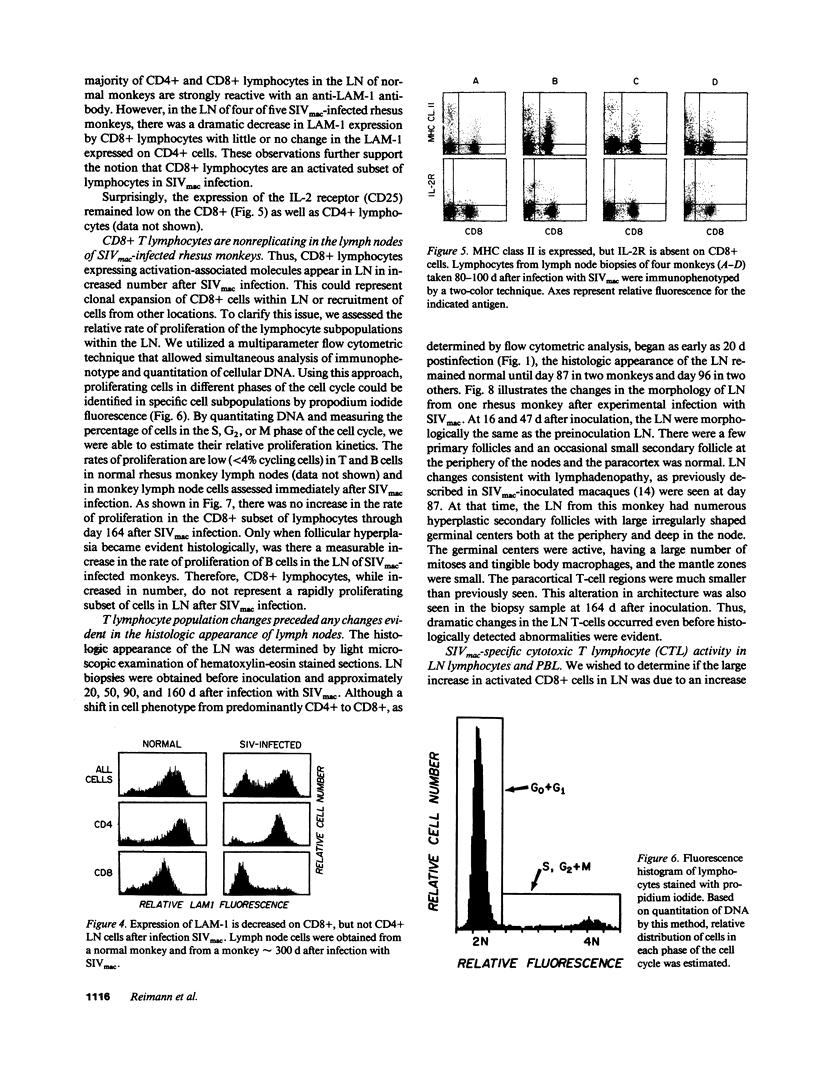
Although alterations in T lymphocyte subset distribution and function in the peripheral blood of HIV-infected humans are well defined, the extent to which these reflect changes in other lymphoid compartments is unclear. We have characterized the coincident changes in PBL and lymph nodes (LN)1 after simian immunodeficiency virus of macaques (SIVmac) infection of rhesus monkeys. Whereas no consistent change in CD8+ PBL was noted during the first 60 d after infection, CD8+ lymphocytes increased significantly in number in LN. These CD8+ LN lymphocytes exhibited an increased expression of MHC class II and a decreased expression of leukocyte adhesion molecule-1, suggesting that they were activated, but interestingly did not express CD25 (IL-2 receptor). Moreover, there was no evidence that these CD8+ LN cells were proliferating, suggesting that they had migrated to the LN. These changes in the LN CD8+ lymphocyte population preceded any detectable change in the light microscopic appearance of the LN. When SIVmac-specific effector T cell responses were assessed, the magnitude of virus-specific effector activity was nearly identical in the PBL and LN of each monkey studied. However, the presence of SIVmac-specific effector cells in the LN did not correlate with the presence of CD8+, MHC class II+ cells. These findings suggest that this numerically important CD8+ lymphocyte subpopulation may serve a regulatory function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autran B., Mayaud C. M., Raphael M., Plata F., Denis M., Bourguin A., Guillon J. M., Debre P., Akoun G. Evidence for a cytotoxic T-lymphocyte alveolitis in human immunodeficiency virus-infected patients. AIDS. 1988 Jun;2(3):179–183. [PubMed] [Google Scholar]
- Carney W. P., Rubin R. H., Hoffman R. A., Hansen W. P., Healey K., Hirsch M. S. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981 Jun;126(6):2114–2116. [PubMed] [Google Scholar]
- Chalifoux L. V., Ringler D. J., King N. W., Sehgal P. K., Desrosiers R. C., Daniel M. D., Letvin N. L. Lymphadenopathy in macaques experimentally infected with the simian immunodeficiency virus (SIV). Am J Pathol. 1987 Jul;128(1):104–110. [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Frankfurt O. S. Flow cytometric analysis of double-stranded RNA content distributions. J Histochem Cytochem. 1980 Jul;28(7):663–669. doi: 10.1177/28.7.6156201. [DOI] [PubMed] [Google Scholar]
- Giorgi J. V., Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989 Jul;52(1):10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Hoffenbach A., Langlade-Demoyen P., Dadaglio G., Vilmer E., Michel F., Mayaud C., Autran B., Plata F. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J Immunol. 1989 Jan 15;142(2):452–462. [PubMed] [Google Scholar]
- Joly P., Guillon J. M., Mayaud C., Plata F., Theodorou I., Denis M., Debre P., Autran B. Cell-mediated suppression of HIV-specific cytotoxic T lymphocytes. J Immunol. 1989 Oct 1;143(7):2193–2201. [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Wilson D. B. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med. 1977 Mar 1;145(3):508–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. D., Lord C. I., Stallard V., Mazzara G. P., Letvin N. L. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990 Jan 1;144(1):122–128. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Nicholson J. K., McDougal J. S., Spira T. J., Cross G. D., Jones B. M., Reinherz E. L. Immunoregulatory subsets of the T helper and T suppressor cell populations in homosexual men with chronic unexplained lymphadenopathy. J Clin Invest. 1984 Jan;73(1):191–201. doi: 10.1172/JCI111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., De Maria A., Koenig S., Butini L., Moss B., Baseler M., Lane H. C., Fauci A. S. CD8+ T lymphocytes of patients with AIDS maintain normal broad cytolytic function despite the loss of human immunodeficiency virus-specific cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4818–4822. doi: 10.1073/pnas.87.12.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Koenig S., Baseler M., Lane H. C., Fauci A. S. Defective clonogenic potential of CD8+ T lymphocytes in patients with AIDS. Expansion in vivo of a nonclonogenic CD3+CD8+DR+CD25- T cell population. J Immunol. 1990 Mar 1;144(5):1696–1704. [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Sharpless T., Traganos F., Darzynkiewicz Z., Melamed M. R. Flow cytofluorimetry: discrimination between single cells and cell aggregates by direct size measurements. Acta Cytol. 1975 Nov-Dec;19(6):577–581. [PubMed] [Google Scholar]
- Sohen S., Rothstein D. M., Tallman T., Gaudette D., Schlossman S. F., Morimoto C. The functional heterogeneity of CD8+ cells defined by anti-CD45RA (2H4) and anti-CD29 (4B4) antibodies. Cell Immunol. 1990 Jun;128(1):314–328. doi: 10.1016/0008-8749(90)90028-p. [DOI] [PubMed] [Google Scholar]
- Spertini O., Kansas G. S., Reimann K. A., Mackay C. R., Tedder T. F. Function and evolutionary conservation of distinct epitopes on the leukocyte adhesion molecule-1 (TQ-1, Leu-8) that regulate leukocyte migration. J Immunol. 1991 Aug 1;147(3):942–949. [PubMed] [Google Scholar]
- Tabi Z., Lynch F., Ceredig R., Allan J. E., Doherty P. C. Virus-specific memory T cells are Pgp-1+ and can be selectively activated with phorbol ester and calcium ionophore. Cell Immunol. 1988 May;113(2):268–277. doi: 10.1016/0008-8749(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Tedder T. F., Penta A. C., Levine H. B., Freedman A. S. Expression of the human leukocyte adhesion molecule, LAM1. Identity with the TQ1 and Leu-8 differentiation antigens. J Immunol. 1990 Jan 15;144(2):532–540. [PubMed] [Google Scholar]
- Tomkinson B. E., Wagner D. K., Nelson D. L., Sullivan J. L. Activated lymphocytes during acute Epstein-Barr virus infection. J Immunol. 1987 Dec 1;139(11):3802–3807. [PubMed] [Google Scholar]
- Tsubota H., Lord C. I., Watkins D. I., Morimoto C., Letvin N. L. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989 Apr 1;169(4):1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Westermann J., Pabst R. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol Today. 1990 Nov;11(11):406–410. doi: 10.1016/0167-5699(90)90160-b. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Burns B. F., Dorfman R. F., Warnke R. A. In situ quantitation of lymph node helper, suppressor, and cytotoxic T cell subsets in AIDS. Blood. 1986 Mar;67(3):596–603. [PubMed] [Google Scholar]
- Yamamoto H., Miller M. D., Watkins D. I., Snyder G. B., Chase N. E., Mazzara G. P., Gritz L., Panicali D. L., Letvin N. L. Two distinct lymphocyte populations mediate simian immunodeficiency virus envelope-specific target cell lysis. J Immunol. 1990 Dec 1;145(11):3740–3746. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Stachel D., Schlunk T., Gürtler L., Schramm W., Fröschl M., Bogner J. R., Riethmüller G. Class II (DR) antigen expression on CD8+ lymphocyte subsets in acquired immune deficiency syndrome (AIDS). J Clin Immunol. 1988 Nov;8(6):473–478. doi: 10.1007/BF00916953. [DOI] [PubMed] [Google Scholar]