Abstract
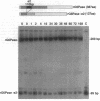
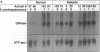
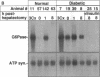
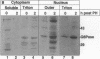
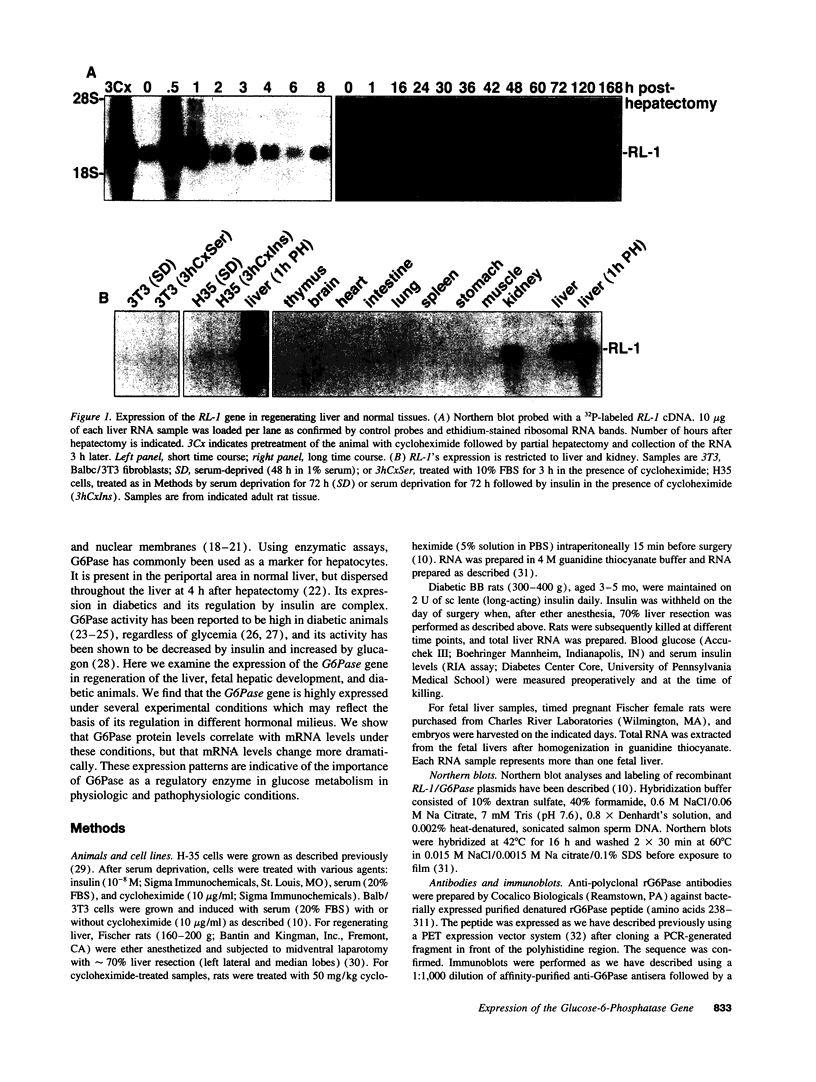
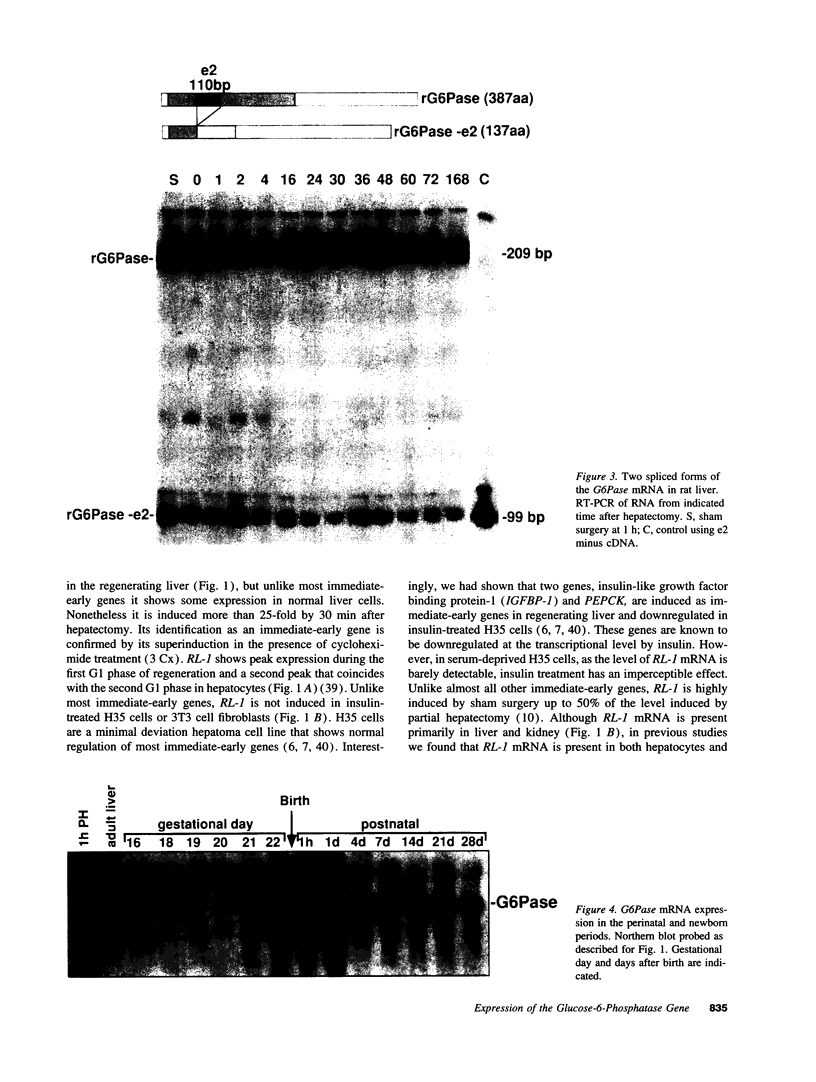
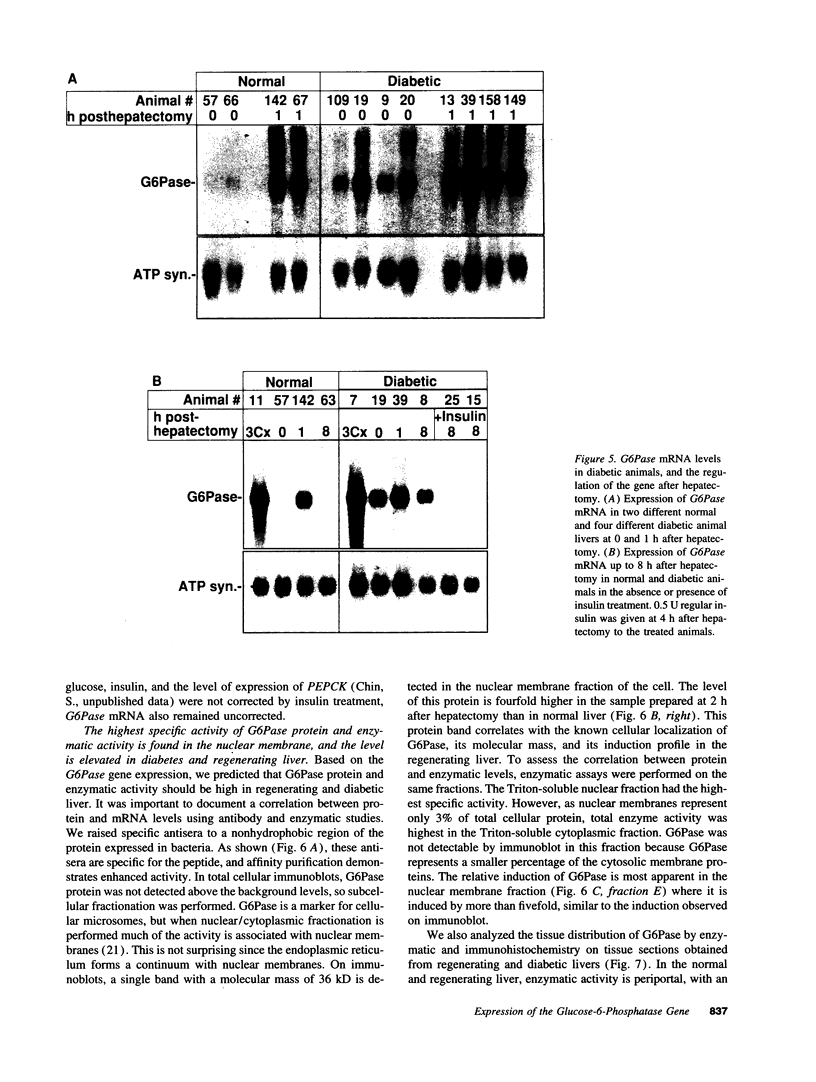
The regenerating liver after partial hepatectomy is one of the few physiologic models of cellular proliferation in the adult animal. During hepatic regeneration, the animal is able to maintain metabolic homeostasis despite the acute loss of two thirds of hepatic tissue. In examining the molecular mechanisms regulating hepatic regeneration, we isolated novel immediate-early genes that are rapidly induced as the remnant liver undergoes the transition from its normal quiescent state into the G1 phase of the cell cycle. One of the most rapidly and highly induced genes which we initially termed RL-1, encodes rat glucose-6-phosphatase (rG6Pase). G6Pase mRNA peaks at 30 min and 36-48 h after hepatectomy correlating with the first and second rounds of cell division. This finding is compatible with studies that showed that G6Pase enzyme activity increases during liver regeneration. However, the increase in G6Pase mRNA is much more dramatic, indicating that it is a more sensitive indicator of this regulation. G6Pase gene expression peaks in the perinatal time period in the liver and remains elevated during the first month of life. The expression of the G6Pase gene is also dramatically elevated in BB diabetic rats, again higher than the enzyme elevation, and its relative induction after partial hepatectomy is blunted in these animals. Insulin treatment of partially hepatectomized diabetic animals downregulates the expression of G6Pase mRNA. Using specific antibodies against G6Pase, we detect a 36-kD G6Pase protein, and its level is elevated in regenerating and diabetic livers. The pattern of G6Pase mRNA expression appears to reflect similar changes in insulin and glucagon levels which accompany diabetes and hepatic proliferation. The elevation of G6Pase expression in these conditions is indicative of its importance as a regulator of glucose homeostasis in normal and abnormal physiologic states.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barra R., Hall J. C. Liver regeneration in normal and alloxan-induced diabetic rats. J Exp Zool. 1977 Jul;201(1):93–99. doi: 10.1002/jez.1402010111. [DOI] [PubMed] [Google Scholar]
- Barzilai N., Rossetti L. Role of glucokinase and glucose-6-phosphatase in the acute and chronic regulation of hepatic glucose fluxes by insulin. J Biol Chem. 1993 Nov 25;268(33):25019–25025. [PubMed] [Google Scholar]
- Brinkmann A., Katz N., Sasse D., Jungermann K. Increase of the gluconeogenic and decrease of the glycolytic capacity of rat liver with a change of the metabolic zonation after partial hepatectomy. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1561–1571. doi: 10.1515/bchm2.1978.359.2.1561. [DOI] [PubMed] [Google Scholar]
- Bucher M. L., Swaffield M. N. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1157–1160. doi: 10.1073/pnas.72.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell A., Burchell B. Identification and purification of a liver microsomal glucose 6-phosphatase. Biochem J. 1982 Sep 1;205(3):567–573. doi: 10.1042/bj2050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. M., Young K. M., Hutson N. J., Brumley F. T., Exton J. H. Hepatic metabolism of genetically diabetic (db/db) mice. I. Carbohydrate metabolism. Am J Physiol. 1975 Dec;229(6):1702–1712. doi: 10.1152/ajplegacy.1975.229.6.1702. [DOI] [PubMed] [Google Scholar]
- Christ B., Probst I., Jungermann K. Antagonistic regulation of the glucose/glucose 6-phosphate cycle by insulin and glucagon in cultured hepatocytes. Biochem J. 1986 Aug 15;238(1):185–191. doi: 10.1042/bj2380185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. H., Cressman D. E., Laz T. M., Abrams C. S., Taub R. PRL-1, a unique nuclear protein tyrosine phosphatase, affects cell growth. Mol Cell Biol. 1994 Jun;14(6):3752–3762. doi: 10.1128/mcb.14.6.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. H., Du K., Lee V. M., Mohn K. L., Haber B. A., Tewari D. S., Taub R. Novel delayed-early and highly insulin-induced growth response genes. Identification of HRS, a potential regulator of alternative pre-mRNA splicing. J Biol Chem. 1993 Jul 15;268(20):15185–15192. [PubMed] [Google Scholar]
- Eritani N., Fukuda H., Matsumura Y. Lipogenic enzyme gene expression in rat liver during development after birth. J Biochem. 1993 May;113(5):519–525. doi: 10.1093/oxfordjournals.jbchem.a124076. [DOI] [PubMed] [Google Scholar]
- Fausto N., Mead J. E. Regulation of liver growth: protooncogenes and transforming growth factors. Lab Invest. 1989 Jan;60(1):4–13. [PubMed] [Google Scholar]
- GRISHAM J. W. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962 Aug;22:842–849. [PubMed] [Google Scholar]
- Gardner L. B., Liu Z., Barrett E. J. The role of glucose-6-phosphatase in the action of insulin on hepatic glucose production in the rat. Diabetes. 1993 Nov;42(11):1614–1620. doi: 10.2337/diab.42.11.1614. [DOI] [PubMed] [Google Scholar]
- Haber B. A., Mohn K. L., Diamond R. H., Taub R. Induction patterns of 70 genes during nine days after hepatectomy define the temporal course of liver regeneration. J Clin Invest. 1993 Apr;91(4):1319–1326. doi: 10.1172/JCI116332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuret J., Bell H., Cohen P. Identification of high levels of protein phosphatase-1 in rat liver nuclei. FEBS Lett. 1986 Jul 28;203(2):197–202. doi: 10.1016/0014-5793(86)80741-4. [DOI] [PubMed] [Google Scholar]
- Lange A. J., Argaud D., el-Maghrabi M. R., Pan W., Maitra S. R., Pilkis S. J. Isolation of a cDNA for the catalytic subunit of rat liver glucose-6-phosphatase: regulation of gene expression in FAO hepatoma cells by insulin, dexamethasone and cAMP. Biochem Biophys Res Commun. 1994 May 30;201(1):302–309. doi: 10.1006/bbrc.1994.1702. [DOI] [PubMed] [Google Scholar]
- Lavoie L., Dimitrakoudis D., Marette A., Annabi B., Klip A., Vranic M., van de Werve G. Opposite effects of hyperglycemia and insulin deficiency on liver glycogen synthase phosphatase activity in the diabetic rat. Diabetes. 1993 Feb;42(2):363–366. doi: 10.2337/diab.42.2.363. [DOI] [PubMed] [Google Scholar]
- Lee J., Greenbaum L., Haber B. A., Nagle D., Lee V., Miles V., Mohn K. L., Bucan M., Taub R. Structure and localization of the IGFBP-1 gene and its expression during liver regeneration. Hepatology. 1994 Mar;19(3):656–665. doi: 10.1002/hep.1840190317. [DOI] [PubMed] [Google Scholar]
- Lei K. J., Shelly L. L., Pan C. J., Sidbury J. B., Chou J. Y. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type 1a. Science. 1993 Oct 22;262(5133):580–583. doi: 10.1126/science.8211187. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Miethke H., Wittig B., Nath A., Zierz S., Jungermann K. Metabolic zonation in liver of diabetic rats. Zonal distribution of phosphoenolpyruvate carboxykinase, pyruvate kinase, glucose-6-phosphatase and succinate dehydrogenase. Biol Chem Hoppe Seyler. 1985 May;366(5):493–501. doi: 10.1515/bchm3.1985.366.1.493. [DOI] [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Hsu J. C., Melby A. E., Bravo R., Taub R. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol Cell Biol. 1991 Jan;11(1):381–390. doi: 10.1128/mcb.11.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Melby A. E., Taub R. Immediate-early gene expression differs between regenerating liver, insulin-stimulated H-35 cells, and mitogen-stimulated Balb/c 3T3 cells. Liver-specific induction patterns of gene 33, phosphoenolpyruvate carboxykinase, and the jun, fos, and egr families. J Biol Chem. 1990 Dec 15;265(35):21914–21921. [PubMed] [Google Scholar]
- Mohn K. L., Melby A. E., Tewari D. S., Laz T. M., Taub R. The gene encoding rat insulinlike growth factor-binding protein 1 is rapidly and highly induced in regenerating liver. Mol Cell Biol. 1991 Mar;11(3):1393–1401. doi: 10.1128/mcb.11.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Granner D. K. Regulation of gene expression by insulin. Biochem J. 1991 Sep 15;278(Pt 3):609–619. doi: 10.1042/bj2780609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa J. L., Bartrons R., Tauler A. Gene expression of regulatory enzymes of glycolysis/gluconeogenesis in regenerating rat liver. Biochem J. 1992 Oct 1;287(Pt 1):113–116. doi: 10.1042/bj2870113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa J. L., Tauler A., Lange A. J., Pilkis S. J., Bartrons R. Transcriptional and posttranscriptional regulation of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase during liver regeneration. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3746–3750. doi: 10.1073/pnas.89.9.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Kelsey G., Schedl A., Schmid E., Thies E., Schütz G. Deficiency of an enzyme of tyrosine metabolism underlies altered gene expression in newborn liver of lethal albino mice. Genes Dev. 1992 Aug;6(8):1430–1443. doi: 10.1101/gad.6.8.1430. [DOI] [PubMed] [Google Scholar]
- Rymsa B., de Groot H. Partial purification of rat liver microsomal glucose-6-phosphatase on hydroxylapatite. Biol Chem Hoppe Seyler. 1988 Feb;369(2):115–121. doi: 10.1515/bchm3.1988.369.1.115. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly L. L., Lei K. J., Pan C. J., Sakata S. F., Ruppert S., Schutz G., Chou J. Y. Isolation of the gene for murine glucose-6-phosphatase, the enzyme deficient in glycogen storage disease type 1A. J Biol Chem. 1993 Oct 15;268(29):21482–21485. [PubMed] [Google Scholar]
- Speth M., Schulze H. U. The purification of a detergent-soluble glucose-6-phosphatase from rat liver. Eur J Biochem. 1992 Sep 15;208(3):643–650. doi: 10.1111/j.1432-1033.1992.tb17230.x. [DOI] [PubMed] [Google Scholar]
- Taub R., Roy A., Dieter R., Koontz J. Insulin as a growth factor in rat hepatoma cells. Stimulation of proto-oncogene expression. J Biol Chem. 1987 Aug 5;262(22):10893–10897. [PubMed] [Google Scholar]
- Teutsch H. F. Improved method for the histochemical demonstration of glucose-6-phosphatase activity. Histochemistry. 1978 Aug 25;57(2):107–117. doi: 10.1007/BF00496675. [DOI] [PubMed] [Google Scholar]