Abstract
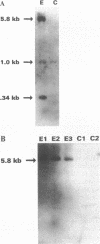
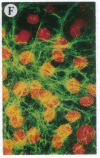
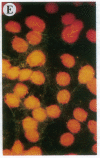
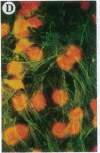
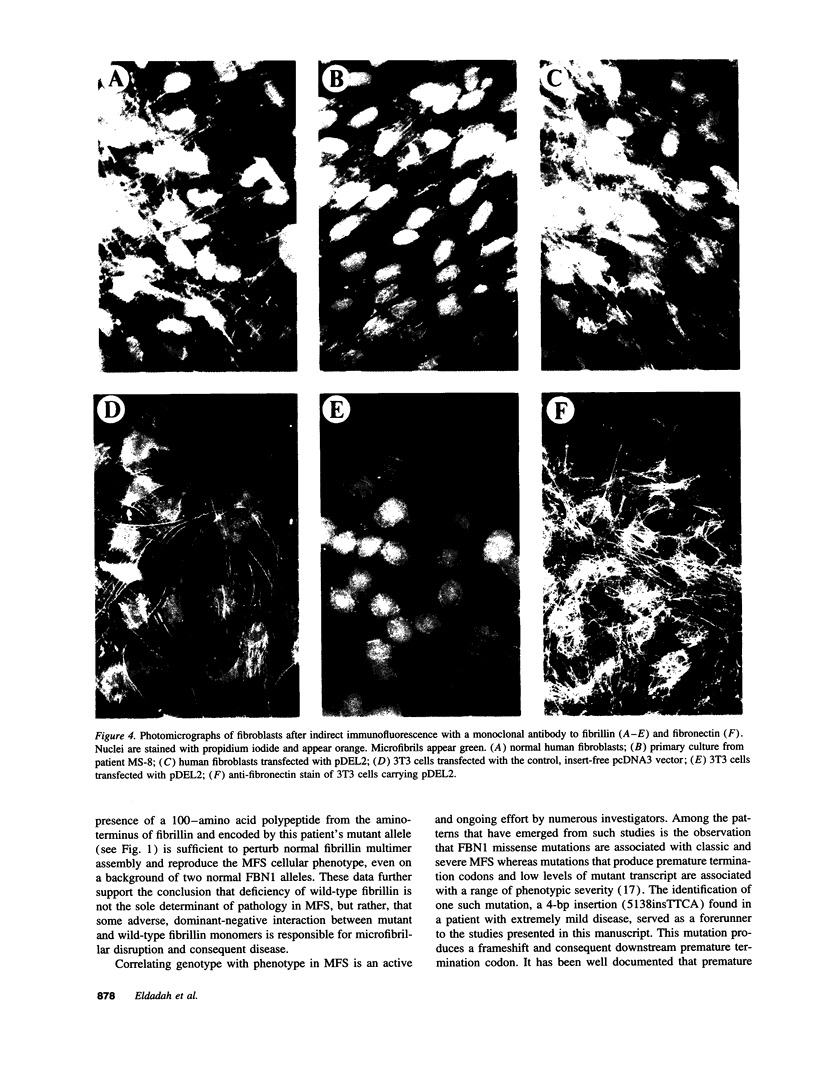
The Marfan syndrome (MFS) is a connective tissue disorder inherited as an autosomal dominant trait and caused by mutations in the gene encoding fibrillin, a 350-kD glycoprotein that multimerizes to form extracellular microfibrils. It has been unclear whether disease results from a relative deficiency of wild-type fibrillin; from a dominant-negative effect, in which mutant fibrillin monomers disrupt the function of the wild-type protein encoded by the normal allele; or from a dynamic and variable interplay between these two pathogenetic mechanisms. We have now addressed this issue in a cell culture system. A mutant fibrillin allele from a patient with severe MFS was expressed in normal human and murine fibroblasts by stable transfection. Immunohistochemical analysis of the resultant cell lines revealed markedly diminished fibrillin deposition and disorganized microfibrillar architecture. Pulse-chase studies demonstrated normal levels of fibrillin synthesis but substantially reduced deposition into the extracellular matrix. These data illustrate that expression of a mutant fibrillin allele, on a background of two normal alleles, is sufficient to disrupt normal microfibrillar assembly and reproduce the MFS cellular phenotype. This underscores the importance of the fibrillin amino-terminus in normal microfibrillar assembly and suggests that expression of the human extreme 5' fibrillin coding sequence may be sufficient, in isolation, to produce an animal model of MFS. Lastly, this substantiation of a dominant-negative effect offers mutant allele knockout as a potential strategy for gene therapy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama T., Francke U., Dietz H. C., Furthmayr H. Quantitative differences in biosynthesis and extracellular deposition of fibrillin in cultured fibroblasts distinguish five groups of Marfan syndrome patients and suggest distinct pathogenetic mechanisms. J Clin Invest. 1994 Jul;94(1):130–137. doi: 10.1172/JCI117298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T., Tynan K., Dietz H. C., Francke U., Furthmayr H. Missense mutations impair intracellular processing of fibrillin and microfibril assembly in Marfan syndrome. Hum Mol Genet. 1993 Dec;2(12):2135–2140. doi: 10.1093/hmg/2.12.2135. [DOI] [PubMed] [Google Scholar]
- Cheng J., Fogel-Petrovic M., Maquat L. E. Translation to near the distal end of the penultimate exon is required for normal levels of spliced triosephosphate isomerase mRNA. Mol Cell Biol. 1990 Oct;10(10):5215–5225. doi: 10.1128/mcb.10.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. M., Wilkinson A. J., Baron M., Pastore A., Tappin M. J., Campbell I. D., Gregory H., Sheard B. The solution structure of human epidermal growth factor. 1987 May 28-Jun 3Nature. 327(6120):339–341. doi: 10.1038/327339a0. [DOI] [PubMed] [Google Scholar]
- Corson G. M., Chalberg S. C., Dietz H. C., Charbonneau N. L., Sakai L. Y. Fibrillin binds calcium and is coded by cDNAs that reveal a multidomain structure and alternatively spliced exons at the 5' end. Genomics. 1993 Aug;17(2):476–484. doi: 10.1006/geno.1993.1350. [DOI] [PubMed] [Google Scholar]
- Davis C. G. The many faces of epidermal growth factor repeats. New Biol. 1990 May;2(5):410–419. [PubMed] [Google Scholar]
- Dietz H. C., Cutting G. R., Pyeritz R. E., Maslen C. L., Sakai L. Y., Corson G. M., Puffenberger E. G., Hamosh A., Nanthakumar E. J., Curristin S. M. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991 Jul 25;352(6333):337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., McIntosh I., Sakai L. Y., Corson G. M., Chalberg S. C., Pyeritz R. E., Francomano C. A. Four novel FBN1 mutations: significance for mutant transcript level and EGF-like domain calcium binding in the pathogenesis of Marfan syndrome. Genomics. 1993 Aug;17(2):468–475. doi: 10.1006/geno.1993.1349. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Pyeritz R. E., Puffenberger E. G., Kendzior R. J., Jr, Corson G. M., Maslen C. L., Sakai L. Y., Francomano C. A., Cutting G. R. Marfan phenotype variability in a family segregating a missense mutation in the epidermal growth factor-like motif of the fibrillin gene. J Clin Invest. 1992 May;89(5):1674–1680. doi: 10.1172/JCI115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glesby M. J., Pyeritz R. E. Association of mitral valve prolapse and systemic abnormalities of connective tissue. A phenotypic continuum. JAMA. 1989 Jul 28;262(4):523–528. [PubMed] [Google Scholar]
- Handford P. A., Mayhew M., Baron M., Winship P. R., Campbell I. D., Brownlee G. G. Key residues involved in calcium-binding motifs in EGF-like domains. Nature. 1991 May 9;351(6322):164–167. doi: 10.1038/351164a0. [DOI] [PubMed] [Google Scholar]
- Hewett D. R., Lynch J. R., Smith R., Sykes B. C. A novel fibrillin mutation in the Marfan syndrome which could disrupt calcium binding of the epidermal growth factor-like module. Hum Mol Genet. 1993 Apr;2(4):475–477. doi: 10.1093/hmg/2.4.475. [DOI] [PubMed] [Google Scholar]
- Hollister D. W., Godfrey M., Sakai L. Y., Pyeritz R. E. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med. 1990 Jul 19;323(3):152–159. doi: 10.1056/NEJM199007193230303. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Pulkkinen L., Savolainen A., Kaitila I., Peltonen L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N Engl J Med. 1990 Oct 4;323(14):935–939. doi: 10.1056/NEJM199010043231402. [DOI] [PubMed] [Google Scholar]
- Keene D. R., Maddox B. K., Kuo H. J., Sakai L. Y., Glanville R. W. Extraction of extendable beaded structures and their identification as fibrillin-containing extracellular matrix microfibrils. J Histochem Cytochem. 1991 Apr;39(4):441–449. doi: 10.1177/39.4.2005373. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Shuttleworth C. A. The role of calcium in the organization of fibrillin microfibrils. FEBS Lett. 1993 Dec 27;336(2):323–326. doi: 10.1016/0014-5793(93)80829-j. [DOI] [PubMed] [Google Scholar]
- Lee B., Godfrey M., Vitale E., Hori H., Mattei M. G., Sarfarazi M., Tsipouras P., Ramirez F., Hollister D. W. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991 Jul 25;352(6333):330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Maddox B. K., Sakai L. Y., Keene D. R., Glanville R. W. Connective tissue microfibrils. Isolation and characterization of three large pepsin-resistant domains of fibrillin. J Biol Chem. 1989 Dec 15;264(35):21381–21385. [PubMed] [Google Scholar]
- Maslen C. L., Corson G. M., Maddox B. K., Glanville R. W., Sakai L. Y. Partial sequence of a candidate gene for the Marfan syndrome. Nature. 1991 Jul 25;352(6333):334–337. doi: 10.1038/352334a0. [DOI] [PubMed] [Google Scholar]
- Milewicz D. M., Pyeritz R. E., Crawford E. S., Byers P. H. Marfan syndrome: defective synthesis, secretion, and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest. 1992 Jan;89(1):79–86. doi: 10.1172/JCI115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Colige A., Helminen H., Khillan J. S., Pereira R., Vandenberg P. Mutations in type 1 procollagen that cause osteogenesis imperfecta: effects of the mutations on the assembly of collagen into fibrils, the basis of phenotypic variations, and potential antisense therapies. J Bone Miner Res. 1993 Dec;8 (Suppl 2):S489–S492. doi: 10.1002/jbmr.5650081311. [DOI] [PubMed] [Google Scholar]
- Pyeritz R. E., McKusick V. A. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979 Apr 5;300(14):772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Pereira L., Zhang H., Lee B. The fibrillin-Marfan syndrome connection. Bioessays. 1993 Sep;15(9):589–594. doi: 10.1002/bies.950150904. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Glanville R. W., Bächinger H. P. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991 Aug 5;266(22):14763–14770. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]