Abstract
Glia maturation factor (GMF), a primarily CNS localized protein was discovered and characterized in our laboratory. We previously demonstrated that GMF is the upstream regulator for excessive production and release of proinflammatory cytokines /chemokines in brain cells leading to the destruction of oligodendrocytes, the myelin forming cells, and neurons. We also reported that mice lacking endogenous GMF (GMF-deficient, GMF-KO) were resistant to myelin oligodendrocyte glycoprotein peptide 35–55 (MOG35-55) induced EAE, since immunization induced only delayed EAE with diminished severity. In the present study we show that a replication-defective adenovirus-GMF construct caused expression of GMF in CNS of GMF-KO mice and reinstated MOG35-55 induced early and severe EAE. Our results show that MOG35-55 immunization caused only a muted EAE and inflammation/demyelination in mice lacking endogenous GMF. The diminished incidence of EAE in GMF-KO mice was consistent with the significantly reduced expressions of cytokines/chemokines. The muted severity of EAE in GMF-KO mice was restored to full blown levels upon reintroduction of GMF using an adeno-GMF-virus (Adv-GMF) vector. Consistent with the clinical findings, histological examination of the CNS of mice with EAE revealed profound differences between wild type (Wt), GMF-KO, and GMF-KO mice with re-introduced GMF (GMF-KO +Adv-GMF). Spinal cord sections from mice with EAE were analyzed for the infiltration of mononuclear cells (inflammation) and myelin loss (demyelination). In Wt mice, 40% of spinal cord quadrants were positive for demyelination and 45% of spinal cord quadrants were positive for inflammation at the peak of EAE. Drastically reduced infiltrates (15%) and demyelination (10%) was found in GMF-KO mice that developed reduced severity of EAE. Upon GMF reintroduction in GMF-KO mice, MOG35-55 immunization caused extensive monocytes infiltration (48%) and demyelination (46%), similar to that observed in the immunized Wt mice. The levels of cytokine/chemokine in the spinal cords of mice at three time points, corresponding to the onset, peak severity and recovery period of EAE, show a distinct pattern of very large increases in IFN-γ, TNF-α, GM-CSF and MCP-1 in Wt and GMF-KO +Adv-GMF mice compared to GMF-KO and GMF-KO +Adv-LacZ mice.
Keywords: Experimental autoimmune encephalomyelitis (EAE), Multiple sclerosis (MS), Glia maturation factor (GMF), Myelin oligodendrocyte glycoprotein 35–55 (MOG 35-55), Neuro inflammation, Cytokine/chemokine
INTRODUCTION
Multiple sclerosis (MS) is a disabling inflammatory demyelinating autoimmune disease of the central nervous system (CNS) that affects an estimated 350,000 Americans and over a million individuals worldwide. It is one of the main causes of neurological disabilities among young adults. Pathologically, MS is characterized by the loss of the myelin sheath around neurons, gliosis and death of neurons (Al-Omaishi et al., 1999). Much of our current knowledge about contributing factors of MS is based on animal models of experimental autoimmune encephalomyelitis (EAE), such as: C57Bl/6/ MOG35-55, SJL/PLP139-151 and adoptive transfer-EAE (AT-EAE). Several theories for the pathogenesis of MS exist and implicate transendothelial infiltration of reactive T cells, proinflammatory cytokines/chemokines, as well as activated microglia and astrocytes in CNS. Additionally, the natural process of remyelination is also disturbed (Jurewicz et al., 2007; Karnezis et al., 2004). Since the pathogenesis of MS is not clear, an effective and definitive treatment is not available so far.
Glia maturation factor (GMF), a primarily CNS localized protein, was isolated, sequenced and cloned in our laboratory (Kaplan et al., 1991; Lim et al., 1989; Lim et al., 1990; Zaheer et al., 1993; Zaheer et al., 1995a; Zaheer et al., 1995b). We reported earlier that GMF is a major immunomodulator and required for induced expression of GM-CSF in astrocytes. The GMF-dependent expression of GM-CSF then induces expression of a number of proinflammatory cytokines/chemokines in microglia that leads to the death of oligodendrocytes, the myelin producing cells (Zaheer et al., 2007b). We also reported that, compared to wild type mice, GMF-deficient mice (GMF-KO) were resistant to actively induced and passively transferred EAE and immunization failed to induce large increases in GM-CSF and other proinflammatory molecules in the CNS of GMF-KO mice (Menon et al., 2007; Zaheer et al., 2007a; Zaheer et al., 2007c; Zaheer et al., 2008; Zaheer et al., 2007d). In this report, we provide further convincing evidences for a major role of GMF in progression of severe EAE pathology induced by MOG35-55 peptide. Our present results demonstrate that the overexpression of GMF in the CNS significantly increases inflammatory cell infiltration, demyelination, and proinflammatory cytokine/chemokine production in MOG-induced EAE.
EXPERIMENTAL PROCEDURES
Reagents
Myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55), complete Freund’s adjuvant and pertussis toxin were purchased from Sigma-Aldrich, St. Louis, MO. Adenovirus constructs were prepared at the University of Iowa Gene Transfer Vector Core as described earlier (Davidson et al., 1993; Davidson and Breakefield, 2003; Lim et al., 1998), using a replication-defective adenovirus (serotype 5) vector. The constructs contained either a full-length GMF cDNA (Adv-GMF), or a cytoplasmic lacZ cDNA (Adv-LacZ). G2-09 was a monoclonal antibody (IgG1) against GMF and was affinity-purified with protein-A. ELISA kits for mouse TNF-α (Cat # KMC3011), IFN-γ (Cat # KMC4021), GM-CSF (Cat # KMC2011) and MCP-1 (monocyte chemoattractant protein-1, Cat # KMC0061) were obtained from Biosource International, CA.
GMF-deficient mice
GMF-deficient (GMF knockout) mice were originally produced by homologous recombination with over 80% of the amino acid sequence deleted, as previously described (Lim et al., 2004; Zaheer et al., 2004). GMF- knockout (GMF-KO) mice were maintained by backcross breeding to C57BL/6 for eleven generations at The University of Iowa, Animal Care and Use facility. Control wild type (C57BL/6) mice were purchased from Harlan Sprague Dawley, Inc., Indianapolis, IN. The animals were cared for in accordance with the guidelines approved by the IACUC and National Institutes of Health.
Induction of EAE
C57BL/6 mice were purchased from Harlan Sprague Dawley, Inc., Indianapolis, IN. Mice were maintained in the animal colony at The University of Iowa and used in accordance with the guidelines approved by the IACUC and National Institutes of Health. For active induction of EAE, C57BL/6, 8-10 week-old, female mice were immunized with subcutaneous injection of 150 μg encephalitogenic myelin oligodendrocyte glycoprotein peptide 35-55 (MOG35-55) in 100 μl PBS and mixed with 100 μl of complete Freund’s adjuvant (CFA). Mice were boosted day 0 and day 2 with i.p. injection of 300 ng pertussis toxin. Control mice received identical injections without MOG35-55. The mice were observed for 36 days following immunization and weighed and scored daily in a double blinded fashion according to the scoring scale of 0 to 5, score 0, no disease; score 1, tail weakness; score 2, weakness in hind limb; score 3, complete hind limb paralysis; score 4, hind limb paralysis with fore limb weakness or paralysis; and score 5, moribund or deceased.
Adenoviral gene delivery in mice
Mice were administered recombinant adenoviruses that expressed either the GMF (Adv-GMF) or the bacterial reporter gene lacZ (Adv. LacZ). We randomly assigned 12 mice per group and four doses (50 μl; 1 × 10 7 particles per μl) administered by intravenous injection (i.v). First dose was given at day 3 post MOG-immunization, a time at which the brain-blood barrier is breached, followed by every second day (day 5, 7, and 9 post immunizations). Mice were compared for the disease with respect to rapidity of onset, severity, and disease duration. The severity of the disease was scored daily on a scale of 0-5.
Histological assessment
At the peak of the disease, three mice from each experimental group were anesthetized by intraperitoneal injection of sodium pentobarbital and transcardially perfused with PBS and by 4% paraformaldehyde in phosphate buffer as described earlier (Thangavel et al., 2008a; 2009a; Thangavel et al., 2008b; 2009b; Zaheer et al., 2007a; Zaheer et al., 2007c). Spinal cords were assessed for inflammation and demyelination essentially as described earlier (Zaheer et al., 2007a; Zaheer et al., 2007c). Five micrometer thick transverse sections (five sections per mouse) were taken from cervical, upper thoracic, lower thoracic, and lumbar regions of spinal cord. The sections were stained with hematoxylin and eosin to reveal infiltrating inflammatory cells (with morphological characteristics of lymphocytes, granulocytes, macrophages, microglia and astrocytes) and with Luxol fast blue for the evidence of demyelination. Each spinal cord section further subdivided into anterior, posterior and two lateral columns (4 quadrants). The signs of inflammation and demyelination in the spinal cord sections were scored under microscope in a blinded manner by two examiners. Each quadrant showing the infiltration of mononuclear cells was assigned a score of one inflammation and the quadrant that showed perivascular lesion and loss of myelin staining a score of one demyelination. Thus, each animal will have a potential maximum score of 80. The pathologic score (inflammation or demyelination) for each group were expressed as the percentage positive over the total number of quadrants examined (Bright et al., 1998; Bright et al., 1999a; Bright et al., 1999b; Zaheer et al., 2007a; Zaheer et al., 2007c).
Enzyme-linked immunosorbent assay (ELISA)
The analysis of IFN-γ, TNF-α, GM-CSF, and MCP-1 protein concentration was estimated by sandwich immuno-assay procedure as specified in the manufacturer’s protocol. Briefly, to 96-well microtiter ELISA plates pre-coated with anti-cytokine capture antibodies, the cytokine standard and samples were added and incubated overnight at 4°C followed by washing. Corresponding biotinylated antibodies, horseradish peroxidase-conjugated streptovidin and TMB substrate used to develop a yellow color and read by a microplate reader at 450 nm. The concentration of cytokine was estimated from a standard curve generated with each run. The lower detection limits of these ELISA are in the range of 8-12 pg/ml. ELISA data are presented as mean values ± standard deviations and represent more than three independent experiments with similar results.
Statistical analysis
Statistical significance was assessed with one-way ANOVA followed by Tukey’s procedure using SigmaStat software (SPP, Chicago, IL). A value of p< 0.05 was considered statistically significant.
RESULTS
Efficient and sustained restoration of GMF expression in GMF-KO mice via adeno-GMF viral vector delivery
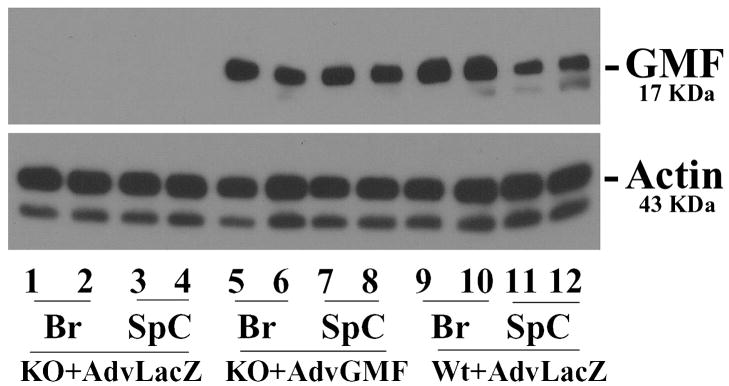
To investigate whether overexpression of GMF in mice can mediate a variety of deleterious events leading to inflammation and demyelination under stressful conditions such as immunization with encephalitogenic MOG35-55, we reintroduced GMF in the CNS of GMF-KO mice using a replication-defective adenovirus vector containing a full length GMF cDNA (Adv-GMF) or a control cytoplasmic lacZ cDNA (Adv-LacZ). Adenoviral vectors expressing either GMF or control LacZ were administered by four intravenous in (i.v) injections (day 3, 5, 7, and 9) in GMF-KO mice starting day 3 after MOG35-55 immunization, a time at which the brain-blood barrier is breached. Expression of GMF in the brain and spinal cord of the mice was determined at the peak severity of EAE. Results (Figure 1) show that viral vector-mediated introduction of GMF led to substantial and sustained expression of GMF in the brain and spinal cord of GMF-KO mice whereas Adv-LacZ control had no effect. These results clearly demonstrate that a robust and sustained expression of GMF in the CNS of mice could be achieved using adenoviral vector delivery system.
Figure 1. Expression of GMF in brain and spinal cord of EAE mice.
Mice were injected with a replication-defective adenovirus vector containing a full length GMF cDNA (Adv-GMF) or cytoplasmic lacZ cDNA (Adv-LacZ) and two mice in each group were analyzed by Western blot analysis at the peak of EAE. Brain or spinal cord tissue homogenates (20 μg protein per lane) were subjected to SDS-polyacrylamide gel electrophoresis followed by electroblotting. The blots were probed with a monoclonal anti-GMF (G2-09) antibody and actin antibody. β-Actin served as an internal marker showing equal sample loading. The data shown are representative of at least three experiments. Br, brain; SpC, spinal cord
Reintroduction of GMF in GMF-KO mice via adenoviral vector delivery significantly increases susceptibility to MOG35-55- induced EAE
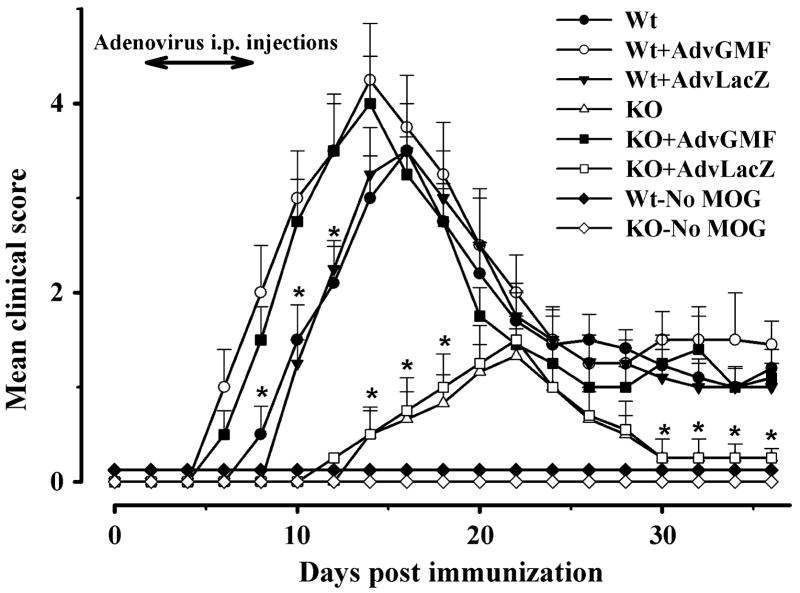
Next, we examined whether virally introduced GMF in Wt and GMF-KO mice could increase susceptibility to MOG35-55 induced EAE. The time courses of onset and progression of EAE are illustrated in Figure 2. The severity of EAE was measured in terms of mean clinical score. As expected, no EAE developed in Wt or GMF-KO mice that were immunized without MOG35-55 (Wt-no MOG or KO-no MOG, respectively). The mean clinical score of the disease started to increase with a lag period of 7 days (onset time) in immunized Wt mice (Figure 2), reached to a peak of 3.5 ± 0.5 at day 16 then declined to about 1.0 during 16 to 36 days. The onset time was remarkably delayed to 12 days in identically immunized GMF-KO mice. EAE of a minor severity with a mean clinical score of only 1.3 ± 0.2 developed at day 22 after immunization in GMF-KO mice and then returned to the level of non-immunized mice at 30-35 days. A dramatic reversal of EAE onset time and severity was observed when GMF was reintroduced by adeno-GMF in immunized GMF-KO mice (KO+Adv-GMF). The onset time was decreased to the earliest time (5 days), and a very severe EAE with maximum mean score of 4.0 ± 0.5 was developed in KO+AdvGMF mice at day 14 post MOG-immunization. The peak clinical score in GMF-KO mice was significantly lower compared to the Wt and KO+AdvGMF mice but the peak scores did not differ statistically between Wt and KO+AdvGMF. The time courses of EAE onset and progression of EAE severities in immunized Wt mice transfected with Adv-GMF (Wt+AdvGMF) and in KO+AdvGMF mice were not distinguishable. Transfection with control Adv-LacZ in Wt or GMF-KO mice had no effect on the onset or the severity of EAE. These results show that susceptibility to MOG35-55 induced EAE can be altered by GMF-overexpression and that the increased susceptibility to EAE in GMF-KO mice is a direct result of GMF overexpression.
Figure 2. Overexpression of GMF exacerbates EAE induced by MOG35–55 peptide.
Mice were immunized with MOG35–55 peptide and monitored for the development of disease. Adv-GMF or Adv-LacZ was injected intravenously every day, starting at day 2 to day 8 after immunization with MOG35–55 peptide. Mean clinical score of MOG-induced EAE in Wt and GMF-KO (KO) along with Adv-GMF or Adv-LacZ injected mice (n = 10 for each group). Note this is a representative of three independent experiments.
Histological findings in the spinal cords from mice with actively induced EAE
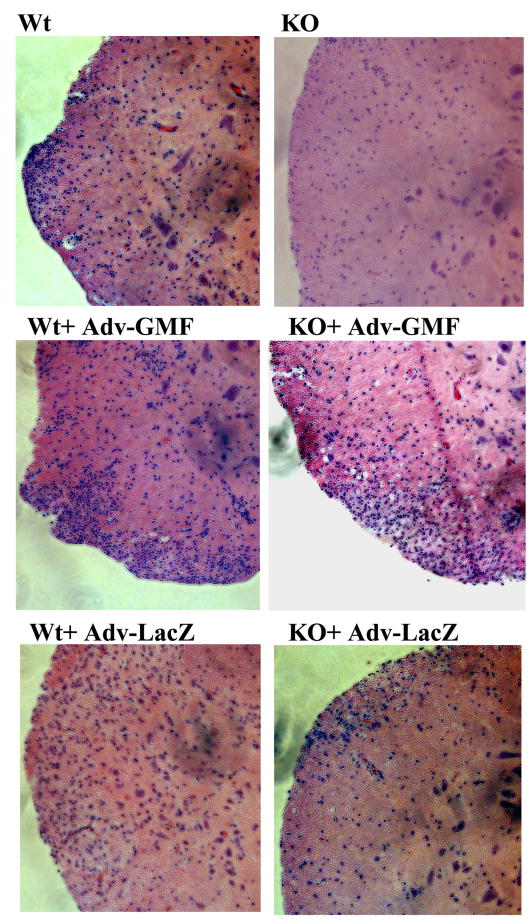
The infiltration of inflammatory mononuclear cells into the CNS precedes the development of the clinical symptoms generally during 3 to 5 days post MOG35-55 immunization in Wt mice. Therefore, the histology observations of spinal cords were done at peak of EAE for a meaningful comparison in immunized mice injected with Adv-GMF or control Adv-LacZ and representative photomicrographs are shown in Figure 3. The inflammatory infiltrates are noticeably low in the spinal cords of immunized GMF-KO and KO-LacZ mice, which did not develop severe EAE, compared to the excessive infiltrations in WT, Wt+AdvGMF, Wt+AdvLacZ and KO-AdvGMF mice, all of which developed severe EAE as was seen in Figure 2. This analysis revealed that GMF-KO mice when challenged with encephalitogenic MOG35-55 peptide resulted in no manifestation of infiltration of inflammatory cells in the spinal cord compared to its wild type counter parts. These results suggest that GMF is critical to the development of EAE.
Figure 3. Overexpression of GMF significantly increases the inflammation in MOG35–55 induced EAE.
Animals were sacrificed at day 12 -post immunization with MOG35-55 and spinal cord inflammation was assessed by hematoxylin and eosin (H&E) staining. Representative microscopic photographs of the spinal cord showing significantly increased infiltration of inflammatory mononuclear cells in Wt and GMF-KO mice injected with Adv-GMF as compared to control-Adv-LacZ-injected mice at the peak of EAE. (Original magnification: X10; n =3 for each group)
GMF-KO mice with reintroduced GMF show increased inflammation and demyelination in CNS following MOG35-55 immunization
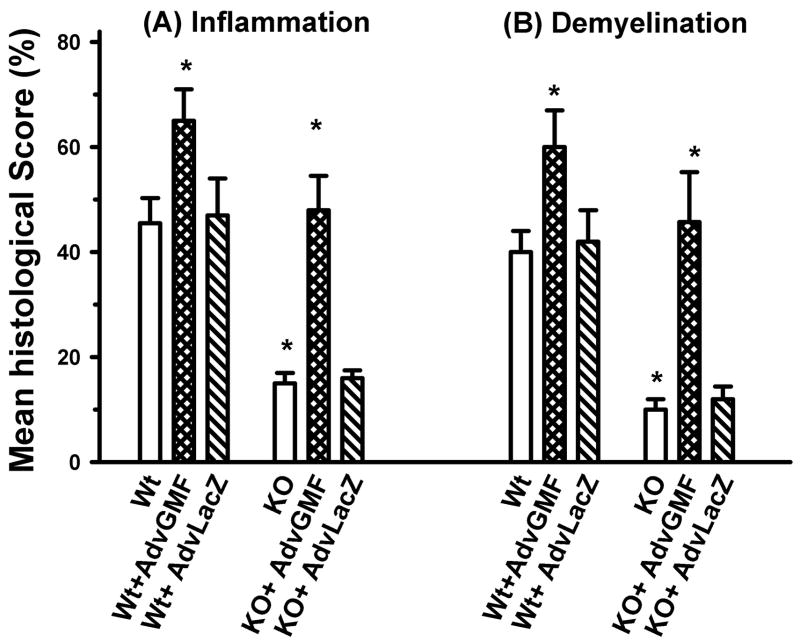
Figure 4 shows significant quantitative differences in inflammation and demyelination scores in the spinal cords of immunized Wt and GMF-KO mice at the peak EAE. The inflammation scores in Wt, Wt+AdvGMF, Wt+AdvLacZ and KO+AdvGMF mice were 45 ± 5, 65 ± 5, 47 ± 7 and 50 ± 6, respectively. These scores were about 3 fold or more than the scores in KO and KO+AdvLacZ, 15 ± 2 and 16 ± 2, respectively. Similarly, the lowest scores of demyelination, 10 ± 2 and 12 ± 3 were recorded at peak of EAE in GMF-KO and KO+AdvLacZ mice which did not develop severe EAE. The corresponding demyelination scores in Wt, Wt+AdvGMF, Wt+AdvLacZ and KO+AdvGMF mice which developed severe EAE were 40 ± 4, 65 ± 6, 42 ± 6 and 45 ± 7, respectively. These results show that MOG35-55 induced inflammation and demyelination can be altered by GMF-overexpression.
Figure 4. Histopathological scores for inflammation and demyelination in MOG35-55 induced EAE.
Mice were injected with Adv-GMF or Adv-LacZ as described in Figure 2. The spinal cord sections are assessed for inflammation and demyelination in EAE mice. At the peak of the disease, five-micrometer thick transverse sections (five sections) from cervical, upper thoracic, lower thoracic and lumbar regions of spinal cord were stained. Each section was further subdivided into anterior, posterior and two lateral columns (4 quadrants). Each quadrant showing the infiltration of mononuclear cells was assigned a score of one inflammation and the quadrant that showed perivascular lesion and loss of myelin staining a score of one demyelination. Thus, each animal had a potential maximum score of 80. The average number of quadrants examined per mouse was 16. The pathologic score (inflammation or demyelination) for each group was expressed as the percentage positive over the total number of quadrants examined. Differences between Wt, GMF-KO, and GMF-KO+ Adv-GMF mice were highly significant (P < 0.001) at the peak of EAE.
GMF-KO mice with reintroduced GMF show increased levels of proinflammatory cytokine and chemokine expression in CNS following MOG35-55 immunization
TNF-α, IFN-γ, GM-CSF and MCP-1 levels in spinal cord homogenates were determined by ELISA in EAE mice at the onset, at the peak and recovery periods of the disease. Data represents the mean ± S.D. from three independent experiments, each with triplicate measurements. The levels of all the proinflammatory cytokines and chemokine measured, including IFN-γ, TNF-α, MCP-1 and GM-CSF, increased significantly, at all the three time intervals in immunized Wt, Wt+AdvGMF, Wt+AdvLacZ and KO+AdvGMF mice that developed severe EAE compared to GMF-KO and KO+AdvLacZ mice that were resistant to the EAE (Figure 5). The increases were excessive and maximum at the peak EAE then declined to about the onset levels during the recovery period. This quantitative comparison of inflammatory cytokines and chemokines in the CNS indicated that adenoviral expression of GMF significantly enhanced their production following MOG35-55 immunization.
Figure 5. Expression of pro inflammatory cytokines/chemokines in the spinal cord of MOG35-55 induced EAE mice.
The lumbar and lower thoracic spinal cord tissue (100 mg wet weight) was homogenized and TNF-α, IFN-γ, GM-CSF and MCP-1 levels in spinal cord homogenates were determined by ELISA in EAE mice at the onset, at the peak and recovery period of the disease. The results are obtained from three pools of material, each consisting of three mice.
DISCUSSION
Although some genetic risk factors were identified recently (Akkad et al., 2009), the etiology of MS is not fully understood. CD4+ myelin-reactive Th1 cells are believed to be responsible for the induction and progression of MS and EAE (Steinman, 1996). The myelin reactive T cells and inflammatory cells infiltrate the CNS and produce copious amounts of proinflammatory cytokines / chemokines in association with activated astrocytes and microglial cells leading to demyelination and loss of neural cells (Sorensen and Ransohoff, 1998). By overexpressing GMF in primary cultures of brain cells, we previously reported that GMF is an upstream mediator for the induction of granulocyte-macrophage-colony stimulating factor (GM-CSF) via p38 MAPK and NF-kB signaling pathways (Zaheer et al., 2007b). And that the GMF-dependent induction of GM-CSF was sufficient for the production of proinflammatory cytokines/chemokines (Zaheer et al., 2007b) in microglia resulting in the subsequent destruction of neurons and oligodendroglia, the myelin producing cells. In our earlier studies, we used gene-targeted mice lacking GMF, a brain specific protein associated with CNS inflammation. GMF-deficient mice are viable, fertile, and showed no gross anatomical or neuronal abnormalities. Using GMF-KO mice, we showed that in contrast to wild type (GMF-efficient) mice, the productions of proinflammatory cytokines and chemokines were attenuated in MOG immunized GMF-KO mice and the immunization failed to induce severe EAE (Zaheer et al., 2007a; Zaheer et al., 2007c; Zaheer et al., 2007d).
In the present studies, we establish the crucial role of GMF in MOG35-55 induced onset and progression of EAE disease, EAE associated production of inflammatory cytokines / chemokines, inflammatory CNS infiltrate and demyelination by restoring GMF expression in GMF-KO mice via an efficient adenoviral vector delivery system. Our results show that overexpression of GMF in the CNS of GMF-KO mice completely reversed the resistance to early onset and progression of severe EAE in immunized GMF-KO mice. There was no difference in the EAE courses of wild type and GMF-KO mice, both injected with the same Adv-GMF construct. The control Adv-LacZ construct was without effect. These results suggest that the onset time and severity of EAE in immunized mice are closely dependent on the levels of GMF and that the control adenovirus vector did not influence the effects of GMF. The restoration of EAE severity by GMF overexpression in GMF-KO mice also increased the EAE associated inflammatory infiltration of mononuclear cells, demyelination scores and proinflammatory cytokine/chemokine levels to the extent that reflects the induced EAE severities in Wt mice. Our data clearly demonstrates that either the overexpression of GMF in Wt mice or the restoration of GMF expression in GMF-KO mice via adenoviral vector delivery will exacerbate MOG-induced EAE. Taken together, these results strongly suggest that GMF plays a prominent pathophysiological role in the onset and progression of severity of MOG-induced EAE. As a consequence, inhibition or neutralization of GMF could suppress the full spectrum of pathophysiology of EAE. The intervention with GMF neutralizing anti-body or GMF specific siRNA could prove the rational, effective and non-toxic approach for treating EAE/MS disease since GMF is not required for survival or normal functions. The absence of GMF does not prevent antigen stimulated proliferation of T cells and we never observed susceptibility of GMF-KO mice to any opportunistic infection. It is also anticipated that a combined strategy of silencing/neutralizing GMF with other inducers of remyelination in MS, such as LINGO1 antagonists (Mi et al., 2007), will be extremely valuable for the speedy cure and functional recovery from MS disease.
SUMMARY
In summary, we demonstrated that in Wt and in GMF-KO mice, adenoviral mediated overexpression of GMF exacerbates MOG-induced EAE. We also show a significant increase in infiltration of inflammatory mononuclear cells and production of inflammatory cytokines/chemokines in the CNS of these mice following MOG immunization. Our results point to a pathophysiological role for GMF in the progression of EAE.
Table 1.
Clinical features of MOG-induced EAE
| Wt | Wt + Adv-GMF | Wt + Adv-LacZ | GMF-KO | KO + Adv-GMF | KO + Adv-LacZ | |
|---|---|---|---|---|---|---|
| Incidence | 11 / 12 | 12 / 14 | 11 / 12 | 3 / 14 | 9 / 12 | 3 / 12 |
| Day of onset | 7 | 5 | 5 | 12 | 5 | 12 |
| Maximal clinical score | 3.5 | 4.5 | 3.5 | 1.5 | 4.0 | 1.5 |
| Mortality | 2 / 12 | 3 / 14 | 1 / 12 | 0 / 14 | 3 / 12 | 1 / 12 |
Acknowledgments
We thank Krishnakumar Menon, Marcus Ahrens, and Xi Yang, for excellent technical help. We thank Satya Mathur for a critical reading of the manuscript. This work was supported by the Department of Veterans Affairs Merit Review award (to A.Z.) and by the National Institute of Neurological Disorders and Stroke grant NS-47145 (to A.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akkad DA, et al. Variation in the IL7RA and IL2RA genes in German multiple sclerosis patients. J Autoimmun. 2009;32:110–115. doi: 10.1016/j.jaut.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Al-Omaishi J, et al. The cellular immunology of multiple sclerosis. J Leukoc Biol. 1999;65:444–452. doi: 10.1002/jlb.65.4.444. [DOI] [PubMed] [Google Scholar]
- Bright JJ, et al. Prevention of experimental allergic encephalomyelitis via inhibition of IL-12 signaling and IL-12-mediated Th1 differentiation: an effect of the novel anti-inflammatory drug lisofylline. J Immunol. 1998;161:7015–7022. [PubMed] [Google Scholar]
- Bright JJ, et al. Tyrphostin B42 inhibits IL-12-induced tyrosine phosphorylation and activation of Janus kinase-2 and prevents experimental allergic encephalomyelitis. J Immunol. 1999a;162:6255–6262. [PubMed] [Google Scholar]
- Bright JJ, et al. Differential influence of interleukin-12 in the pathogenesis of autoimmune and virus-induced central nervous system demyelination. J Virol. 1999b;73:1637–1639. doi: 10.1128/jvi.73.2.1637-1639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BL, et al. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Davidson BL, et al. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, et al. Soluble Nogo-A, an inhibitor of axonal regeneration, as a biomarker for multiple sclerosis. Neurology. 2007;68:283–287. doi: 10.1212/01.wnl.0000252357.30287.1d. [DOI] [PubMed] [Google Scholar]
- Kaplan R, et al. Molecular cloning and expression of biologically active human glia maturation factor-beta. J Neurochem. 1991;57:483–490. doi: 10.1111/j.1471-4159.1991.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Karnezis T, et al. The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat Neurosci. 2004;7:736–744. doi: 10.1038/nn1261. [DOI] [PubMed] [Google Scholar]
- Lim R, et al. Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci U S A. 1989;86:3901–3905. doi: 10.1073/pnas.86.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, et al. Impaired motor performance and learning in glia maturation factor-knockout mice. Brain Res. 2004;1024:225–232. doi: 10.1016/j.brainres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lim R, et al. Overexpression of glia maturation factor in C6 cells promotes differentiation and activates superoxide dismutase. Neurochem Res. 1998;23:1445–1451. doi: 10.1023/a:1020715126326. [DOI] [PubMed] [Google Scholar]
- Lim R, et al. Complete amino acid sequence of bovine glia maturation factor beta. Proc Natl Acad Sci U S A. 1990;87:5233–5237. doi: 10.1073/pnas.87.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K, et al. Diminished degradation of myelin basic protein by anti-sulfatide antibody and interferon-gamma in myelin from glia maturation factor-deficient mice. Neurosci Res. 2007;58:156–163. doi: 10.1016/j.neures.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat Med. 2007;13:1228–1233. doi: 10.1038/nm1664. [DOI] [PubMed] [Google Scholar]
- Sorensen TL, et al. Etiology and pathogenesis of multiple sclerosis. Semin Neurol. 1998;18:287–294. doi: 10.1055/s-2008-1040879. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Thangavel R, et al. Modular and laminar pathology of Brodmann’s area 37 in Alzheimer’s disease. Neuroscience. 2008a;152:50–55. doi: 10.1016/j.neuroscience.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel R, et al. Loss of nonphosphorylated neurofilament immunoreactivity in temporal cortical areas in Alzheimer’s disease. Neuroscience. 2009a;160:427–433. doi: 10.1016/j.neuroscience.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel R, et al. Posterior parahippocampal gyrus pathology in Alzheimer’s disease. Neuroscience. 2008b;154:667–676. doi: 10.1016/j.neuroscience.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel R, et al. The abnormally phosphorylated tau lesion of early Alzheimer’s disease. Neurochem Res. 2009b;34:118–123. doi: 10.1007/s11064-008-9701-1. [DOI] [PubMed] [Google Scholar]
- Zaheer A, et al. Expression of glia maturation factor beta mRNA and protein in rat organs and cells. J Neurochem. 1993;60:914–920. doi: 10.1111/j.1471-4159.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Zaheer A, et al. Diminished cytokine and chemokine expression in the central nervous system of GMF-deficient mice with experimental autoimmune encephalomyelitis. Brain Res. 2007a;1144:239–247. doi: 10.1016/j.brainres.2007.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer A, et al. Decreased copper-zinc superoxide dismutase activity and increased resistance to oxidative stress in glia maturation factor-null astrocytes. Neurochem Res. 2004;29:1473–1480. doi: 10.1023/b:nere.0000029558.82943.00. [DOI] [PubMed] [Google Scholar]
- Zaheer A, et al. A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem. 2007b;101:364–376. doi: 10.1111/j.1471-4159.2006.04385.x. [DOI] [PubMed] [Google Scholar]
- Zaheer A, et al. Reduced severity of experimental autoimmune encephalomyelitis in GMF-deficient mice. Neurochem Res. 2007c;32:39–47. doi: 10.1007/s11064-006-9220-x. [DOI] [PubMed] [Google Scholar]
- Zaheer A, et al. Glia maturation factor modulates beta-amyloid-induced glial activation, inflammatory cytokine/chemokine production and neuronal damage. Brain Res. 2008;1208:192–203. doi: 10.1016/j.brainres.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer A, et al. Expression of mRNAs of multiple growth factors and receptors by neuronal cell lines: detection with RT-PCR. Neurochem Res. 1995a;20:1457–1463. doi: 10.1007/BF00970594. [DOI] [PubMed] [Google Scholar]
- Zaheer A, et al. Expression of mRNAs of multiple growth factors and receptors by astrocytes and glioma cells: detection with reverse transcription-polymerase chain reaction. Cell Mol Neurobiol. 1995b;15:221–237. doi: 10.1007/BF02073330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaheer S, et al. Glia Maturation Factor Regulation of STAT Expression: A Novel Mechanism in Experimental Autoimmune Encephalomyelitis. Neurochem Res. 2007d;32:2123–2131. doi: 10.1007/s11064-007-9383-0. [DOI] [PubMed] [Google Scholar]