Abstract
Tumor cell migration is mediated by cell autonomous signaling mechanisms as well as paracrine and autocrine factors secreted by activated stromal cells in the tumor microenvironment. Like other members of the ADAM family, the integrin-binding metalloprotease ADAM9 modulates cell-cell and cell-matrix interactions as well as ectodomain shedding of cell surface receptors and ligands, thereby modifying intracellular and extracellular signaling. ADAM9 transcripts are alternatively spliced to express a transmembrane protein (ADAM9-L) and a secreted variant (ADAM9-S). In this study, we show that ADAM9-S promotes breast cancer cell migration in a manner requiring its metalloprotease activity, whereas ADAM9-L suppresses cell migration independent of its metalloprotease activity. Suppression of migration by ADAM9-L requires a functional disintegrin domain and integrin binding. Expression analysis revealed that both ADAM9 isoforms are expressed in breast cancer cell lines and tissues. Therefore, relative levels of membrane-tethered and secreted variants of ADAM9 are a key determinant in manifestation of aggressive migratory phenotypes associated with breast cancer progression.
Keywords: ADAM9, cell migration, breast cancer, metalloprotease, disintegrin
Introduction
One of the hallmarks of advanced breast cancer is the ability of the tumor cells to lose their epithelial phenotype, disengage from neighboring cells, degrade the basement membrane and invade surrounding tissues, ultimately metastasizing to distant organs. The interactions between the tumor and its stromal microenvironment is a key determinant in breast cancer progression from carcinoma in situ to advanced invasive and metastatic carcinoma. Tumor cells proteolyze the basement membrane and stroma to invade the vasculature, and also alter stromal signals to stimulate angiogenesis and alternately release and tether to the extracellular matrix (ECM) to allow for efficient migration. Tumor cells also release growth factors and chemokines that alter the stroma, inducing inflammation, angiogenesis and mechanisms of tissue repair (1, 2).
Proteases play a major signaling role at the tumor-stromal boundary. Enzymes comprising the matrix metalloprotease (MMP) family proteolyze basement membrane and function in pro-migratory signaling mechanisms. Proteolysis by MMPs exposes cryptic sites in ECM constituents such as laminin-5 and collagen IV that in turn promote tumor cell migration (3, 4). Proteolysis of the basement membrane also releases ECM-bound growth factors such as insulin and fibroblast growth factor (FGF) (5, 6). MMPs proteolytically cleave and release the ectodomains of multiple signaling molecules from the cell membrane in a process known as membrane shedding (7). Shedding of substrates such as transforming growth factor-β (TGFβ (8) and tumor necrosis factor-agr; (TNFagr; (9), activates signaling pathways that promote cell migration and survival. The ADAM (A Disintegrin and Metalloprotease) family of proteases has functional similarity to the MMPs in their zinc-binding metalloprotease domains, and are also referred to as the metalloprotease, disintegrin, cysteine-rich (MDC) family. ADAMs control basement membrane proteolysis and shedding of proteins from the cell membrane. Comprising 40 isoforms, the ADAM family of proteases also contain an integrin-binding disintegrin domain. ADAMs function in cell adhesion through their cysteine-rich domains that bind to Syndecans (10) and fibronectin (11). The Src homology-3 (SH3) binding domain in the cytoplasmic regions of some ADAMs mediate signaling by activation of Src and Grb proteins (12). Recent studies point to a functional importance for some ADAM family members in cancer. ADAM12 is expressed in carcinoma and promotes breast cancer progression by inducing the apoptosis of surrounding stromal cells (13). Consequently, ADAM12 protein levels correlate with advanced breast cancer (14, 15). In contrast, the disintegrin domain of ADAM15 inhibits angiogenesis and tumor growth (16). Moreover, ADAM15 expression decreases integrin-mediated ovarian cancer cell adhesion and motility (17).
ADAM9, also known as MDC9 (metalloprotease/disintegrin/cysteine-rich protein 9), MCMP (Myeloma cell metalloprotease) and meltrin-gamma, comprises a subgroup of ADAMs that also contains ADAM12 and ADAM15 (18). The ADAM9 metalloprotease activity cleaves heparin-binding epidermal growth factor (HB-EGF) (19), which binds activates EGF-receptor to promote tumor growth and angiogenesis (20). ADAM9 also cleaves Delta-like 1, a Notch ligand (21), as well as ADAM10 (22, 23). The ADAM9 disintegrin domain is a ligand for specific integrin heterodimers including multiple β1 integrins (24, 25), agr;6β4 (26) and agr;vβ5 integrins (27, 28).
Recent studies have revealed that ADAM9 message is upregulated in breast tumors as well as breast cancer cell lines (29, 30). The chromosomal region of the ADAM9 gene (8p11–12) is amplified in a number of breast cancers and cell lines, and ADAM9 is overexpressed in cell lines encompassing the luminal, basal A, and basal B gene expression clusters (31).
The ADAM9 transcript is alternatively spliced into membrane-bound and secreted isoforms. Alternative splicing of exon 12 in the ADAM9 message leads to a shorter ADAM9-S (ADAM9-secreted) transcript that contains eight unique amino acids not present in ADAM9-L (ADAM9-long). ADAM9-S also lacks the transmembrane and cytoplasmic domains and is a secreted protein (26). ADAM9 is implicated in cancer cell phenotypes as secretion of ADAM9-S promotes invasion of breast cancer cells in Matrigel assays (26). Furthermore, adhesion of keratinocyte cells to recombinant ADAM9 disintegrin-cysteine rich domain increases cell migration, and overexpression of ADAM9 also increases pro-MMP9 expression (25). However, the functional role of ADAM9-L and ADAM9-S in modulating cell migration and invasion in breast cancer cells has not been evaluated.
Here, we use specific ADAM9-L and ADAM9-S antibodies to detect expression of both variants in breast cancer cell lines and breast cancer tissues. Using RNA interference and gene replacement, we show that ADAM9-S and ADAM9-L do not function in a redundant manner in the regulation of cell migration, whereby ADAM9-S promotes and ADAM9-L attenuates migration. These studies identify for the first time an ADAM as a migration suppressor, and have implications for the functional roles of ADAM9 proteins in breast cancer progression.
Materials and Methods
Antibodies and Reagents
Anti-myc antibody purified from the 9B11 hybridoma was purchased from Cell Signaling Technology (Danvers, MA). Anti-β-actin was from Sigma-Aldrich (St Louis, Missouri). Anti-ADAM9-L was from BioMol Research Laboratories Inc (SA-376). Anti-ADAM9-S was designed and produced in collaboration with Pro-Sci Inc. (Poway, CA) as a rabbit-polyclonal antibody based on the immunization peptide sequence CATGLSLKFHAPF. Transient transfections were performed with Lipofectamine 2000 (Invitrogen) or polyethyleimine (Polysciences Inc). pcDNA3-mA9L, pcDNA3-mA9L.EA, pcDNA3-mADAM9L.myc and pcDNA4-hADAM9S.myc have been described previously (12, 26). Point mutants mADAM9L.EA.myc, ADAM9S.EA.myc and ADAM9L.TCE.myc were derived from these vectors via Stratagene site-directed mutagenesis according to the manufacturers instructions and the following primers: ADAM9S.EA 5’ CCAAGATTATGACCCAATGCATGAGCAACAATGG 3’ and 5’ CCATTGTTGCTCATGCATTGGGTCATAATCTTGG 3’; mADAM9L.EA 5’ CCATTGTTGCTCATGCATTGGGGCATAACCTTGG 3’ and 5’ CCAAGGTTATGCCCCAATGCATGAGCAACAATGG 3’; mADAM9L.TCE.myc 5’ GGACCCTGGAGAGCGTGTGAATGCGGC 3’ and 5’ GCCGCATTCACACGTCTCTCCAGGGTCC 3’. Deletion mutations in mADAM9L.Δcyt.myc and mADAM9L.Δdis.myc were derived as follows: To delete the cytoplasmic domain, a KPN1 restriction site was inserted into pcDNA3-mADAM9-L 5’ of the cytoplasmic region with primers 5’ GGCTAACTAGAGAACCCACTGGTACCTGGCTTATCG 3’ and 5’ CGATAAGCCAGGTACCAGTGGGTTCTCTAGTTAGCC 3’. BAMHI from the vector backbone and the inserted KPN1 restriction sites were used to isolate the mADAM9L.Δcyt sequence, which was cloned into digested pCDNA4A/TO/myc/his vector in frame with the Myc tag. To delete the disintegrin domain, KPN1 sites were inserted 5’ and 3’ of the disintegrin domain in pCDNA3-mADAM9L.myc using site-directed mutagenesis using the following sequences : 5’ KPN1 5’ GTGGAGCAAAGAGCGGTACCATGAATTCAGGAGC 3’ and 5’ GCTCCTGAATTCATGGTACCGCTCTTTGCTCCAC 3’; 3’ KPN1 5’ GAAGGAGTGTGAGGGTACCCCATGCTGTGAAGGAAG 3’ and 5’ CTTCCTTCACAGCATGGGGTACCCTCACACTCCTTC 3’. KPN1 digestion was used to isolate and discard the disintegrin sequence, and the vector backbone KPN1 sites were ligated. For RNAi (RNA interference), ADAM9 sequences from the Mission siRNA project (Sigma Aldrich) were cloned into the pLKO.1 vector directing expression of short hairpin RNA (shRNA) (32). The following sequences were used: ADAM9shRNA.1 Sense 5’ CCGGCCCAGAGAAGTTCCTATATATCTCGAGATATATAGGAACTTCTCTGGGTTTTT G 3’ antisense: 5’ AATTCAAAAACCCAGAGAAGTTCCTATATATCTCGAGATATATAGGAACTTCTCTGG 3’; ADAM9shRNA.2 Sense 5’ CCGGGCCAGAATAACAAAGCCTATTCTCGAGAATAGGCTTTGTTATTCTGGCTTT 3’ antisense 5’ AATTCAAAAAGCCAGAATAACAAAGCCTATTCTCGAGAATAGGCTTTGTTATTCTGG C 3’. Second-generation lentiviral packaging plasmids pCMV-dR8.2 dvpr and pCMV-VSVG were obtained from ADDGENE (Cambridge, MA) for lentiviral packaging.
Cell Lines
All cell lines were obtained from ATCC, with the exception of the estrogen-independent human breast cancer SUM-159-PT which has been described (33). BT549, HCC38, and ZR75-1 cells were maintained in RPMI supplemented with 10% fetal bovine serum. MDA-MB-231, HEK293T, MDA-MB-468 and NIH3T3 cells were maintained in DMEM supplemented with 10% fetal bovine serum. CAMA-1 cells were maintained in MEM supplemented with 10% fetal bovine serum. SKBR3 cells were maintained in McCoy’s Modified Medium 5a supplemented with 10% fetal bovine serum. MCF10A cells were maintained in DMEM modified with 5% equine serum, 20ng/ml EGF, 10ug/ml insulin, 100ng/ml cholera toxin, 500ng/ml hydrocortisone, and 1% penicillin and streptomycin. Cell lines were verified by multiple methods including DNA barcoding, gene expression and transcriptome analysis, and were kept in culture for less than 6 months after receipt.
Cell Lysis
Cells were lysed in NP40 lysis buffer (20mM Tris HCl [pH 7.0], 10% glycerol, 1% NP40, 10mM EDTA, 150mM NaCl, 20mM NaF, 5mM sodium pyrophosphate) supplemented with protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was calculated using Bio-Rad Protein Assay solution
Immunoprecipitation
Equal amounts of protein were incubated at 4°C in a 1mL volume with 2μg antibody for 4 h prior to addition of Protein A Sepharose beads. Beads were incubated for 2 h, and then washed with 1% NP40 in PBS three times before elution with 2x Laemmli lysis buffer (125mM Tris [pH6.8] , 23% Glycerol, 4% SDS, 10% β-mercaptoethanol, bromphenol blue) and immunoblotting.
Immunoblotting
Cell lysates were equilibrated using the BioRad Protein Assay solution, and 50–100 μg protein was resolved by SDS PAGE, transferred to nitrocellulose (Hoefer semi-dry transfer system, 160mA, 80–120 min), and blocked in 5% non-fat milk in 1%TBST (1% Tween in TBS). Membranes were incubated with the appropriate antibodies (primary antibodies used at the concentration recommended by manufacturer, ADAM9-S used at 2μg/mL) for 14 h. Cell membranes were washed in 1%TBST and incubated with peroxidase-conjugated anti-mouse or anti-rabbit antibodies (Jackson Immunoresearch) and exposed using high-sensitivity ECL (Millipore).
Lentiviral Infection
pLKO.1 plasmid constructs were co-transfected into HEK293T cells with packaging vectors pCMV-dR8.2 dvpr and pCMV-VSVG using polyethyleimine. 48 h post-transfection, lentiviral particles in supernatant were harvested, passed through a 0.45μM filter and either stored at −80°C or used to infect target cells. Target cells at approximately 80% confluency were infected with virus alone for a period of 4 h at which point they were supplemented with full serum and allowed to recover for 24 h before selection in puromycin in full culture medium for 18 h (2–5 μg/mL).
Transfection of siRNA into BT549 cells
pCS2-(n)-β-galactosidase reporter plasmid (1μg) was transfected alone, or with 200pM of non-targeting or ADAM9 siRNA pools according to the Lipofectamine 2000 protocol (5μl Lipofectamine 2000, 500μl Optimem (Gibco)) into 5×105 BT549 cells per 6cm dish (Corning). Media was replaced with full-serum medium 4 h post-transfection.
Transfection of plasmid DNA
Plasmids of interest and the pCS2-(n)-β-galactosidase reporter plasmid were co-transfected at a ratio of 6.5:1 into non-infected or lentivirally-infected cells 24 h post-infection with Lipofectamine 2000 according to manufacturers’ instructions. Media was replaced with full culture medium for non-infected cells or puromycin selection media 4 h post-transfection. Cells were assayed for migration and protein expression 24 h post-transfection.
Migration Assays
Migration assays were carried out using Transwell chambers (Corning, Acton, Massachusetts) with 8 μM pore membranes. Cells were trypsinized and resuspended in serum-free media containing 0.1% BSA. 1×105 cells were added to the top of each Transwell chamber, and allowed to migrate toward cell-conditioned media for 4–16 h at 37°C depending on the cell type. Cells that had migrated to the lower surface of the membrane were fixed and stained. Lentivirally-infected cells were stained with Hema-3 Protocol Stain (Fisher Scientific). Cells co-transfected with expression plasmids and β-galactosidase were stained with X-galactosidase. Experiments were conducted in triplicate, and equal fields of stained cells were counted in each well and normalized to the plating efficiency or the transfection efficiency. Transfection efficiency was calculated by counting the number of transfected cells per 100,000. Plating efficiency was calculated by counting two wells of 100,000 cells plated in parallel to the migration assays to control for minor discrepancies in plating. Statistical analysis was performed using ANOVA and the InStat statistical analysis software. Cell-conditioned media was harvested from NIH3T3 fibroblast cells grown in full-serum for 48 h to confluency, or HEK293T cells transfected with hADAM9-S expression plasmids and incubated in serum-free media for 48 h.
Integrin-blocking assays
Cells were infected and transfected with plasmid DNA as described above. Prior to migration assay cells were trypsinized and incubated with 5μg/ml of GOH3 antibody for 10 min at room temperature.
Results
ADAM9-S is expressed in breast cancer cell lines and breast tumors
We first determined the expression pattern of ADAM9-S and ADAM9-L in breast cancer cells and breast cancer tissues. To detect specific endogenous isoforms of ADAM-9, a polyclonal antibody directed toward the carboxyl-terminus of ADAM9-L and an ADAM9-S specific polyclonal antibody designed against the unique carboxyl-terminus of human ADAM9-S were used (Fig. 1A). To confirm isoform specificity, C-terminal Myc-tagged murine ADAM9-L and human ADAM9-S were transiently expressed in HEK-293T cells. Equal amounts of protein were immunoblotted with Myc antibody to validate equivalent expression and ADAM9 antibody specificity. The results show that as expected, ADAM9-L is detected by Myc and ADAM9-L antibodies as a pro-ADAM9-L variant that comprises the unprocessed form migrating at 114kDa, and the fully processed mature membrane bound form at 84kDa (Fig. 1B). Expression of ADAM9-S is detected by Myc and ADAM9-S antibodies at 67kDa, as previously shown (26). Importantly, the ADAM9-L antibody does not cross-react with ADAM9-S, and the ADAM9-S antibody does not detect ADAM9-L (Fig. 1B). Therefore, specific antibodies against ADAM9-L and ADAM9-S are specific to the secreted and membrane bound variants of ADAM9. It is important to note that ADAM9-L and ADAM9-S migrate slower on SDS PAGE than their predicted molecular mass due to the presence of cysteine-rich regions as well as glycosylation.
Figure 1. Expression of ADAM9 Isoforms in Breast Cancer Cell Lines and Tissues.
A, A schematic representation of the membrane-bound (ADAM9-L) and secreted (ADAM9-S) isoforms of ADAM9.
B, HEK293T cells transiently transfected with a vector control (pCDNA4), murine ADAM9-L.myc and human ADAM9-S.myc were lysed and proteins resolved on an 8% SDS-PAGE gel. Equivalent lanes were blotted with anti-myc to validate protein expression. Equivalent lanes were blotted with ADAM9-L and ADAM9-S specific antibodies.
C, a panel of cell lines representing breast cancer subtypes were used for immunoblotting with ADAM9-L C-terminal specific antibody, ADAM9-S C-terminal specific antibody, and β actin. # denotes overexpression of ADAM9 and amplified 8p11– 12 , * denotes amplified 8p11.12.
D, SDS-extracted lysates from frozen tumor blocks were immunoblotted with anti-ADAM9-S antibody. BT549 cell lysate was used as control. All results are representative of at least three independent experiments.
To evaluate the expression of ADAM9-S and ADAM9-L in breast cancer cell lines, lysates were collected from BT549, HCC38, MDA-MB-231, SUM159PT, MCF10A, MDA-MB-468, CAMA-1, ZR75-1, MCF-7, and SKBR-3 cells at approximately 80–90% confluence, and ADAM9 expression was determined by immunoblotting. ADAM9-L expression is detected in all cell lines tested (Fig. 1C dark exposure). Importantly, cells previously characterized to overexpress ADAM9 (BT549, HCC38, CAMA-1) (31) reveal a substantially higher level of expression of ADAM9-L compared to other lines (Fig 1C light exposure). In addition, the variance in ADAM9-L bands detected by immunoblotting reflects differences in processing as well as post-translational modifications that include proteolytic cleavage and glycosylation. ADAM9-S endogenous expression is detected in BT549, HCC38, SUM159PT, MCF10A, and MDA-MB-468 cells (Fig 1C). Thus, endogenous ADAM9-L expression is detected in cells of both the luminal and basal breast cancer subtypes, while ADAM9S is predominantly observed in basal-type cells. Notably, CAMA-1, a non-invasive cell type, expresses ADAM9-L, but not ADAM9-S.
To evaluate expression of ADAM9 isoforms in breast cancer tissues, ADAM9-S levels were evaluated in Stage II and III invasive ductal carcinoma tumor and normal breast lysate samples kindly provided by Dr. Gerburg Wulf (Beth Israel Deaconess Medical Center) (34). The results show that ADAM9-S is expressed in breast cancers of both grades as well as normal tissue (Fig 1D).
Overexpression of ADAM9-S promotes breast cancer cell migration
Previous studies have shown that cancer cells exhibit increased translocation through Matrigel in the presence of exogenous recombinant ADAM9-S (26). To evaluate the role of ADAM9 isoforms in cell migration independent of matrix proteolysis, motility was assayed using uncoated Transwells. Plasmid constructs directing the expression of wild type and mutant murine ADAM9-L and human ADAM9-S (Fig. 2A) were transfected into BT549 breast cancer cells. Overexpression of wild-type ADAM9-S significantly increases BT549 migration. In contrast, overexpression of a metalloprotease-deficient ADAM9-S.EA mutant with a Glu to Ala substitution in the metalloprotease active site (35) does not increase migration and is comparable to control transfected cells (Fig. 2B). Similarly, overexpression of the ADAM9-L variant did not promote BT549 cell migration, nor did overexpression of the equivalent metalloprotease-deficient ADAM9L.EA mutant. Therefore, as previously reported, ADAM9-S promotes cell migration in a protease activity-dependent manner. However, this phenotype is not recapitulated by ADAM9-L.
Figure 2. ADAM9-S Promotes Breast Cancer Cell Migration.
A, schematic of human and murine ADAM9 constructs used in migration experiments.
B, migration of BT549 cells overexpressing ADAM9-L and -S. Cells were migrated for 4 h in Transwells. Expression of transfected proteins was confirmed with anti-ADAM9-L C-terminal specific antibody or anti-myc. ADAM9-L is denoted by arrows. Overexpressed murine ADAM9-L migrates slower than endogenous human ADAM9-L. *, p< 0.001, calculated using Anova.
C, migration of BT549 cells stimulated with exogenous ADAM9-S. Cells were migrated for 16 h in Transwells in which the bottom chamber contained media collected from HEK293T cells expressing pCDNA control, ADAM9-S or ADAM9S.EA. Expression of ADAM9-S was confirmed by anti-myc immunoblotting. *, p< 0.05, calculated using Anova. All results are mean +/- SD of triplicate measurements of at least three independent experiments.
To confirm that extracellular ADAM9-S stimulates cell migration, ADAM9-S, ADAM9S.EA or vector control was expressed in HEK293T cells and serum-free conditioned media was collected post-transfection. These supernatants were added to the bottom chamber of uncoated transwells, and BT549 breast cancer cells were added to the top chamber. Under these conditions, BT549 cells require an extended 16 hour incubation period to migrate. However, BT549 cells exhibit increased migration when exposed to ADAM9-S-containing conditioned media in comparison to control-transfected or ADAM9S.EA-transfected media, indicating that exogenous ADAM9-S stimulates haptotactic migration through its metalloprotease activity (Fig 2C).
ADAM9 silencing increases breast cancer cell migration
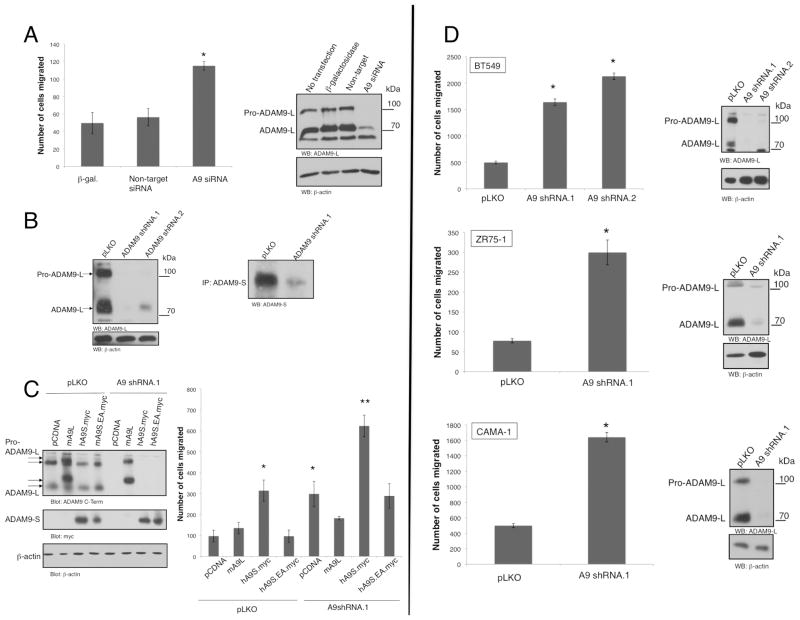
To determine the functional role of endogenous ADAM9 in cell migration, we preformed loss of function experiments using an siRNA oligonucleotide pool targeting five distinct sequences spanning both ADAM9 isoforms. We chose to transiently transfect these siRNA’s into BT549 cells, which express high levels of ADAM9 and are also highly migratory. Surprisingly, ADAM9 siRNA increases migration in BT549 cells as measured by Transwell migration assays, compared to control or non-target siRNA (Fig. 3A). This is concomitant with quantitative silencing of ADAM9-L protein expression. The phenotype of increased migration by ADAM9 siRNA recapitulates that observed upon expression of ADAM9-S (Fig. 2A). To evaluate that this is not due to an off-target effect of the siRNA pool, two distinct lentiviral shRNA constructs were generated to target both isoforms in the 3’ untranslated region of ADAM9 mRNA (ADAM9 shRNA.1) and the cysteine-rich domain (ADAM9 shRNA.2). After lentiviral infection and selection, robust silencing of ADAM9-L and ADAM9-S protein is observed in cells transduced with both sequences as revealed by immunoblotting (Fig. 3B). Importantly, transduction of both shRNA sequences also results in an increase in BT549 cell migration (Fig. 3D). Moreover, increased cell migration resulting from transduction of ADAM9 shRNA is also reproduced in distinct breast cancer cell lines, including CAMA-1 and ZR75-1 (Fig. 3D). Therefore, in different luminal and basal breast cancer type cells silencing ADAM9 expression results in enhanced cell migration observed with ADAM9 shRNA.1 and .2 sequences.
Figure 3. ADAM9 Silencing Increases Cell Migration.
A, BT549 cells were transfected with β-galactosidase alone, or in conjunction with non-targeting siRNA oligonucleotide, or ADAM9 siRNA oligonucleotide pool. Cells were migrated for 16 h. *, p<0.01 , calculated using Anova. Silencing was confirmed by immunoblotting using ADAM9-L C-terminal specific antibody and β-actin.
B, BT549 cells infected with ADAM9 shRNA.1 and shRNA.2 were lysed and immunoblotted against ADAM9-L and ADAM9-S. ADAM9-S was immunoprecipitated from equal cell lysates prior to immunoblotting.
C, BT549 cells were infected with control or ADAM9 shRNA.1 lentiviruses before transfection of pCDNA control, ADAM9-L, ADAM9-S and ADAM9S.EA. Cells were migrated for 4 h. *, p< 0.05, ** p<0.001, calculated using Anova. ADAM9-L immunoblot confirms silencing. ADAM9-S expression is detected with anti-Myc.
D, BT549, CAMA-1 and ZR75-1 cells were infected with control or ADAM9 shRNA.1 or shRNA.2 lentiviruses. Selected cells were migrated in uncoated Transwells for 4 h (BT549) or 16 h. *, p<0.03 , calculated using Anova. Silencing is confirmed by immunoblot with ADAM9-L C-terminal antibody. In all experiments cell counts were normalized and results shown are the mean +/− SD of triplicate measurements of at least three independent experiments.
To further confirm that ADAM9 silencing is responsible for the increase in migration observed with shRNA, a reconstitution experiment was performed by introducing murine ADAM9-L and human ADAM9-S in cells harboring ADAM9 shRNA. The murine ADAM9-L (mADAM9-L) would be predicted to be refractory to silencing using human ADAM9. Consistent with this, expression of mADAM9-L protein was resistant to silencing in cells expressing ADAM9 shRNA, compared to cells with ADAM9 shRNA alone that show a robust reduction of ADAM9-L protein (Fig. 3C). In Transwell assays, expression of mADAM9-L rescues the increase in migration observed with shRNA alone. Therefore, the increase in migration observed upon silencing of ADAM9 is specific to ADAM9-L expression and is not due to off-target effects. The implication is that endogenous ADAM9-L is a suppressor of migration of breast cancer cells.
We also evaluated the contribution of ADAM9-S in the enhanced migration phenotype observed with ADAM9 shRNA. As observed in Fig. 2A, expression of wild type, but not protease-deficient ADAM9-S increases cell migration in control BT549 cells (Fig. 3C). In contrast to the effect of expression of mADAM9-L, the increase in migration induced by ADAM9 silencing is not rescued by expression of ADAM9-S that is refractory to silencing as the shRNA used (ADAM9 shRNA.1) targets the 3’ untranslated region. Instead, expression of ADAM9-S leads to a further increase in the migratory phenotype that is additive to ADAM9 shRNA and ADAM9-S. As expected, this additive effect is not recapitulated by expression of the ADAM9-S protease inactive EA mutant (Fig. 3C). Therefore, ADAM9-S does not phenocopy the migration suppression function of ADAM9-L, and moreover the implication from these results is that suppression of migration is the dominant role of ADAM9 in breast cancer cells.
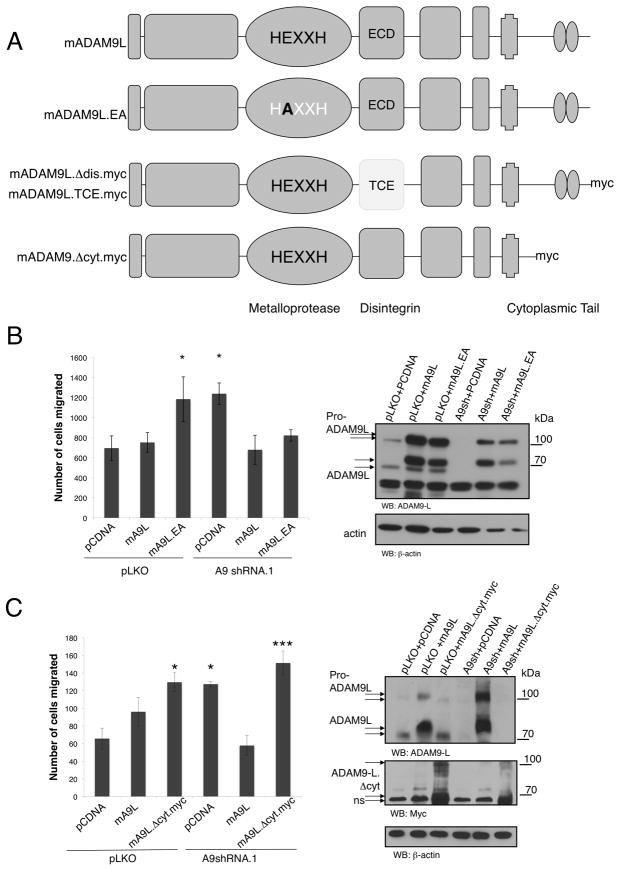
Functional role of ADAM9-L domains in suppression of cell migration
The data thus far demonstrate that ADAM9-S is an enhancer of cell migration, whereas ADAM9-L is a suppressor of migration. We next determined the contribution of the ADAM9-L functional domains in the suppression of migration. We generated mutations or deletions in the metalloprotease (ADAM9-L.EA), disintegrin (ADAM9-L.ΔDis) and cytoplasmic (ADAM9-L.ΔCyt) domains (Fig. 4A). Again the murine ADAM9-L allele was used to generate these mutations as it is refractory to silencing in human breast cancer cells. Wild type and mutant mADAM9-L alleles were introduced into BT549 cells subsequent to silencing with ADAM9 shRNA. As already observed, expression of the wild type ADAM9-L allele rescued the phenotype of increased migration upon ADAM9 silencing (Fig. 4B). This was also observed in cells expressing the metalloprotease-deficient ADAM9-L.EA mutant, indicating that the proteolytic activity of ADAM9-L is dispensable for the suppression of migration. In contrast, there was no rescue of increased migration with the cytoplasmic domain deletion mutant (ADAM9-L.ΔCyt), demonstrating that localization and downstream signaling mediated by the ADAM9-L cytoplasmic domain is required for suppression of cell migration (Fig. 4C).
Figure 4. ADAM9-L Suppresses Cell Migration in a Metalloprotease-independent Manner.
A, schematic representation of murine wild type ADAM9 isoforms and functional domain mutations used to assess the role of each domain in cell migration.
B, BT549 cells were infected with control or A9shRNA.1 lentiviruses before transfection with pCDNA control, mADAM9-L wild type or mADAM9L.EA post-selection. Cells were migrated in uncoated Transwells for 4 h. *, p<0.01, calculated using Anova. Cell lysates were immunoblotted against actin and ADAM9-L to detect both murine (upper arrows) and human (lower arrows) variants.
C, BT549 cells were infected with control or A9shRNA.1 lentiviruses before transfection with pCDNA control, mADAM9-L wild type or mADAM9L.Δcyt post-selection. Cells were migrated in uncoated Transwells for 4 h. . *, p<0.05 , *** p<0.001, calculated using Anova. ADAM9-L expression is detected with anti-Myc, ADAM9 silencing is detected with anti-ADAM9-L, and actin is control. ns, non-specific immunoreactive protein. In all experiments cell counts were normalized to transfection efficiency and results are the mean +/− SD of triplicate measurements of at least three independent experiments.
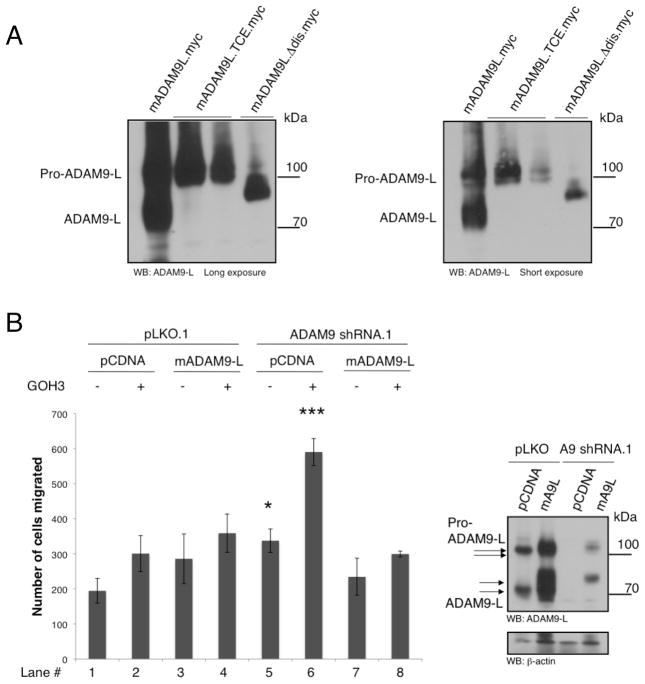
To address the role of the ADAM9-L disintegrin domain and integrin-binding activity, point mutations in the ECD motif as well as whole deletions in the integrin-binding domain were generated. However, expression of these mutant proteins followed by immunoblotting revealed that both mutants are not properly processed from the pro-domain containing form (114 kDa) to the active and mature 84kDa form, compared to wild type ADAM9-L (Fig. 5A). Therefore, we assessed the contribution of the disintegrin domain in the regulation of cell migration using integrin function blocking antibodies. BT549 cells were transduced with ADAM9 shRNA and reconstituted with wild type murine ADAM9-L, followed by treatment with GOH3, an antibody that blocks agr;6-integrin binding to the its ligand laminin (Fig. 5B), or control IgG. GOH3 is also routinely used as direct binding ligand to activate integrin clustering (36). Interestingly, treatment with GOH3 modestly increased the migration of BT549 cells with control pLKO. As predicted by the data thus far, silencing ADAM9 with shRNA increased cell migration compared to control (compare lane 5 to lane 1). However, treatment with GOH3 significantly increased migration (compare lane 6 to lane 5), an effect that was quantitatively rescued by expression of the shRNA-resistant mADAM9-L allele (compare lane 8 to lane 6). This demonstrates that ADAM9-L suppresses cell migration by altering integrin-mediated signals, either at the level of integrin binding, or by interacting with a parallel signaling pathway that engages in cross-talk downstream of integrin receptors.
Figure 5. The ADAM9-L Integrin Binding Activity Modulates Cell Migration.
A, HEK293T cells were transfected with wildtype mADAM9-L, point mutant mADAM9L.TCE.myc, and mADAM9.Δdis.myc. Lysates were immunoblotted with ADAM9-L C-terminal antibody.
B, BT549 cells infected with control PLKO or ADAM9shRNA.1 virus were transfected with pCDNA control or mADAM9L wildtype constructs. Cells were resuspended in 0.01% BSA in PBS with 5μg/mL GOH3 antibody and incubated for 10 min prior to a 4 hr migration assay. Lysates were immunoblotted with ADAM9-L C-terminal antibody to confirm silencing and expression. All results shown are mean +/− SD of triplicate measurements of two independent experiments. p values were calculated using Anova *, p<0.05, ***, p<0.001.
Discussion
The ADAM family of metalloproteases controls multiple phenotypes associated with tumor progression, although the specific role of individual isoforms in tumorigenesis is poorly understood. Several ADAM isoforms, including ADAM9, have been shown to promote cell migration. In this study we identify non-redundant roles for the secreted and membrane-bound ADAM9 variants in breast cancer cell migration. Consistent with previous observations, we show that ADAM9-S can promote breast cancer cell migration in a cell-autonomous manner. Surprisingly, this phenotype is not recapitulated by ADAM9-L, which functions as a migration suppressor.
The ADAM9 gene (8p11.23) is located in the 8p11–12 cluster that is frequently amplified or deleted in breast cancer, with instances of abnormal copy number that correlate with reduced patient survival (37). Although ADAM9 null mice have no overt phenotypes (38), in mouse models of breast, colon and prostate carcinoma such as MMTV-PyMT, APC/Min/+, and W10, overexpression of ADAM9 in neoplastic regions is detected (35). ADAM9 −/− W10 mice have well-differentiated tumors compared to tumors in ADAM9 +/+ W10 (35). Immunohistochemical analysis of ADAM9 expression in breast tumors and cell lines support our finding that secreted ADAM9-S is expressed in breast cancer tissues (29, 30). In our studies the ADAM9-specific antibodies were unable to provide a clear detection of protein expression by immunohistochemistry or immunocytochemistry, but regardless this is the first clear identification of ADAM9-S expression in tumors and cell lines.
Breast cancer cell lines are separated into different clusters based on gene expression profiles (39). Luminal cell lines express genes associated with a more differentiated, non-invasive phenotype, while basal cell lines are less differentiated and more invasive. In a survey of gene expression profiles of 51 breast cancer cell lines, the basal gene cluster was separated into two groups, basal A and B, of which B has a stem-cell like expression profile closest to the clinical “triple-negative” tumor profile (31). Cell lines with ADAM9 amplification and with or without ADAM9 mRNA overexpression were identified in this analysis. In our present study, we find that ADAM9-L is expressed in cells of each gene cluster, while ADAM9-S is expressed primarily in the more aggressive basal genotype (Fig. 1C), correlating with the finding that ADAM9-S promotes cell migration.
Our previous studies had identified ADAM9-S as a pro-migration factor that is secreted by activated liver myofibroblasts in the wound healing response (26). Tumors are sites of chronic wounding, and cancer cells adapt to the wound by increasing cell migration and angiogenesis. The success of tamoxifen treatment in recurring breast cancer with a high stromal content is correlated with elevated levels of ADAM9 (40). Similarly, ADAM9 is upregulated in the stroma surrounding invasive melanoma (41) and colon cancer (26), as well as prostate cancer cells (42, 43). These studies emphasize the importance of ADAM9 function at the tumor-stromal boundary, suggesting that ADAM9 may function downstream of paracrine factors involved in wound healing. We find that while ADAM9-S promotes cell migration via its metalloprotease activity (Fig. 2A), ADAM9-L functions as a migration suppressor in a manner that is independent of proteolytic activity (Fig. 4B). ADAM9-S may proteolyze specific substrates on the cell surface or ECM that are not targeted by ADAM9-L as its localization is restricted by membrane tethering. Consistent with this, cells expressing ADAM9-S have a higher rate of migration than cells exposed to a more diffuse exogenously-added ADAM9-S (Fig. 2A and 2B), indicating that ADAM9-S might function in a cancer cell autonomous manner rather than acting on the tumor stroma. Regardless, the opposing functions of ADAM9-L and ADAM9-S in cell migration suggest that the two splice variants control tissue homeostasis, with isoform-specific functions in response to signals both from neighboring tumor and stromal cells. A recent shRNA screen for cell migration enhancers and suppressors identified ADAM9 as a migration suppressor in non-tumorigenic MCF10A epithelial cells, consistent with our findings (44). Surprisingly, we find that the ADAM9-L metalloprotease domain is dispensable for this phenotype, whereas the cytoplasmic and disintegrin domains are required, indicative of a role for membrane localization and cytoplasmic signaling.
The disintegrin domain of ADAM9 influences cell migration in a cell type-dependent manner. Keratinocytes adhering to recombinant ADAM9-L disintegrin domain exhibit increased migration (25), a result also observed with fibroblasts adhering to recombinant ectodomain (45). In contrast, recombinant disintegrin domain inhibits the adhesion of platelets to collagen I (46), and interacts with multiple integrins resulting in ERK (extracellular regulated kinase) phosphorylation, activation of p38MAPK, cPLA2 and MMP-9 synthesis (25, 27). In breast carcinoma cells, antibody binding to α6-integrin enhances migration, a phenotype that is significantly enhanced in the absence of ADAM9 expression (Fig. 5B). ADAM9-L may control the localization of integrins or Syndecans to specific lipid rafts (47), or alternatively modulate endocytosis of cell surface receptors. ADAM9 has also been shown to interact with and promote the recycling of E-cadherin in colon cancer cells, and this may represent a metalloprotease independent mechanism for ADAM9-L (48).
The opposing roles for ADAM9-S and ADAM9-L as migration enhancers and suppressors in breast cancer cells raises the question as to how the expression profile of each splice variant is regulated. This is predicted to have a major impact in tumor progression as cells overexpressing ADAM9-L would presumably have a reduced migration phenotype. Conversely, any tumors in which ADAM9-S is the predominant isoform would be predicted to have aggressive invasive and metastatic characteristics. Although presently unknown, it is highly likely that one important mechanism is the control of alternative splicing of the ADAM9 message.
In summary, our results show that the secreted and membrane-tethered isoforms of ADAM-9 have opposing functions in breast cancer cell migration. The expression of both isoforms in breast cancer tumors and stromal suggests a model in which the secreted ADAM9-S acts as a migratory stimulant, while ADAM9-L tethered to the plasma membrane binds cell surface proteins and mediates localization and signaling. One important implication from these findings is that isoform-specific functions of secreted and membrane-tethered proteases exist which has obvious consequences for the development of small molecule inhibitors for therapeutic intervention.
Acknowledgments
Grant Support: NIH grants CA096710 (A. Toker); Department of Defense Breast Cancer Research Program Pre-Doctoral Traineeship Award W81XWH 06-1-046 (J. Fry). We thank Dr. Gerburg Wulf for kindly providing tumor tissue samples, Sara Courtneidge for providing ADAM9-L cDNA, Dr. Carl Blobel for providing murine ADAM9-L cDNA and Dr. Isaac Rabinovitz for assistance with the integrin binding studies. We also thank Drs. Marsha Moses, Bruce Zetter and Jeffrey Settleman for guidance, and all members of the Toker laboratory for discussions and advice.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest are disclosed.
References
- 1.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–47. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 2.Zigrino P, Loffek S, Mauch C. Tumor-stroma interactions: their role in the control of tumor cell invasion. Biochimie. 2005;87:321–8. doi: 10.1016/j.biochi.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Rodriguez D, Petitclerc E, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–79. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowlkes JL, Thrailkill KM, Serra DM, Suzuki K, Nagase H. Matrix metalloproteinases as insulin-like growth factor binding protein-degrading proteinases. Prog Growth Factor Res. 1995;6:255–63. doi: 10.1016/0955-2235(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 6.Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271:10079–86. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 7.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8:929–41. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 8.Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J Immunol. 2001;166:4042–8. doi: 10.4049/jimmunol.166.6.4042. [DOI] [PubMed] [Google Scholar]
- 9.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–76. [PMC free article] [PubMed] [Google Scholar]
- 10.Iba K, Albrechtsen R, Gilpin B, et al. The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to beta1 integrin-dependent cell spreading. J Cell Biol. 2000;149:1143–56. doi: 10.1083/jcb.149.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaultier A, Cousin H, Darribere T, Alfandari D. ADAM13 disintegrin and cysteine-rich domains bind to the second heparin-binding domain of fibronectin. J Biol Chem. 2002;277:23336–44. doi: 10.1074/jbc.M201792200. [DOI] [PubMed] [Google Scholar]
- 12.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 13.Kveiborg M, Frohlich C, Albrechtsen R, et al. A role for ADAM12 in breast tumor progression and stromal cell apoptosis. Cancer Res. 2005;65:4754–61. doi: 10.1158/0008-5472.CAN-05-0262. [DOI] [PubMed] [Google Scholar]
- 14.Pories SE, Zurakowski D, Roy R, et al. Urinary metalloproteinases: noninvasive biomarkers for breast cancer risk assessment. Cancer Epidemiol Biomarkers Prev. 2008;17:1034–42. doi: 10.1158/1055-9965.EPI-07-0365. [DOI] [PubMed] [Google Scholar]
- 15.Roy R, Wewer UM, Zurakowski D, Pories SE, Moses MA. ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem. 2004;279:51323–30. doi: 10.1074/jbc.M409565200. [DOI] [PubMed] [Google Scholar]
- 16.Trochon-Joseph V, Martel-Renoir D, Mir LM, et al. Evidence of antiangiogenic and antimetastatic activities of the recombinant disintegrin domain of metargidin. Cancer Res. 2004;64:2062–9. doi: 10.1158/0008-5472.can-03-3272. [DOI] [PubMed] [Google Scholar]
- 17.Beck V, Herold H, Benge A, et al. ADAM15 decreases integrin alphavbeta3/vitronectin-mediated ovarian cancer cell adhesion and motility in an RGD-dependent fashion. Int J Biochem Cell Biol. 2005;37:590–603. doi: 10.1016/j.biocel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Kleino I, Ortiz RM, Huovila AP. ADAM15 gene structure and differential alternative exon use in human tissues. BMC Mol Biol. 2007;8:90. doi: 10.1186/1471-2199-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumi Y, Hirata M, Hasuwa H, et al. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCdelta are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. Embo J. 1998;17:7260–72. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ongusaha PP, Kwak JC, Zwible AJ, et al. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer Res. 2004;64:5283–90. doi: 10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]
- 21.Dyczynska E, Sun D, Yi H, Sehara-Fujisawa A, Blobel CP, Zolkiewska A. Proteolytic processing of delta-like 1 by ADAM proteases. J Biol Chem. 2007;282:436–44. doi: 10.1074/jbc.M605451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkin E, Harris B. A disintegrin and metalloproteinase (ADAM)-mediated ectodomain shedding of ADAM10. J Neurochem. 2009;108:1464–79. doi: 10.1111/j.1471-4159.2009.05907.x. [DOI] [PubMed] [Google Scholar]
- 23.Tousseyn T, Thathiah A, Jorissen E, et al. ADAM10, the Rate-limiting Protease of Regulated Intramembrane Proteolysis of Notch and Other Proteins, Is Processed by ADAMS-9, ADAMS-15, and the {gamma}-Secretase. J Biol Chem. 2009;284:11738–47. doi: 10.1074/jbc.M805894200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahimkar RM, Visaya O, Pollock AS, Lovett DH. The disintegrin domain of ADAM9: a ligand for multiple beta1 renal integrins. Biochem J. 2005;385:461–8. doi: 10.1042/BJ20041133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zigrino P, Steiger J, Fox JW, et al. Role of ADAM-9 disintegrin-cysteine-rich domains in human keratinocyte migration. J Biol Chem. 2007;282:30785–93. doi: 10.1074/jbc.M701658200. [DOI] [PubMed] [Google Scholar]
- 26.Mazzocca A, Coppari R, De Franco R, et al. A secreted form of ADAM9 promotes carcinoma invasion through tumor-stromal interactions. Cancer Res. 2005;65:4728–38. doi: 10.1158/0008-5472.CAN-04-4449. [DOI] [PubMed] [Google Scholar]
- 27.Karadag A, Zhou M, Croucher PI. ADAM-9 (MDC-9/meltrin-gamma), a member of the a disintegrin and metalloproteinase family, regulates myeloma-cell-induced interleukin-6 production in osteoblasts by direct interaction with the alpha(v)beta5 integrin. Blood. 2006;107:3271–8. doi: 10.1182/blood-2005-09-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maschler S, Wirl G, Spring H, et al. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–41. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 29.Lendeckel U, Kohl J, Arndt M, Carl-McGrath S, Donat H, Rocken C. Increased expression of ADAM family members in human breast cancer and breast cancer cell lines. J Cancer Res Clin Oncol. 2005;131:41–8. doi: 10.1007/s00432-004-0619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Shea C, McKie N, Buggy Y, et al. Expression of ADAM-9 mRNA and protein in human breast cancer. Int J Cancer. 2003;105:754–61. doi: 10.1002/ijc.11161. [DOI] [PubMed] [Google Scholar]
- 31.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moffat J, Grueneberg DA, Yang X, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 33.Flanagan L, Van Weelden K, Ammerman C, Ethier SP, Welsh J. SUM-159PT cells: a novel estrogen independent human breast cancer model system. Breast Cancer Res Treat. 1999;58:193–204. doi: 10.1023/a:1006331716981. [DOI] [PubMed] [Google Scholar]
- 34.Wulf GM, Ryo A, Wulf GG, et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. Embo J. 2001;20:3459–72. doi: 10.1093/emboj/20.13.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peduto L, Reuter VE, Shaffer DR, Scher HI, Blobel CP. Critical function for ADAM9 in mouse prostate cancer. Cancer Res. 2005;65:9312–9. doi: 10.1158/0008-5472.CAN-05-1063. [DOI] [PubMed] [Google Scholar]
- 36.Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992;117:671–8. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chin K, DeVries S, Fridlyand J, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–41. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Weskamp G, Cai H, Brodie TA, et al. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol Cell Biol. 2002;22:1537–44. doi: 10.1128/mcb.22.5.1537-1544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perou CM, Jeffrey SS, van de Rijn M, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci U S A. 1999;96:9212–7. doi: 10.1073/pnas.96.16.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sieuwerts AM, Meijer-van Gelder ME, Timmermans M, et al. How ADAM-9 and ADAM-11 differentially from estrogen receptor predict response to tamoxifen treatment in patients with recurrent breast cancer: a retrospective study. Clin Cancer Res. 2005;11:7311–21. doi: 10.1158/1078-0432.CCR-05-0560. [DOI] [PubMed] [Google Scholar]
- 41.Zigrino P, Mauch C, Fox JW, Nischt R. Adam-9 expression and regulation in human skin melanoma and melanoma cell lines. Int J Cancer. 2005;116:853–9. doi: 10.1002/ijc.21087. [DOI] [PubMed] [Google Scholar]
- 42.Shigemura K, Sung SY, Kubo H, et al. Reactive oxygen species mediate androgen receptor- and serum starvation-elicited downstream signaling of ADAM9 expression in human prostate cancer cells. Prostate. 2007;67:722–31. doi: 10.1002/pros.20565. [DOI] [PubMed] [Google Scholar]
- 43.Sung SY, Kubo H, Shigemura K, et al. Oxidative Stress Induces ADAM9 Protein Expression in Human Prostate Cancer Cells. Cancer Res. 2006;66:9519–26. doi: 10.1158/0008-5472.CAN-05-4375. [DOI] [PubMed] [Google Scholar]
- 44.Simpson KJ, Selfors LM, Bui J, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–38. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 45.Nath D, Slocombe PM, Webster A, Stephens PE, Docherty AJ, Murphy G. Meltrin gamma(ADAM-9) mediates cellular adhesion through alpha(6)beta(1 )integrin, leading to a marked induction of fibroblast cell motility. J Cell Sci. 2000;113 ( Pt 12):2319–28. doi: 10.1242/jcs.113.12.2319. [DOI] [PubMed] [Google Scholar]
- 46.Cominetti MR, Martin AC, Ribeiro JU, et al. Inhibition of platelets and tumor cell adhesion by the disintegrin domain of human ADAM9 to collagen I under dynamic flow conditions. Biochimie. 2009;91:1045–52. doi: 10.1016/j.biochi.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Thodeti CK, Albrechtsen R, Grauslund M, et al. ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J Biol Chem. 2003;278:9576–84. doi: 10.1074/jbc.M208937200. [DOI] [PubMed] [Google Scholar]
- 48.Hirao T, Nanba D, Tanaka M, et al. Overexpression of ADAM9 enhances growth factor-mediated recycling of E-cadherin in human colon cancer cell line HT29 cells. Exp Cell Res. 2006;312:331–9. doi: 10.1016/j.yexcr.2005.10.032. [DOI] [PubMed] [Google Scholar]