Abstract
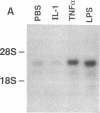
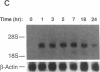
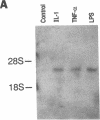
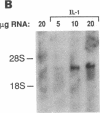
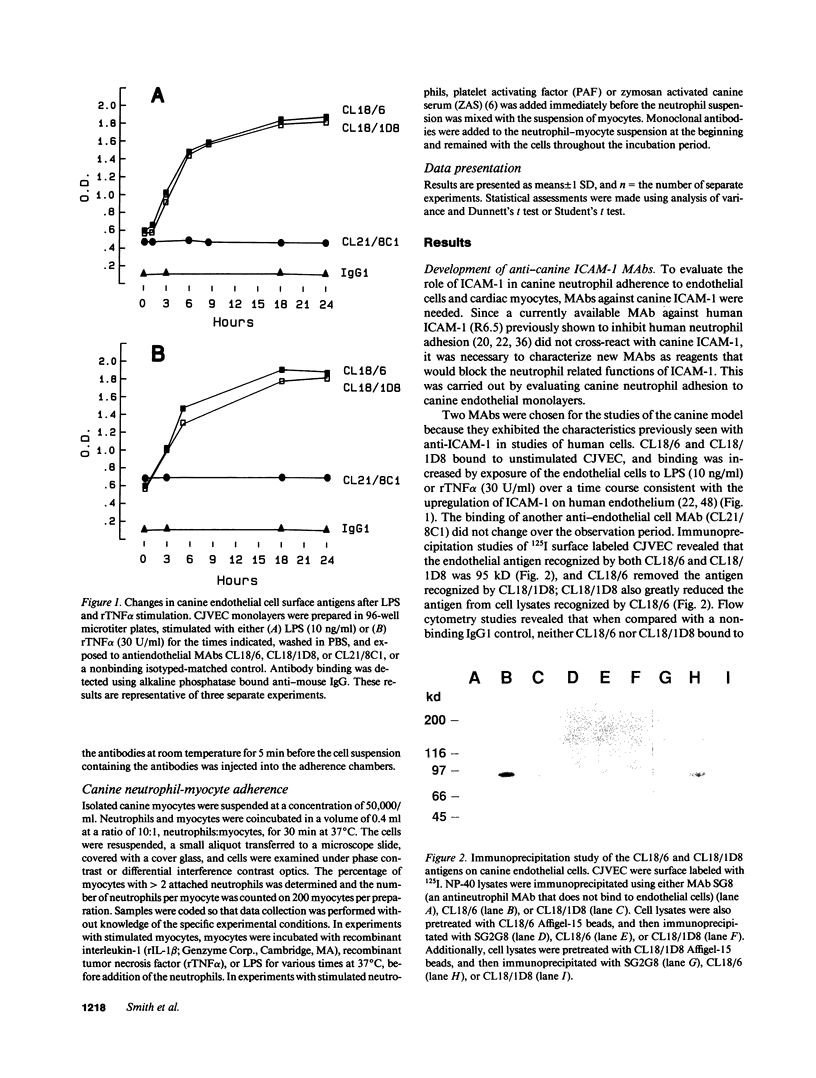
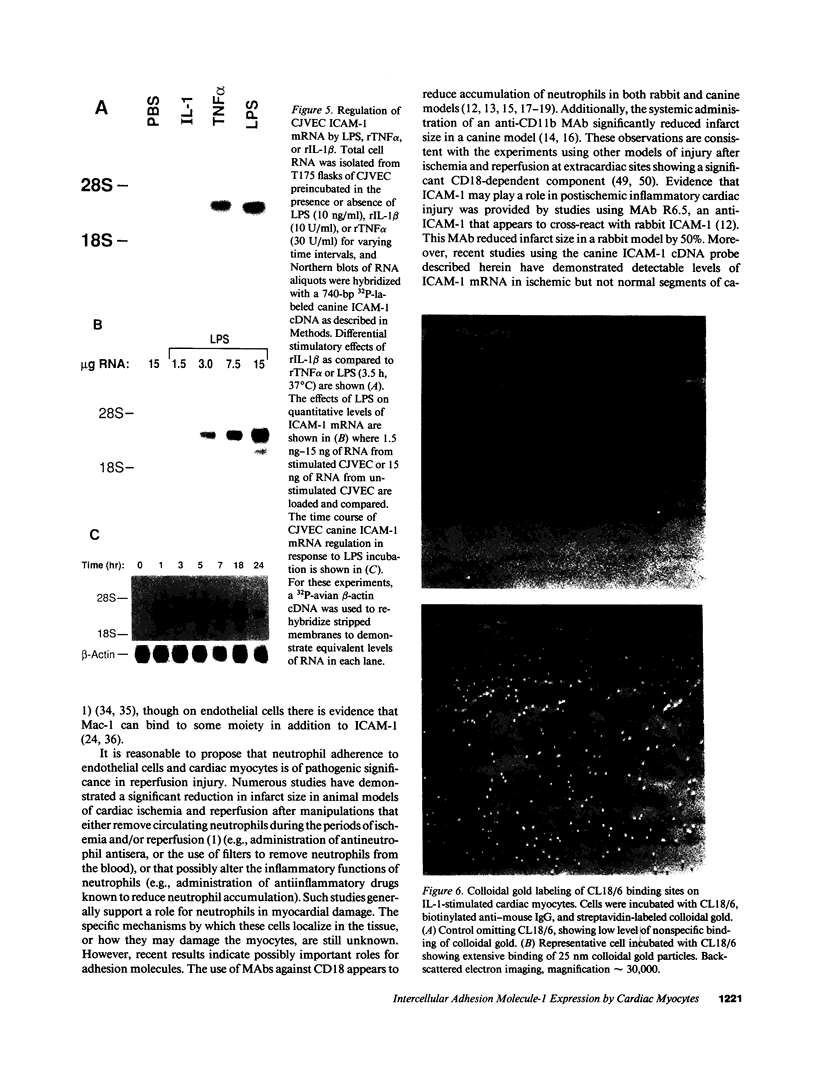
The adhesiveness of isolated canine cardiac myocytes for neutrophils is greatly increased by stimulation with cytokines such as tumor necrosis factor alpha (TNF alpha). Since this adhesion is significantly inhibited by an anti-CD18 MAb, experiments were performed to test the hypothesis that the newly expressed adhesion molecule on the cardiac myocytes was intercellular adhesion molecule-1 (ICAM-1). A newly developed MAb, CL18/6, was found to exhibit the functional and binding characteristics with canine neutrophils and canine jugular vein endothelial cells expected of an antibody recognizing ICAM-1. MAb CL18/6 also bound to isolated cardiac myocytes after stimulation of the myocytes with cytokines, and it blocked by greater than 90% the adhesion of neutrophils to stimulated myocytes. A partial cDNA clone for canine ICAM-1 was isolated, and ICAM-1 mRNA was found to be increased in both endothelial cells and cardiac myocytes after cytokine stimulation. Cytokines that both increased the CL18/6-inhibitable adhesion of neutrophils to myocytes and induced expression of ICAM-1 were IL-1 beta, TNF alpha, and LPS. These results are consistent with the conclusion that canine endothelial cells and cardiac myocytes express ICAM-1 in response to cytokine stimulation, and that ICAM-1 functions as an adhesive molecule for neutrophils on both cell types.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Freeman K. L., Heerdt B., Hughes B. J., Jack R. M., Smith C. W. Abnormal stimulated adherence of neonatal granulocytes: impaired induction of surface Mac-1 by chemotactic factors or secretagogues. Blood. 1987 Sep;70(3):740–750. [PubMed] [Google Scholar]
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Ballantyne C. M., O'Brien W. E., Beaudet A. L. Nucleotide sequence of the cDNA for murine intercellular adhesion molecule-1 (ICAM-1). Nucleic Acids Res. 1989 Jul 25;17(14):5853–5853. doi: 10.1093/nar/17.14.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer W. J., Smith C. W., Michael L. H., Rossen R. D., Hughes B. J., Entman M. L., Anderson D. C. Canine neutrophil activation by cardiac lymph obtained during reperfusion of ischemic myocardium. Circ Res. 1989 Dec;65(6):1751–1762. doi: 10.1161/01.res.65.6.1751. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Peterson M. A., Dobbs A., Schmid-Schönbein G. W. Accumulation of polymorphonuclear leukocytes during 3-h experimental myocardial ischemia. Am J Physiol. 1986 Jul;251(1 Pt 2):H93–100. doi: 10.1152/ajpheart.1986.251.1.H93. [DOI] [PubMed] [Google Scholar]
- Entman M. L., Youker K., Shappell S. B., Siegel C., Rothlein R., Dreyer W. J., Schmalstieg F. C., Smith C. W. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J Clin Invest. 1990 May;85(5):1497–1506. doi: 10.1172/JCI114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ford J. W., Burkel W. E., Kahn R. H. Isolation of adult canine venous endothelium for tissue culture. In Vitro. 1981 Jan;17(1):44–50. doi: 10.1007/BF02618029. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M. Consequences of leukocyte-vessel wall interactions in inflammatory and immune reactions. Semin Thromb Hemost. 1987 Oct;13(4):434–444. doi: 10.1055/s-2007-1003520. [DOI] [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Hook B. G., Abrams G. D., Kunkel S. L., Lucchesi B. R. Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am Heart J. 1986 Oct;112(4):682–690. doi: 10.1016/0002-8703(86)90461-8. [DOI] [PubMed] [Google Scholar]
- Kamb A., Weir M., Rudy B., Varmus H., Kenyon C. Identification of genes from pattern formation, tyrosine kinase, and potassium channel families by DNA amplification. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4372–4376. doi: 10.1073/pnas.86.12.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. K., Detmers P. A., Levin S. M., Wright S. D. Transient adhesion of neutrophils to endothelium. J Exp Med. 1989 May 1;169(5):1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. K., Van Seventer G. A., Levin S. M., Wright S. D. Two leukocyte receptors (CD11a/CD18 and CD11b/CD18) mediate transient adhesion to endothelium by binding to different ligands. J Immunol. 1989 Nov 15;143(10):3325–3329. [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rossen R. D., Swain J. L., Michael L. H., Weakley S., Giannini E., Entman M. L. Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle. A possible initiator of an extra myocardial mechanism of ischemic injury. Circ Res. 1985 Jul;57(1):119–130. doi: 10.1161/01.res.57.1.119. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Engler R. L. Granulocytes as active participants in acute myocardial ischemia and infarction. Am J Cardiovasc Pathol. 1987 Jan;1(1):15–30. [PubMed] [Google Scholar]
- Shappell S. B., Toman C., Anderson D. C., Taylor A. A., Entman M. L., Smith C. W. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990 Apr 1;144(7):2702–2711. [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Fantone J. C., Mickelson J. K., Griffin J. D., Lucchesi B. R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988 Feb;81(2):624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Mickelson J. K., Fantone J. C., Gallagher K. P., Lee K. A., Tamura Y., Cronin M., Lucchesi B. R. Sustained limitation of myocardial reperfusion injury by a monoclonal antibody that alters leukocyte function. Circulation. 1990 Jan;81(1):226–237. doi: 10.1161/01.cir.81.1.226. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Tonnesen M. G., Anderson D. C., Springer T. A., Knedler A., Avdi N., Henson P. M. Adherence of neutrophils to cultured human microvascular endothelial cells. Stimulation by chemotactic peptides and lipid mediators and dependence upon the Mac-1, LFA-1, p150,95 glycoprotein family. J Clin Invest. 1989 Feb;83(2):637–646. doi: 10.1172/JCI113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder N. B., Fouty B. W., Winn R. K., Harlan J. M., Rice C. L. Role of neutrophils in generalized reperfusion injury associated with resuscitation from shock. Surgery. 1989 Sep;106(3):509–516. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. A., McIntyre T. M. Neutrophil adherence to human endothelium in vitro occurs by CDw18 (Mo1, MAC-1/LFA-1/GP 150,95) glycoprotein-dependent and -independent mechanisms. J Clin Invest. 1988 Feb;81(2):531–537. doi: 10.1172/JCI113351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorgeril M., Basmadjian A., Lavallée M., Clément R., Millette D., Rousseau G., Latour J. G. Influence of leukopenia on collateral flow, reperfusion flow, reflow ventricular fibrillation, and infarct size in dogs. Am Heart J. 1989 Mar;117(3):523–532. doi: 10.1016/0002-8703(89)90724-2. [DOI] [PubMed] [Google Scholar]